Abstract
Fibromyalgia is a chronic pain syndrome characterized by widespread pain, fatigue, and memory and mood disturbances. Despite advances in our understanding of the underlying pathophysiology, treatment is often challenging. New research indicates that changes in functional connectivity between brain regions, as can be measured by magnetic resonance imaging (fcMRI) of the resting state, may underlie the pathogenesis of this and other chronic pain states. As such, this parameter may be able to be used to monitor changes in brain function associated with pharmacological treatment, and might also be able to predict treatment response.
We performed a resting state fcMRI trial using a randomized, placebo-controlled, cross-over design to investigate mechanisms of action of milnacipran (MLN), a selective serotonin and norepinephrine reuptake inhibitor (SNRI), in fibromyalgia patients. Our aim was to identify functional connectivity patterns at baseline that would differentially predict treatment response to MLN as compared to placebo. Since preclinical studies of MLN suggest that this medication works by augmenting antinociceptive processes, we specifically investigated brain regions known to be involved in pain inhibition.
15 fibromyalgia patients completed the study, consisting of 6 weeks of drug and placebo intake (order counterbalanced) with an interspersed 2 week wash out period. As a main finding we report that reductions in clinical pain scores during MLN were associated with decreased functional connectivity between pro-nociceptive regions and antinociceptive pain regions at baseline, specifically between the rostral part of the anterior cingulate cortex (ACC) and the insular cortex (IC), as well as between the periaqueductal gray (PAG) and the IC: patients with lower preexisting functional connectivity had the greatest reduction in clinical pain. This pattern was not observed for the placebo period. However a more robust placebo response was associated with lower baseline functional connectivity between the ACC and the dorsolateral prefrontal cortex.
This study indicates that ACC–IC connectivity might play a role in the mechanism of action of MLN, and perhaps more importantly fcMRI might be a useful tool to predict pharmacological treatment response.
Keywords: Chronic pain, Fibromyalgia, SNRI, fMRI, Functional connectivity
Abbreviations: 5-HT, serotonin; ACC, anterior cingulate cortex; BPI, brief pain inventory; CNS, central nervous system; DMN, default mode network; DLPFC, dorsolateral prefrontal cortex; fMRI, functional magnetic resonance imaging; fcMRI, functional connectivity magnetic resonance imaging; IC, insular cortex; FEW, family wise error; MCC, mid-cingulate cortex; IPL, inferior parietal lobule; MLN, milnacipran; NE, norepinephrine; PAG, periaqueductal gray; PCC, posterior cingulate cortex; QST, quantitative sensory testing; rs-fc, resting state functional connectivity; SNRI, selective serotonin and norepinephrine reuptake inhibitor; SPM, statistical parametric mapping; TMS, transcranial magnetic stimulation
Highlights
-
•
Resting brain connectivity predicts clinical pain response to milnacipran.
-
•
Different brain connectivity patterns predicted response to placebo treatment.
-
•
Personalized analgesic therapy may be facilitated by brain imaging.
1. Introduction
Fibromyalgia is a chronic pain syndrome of unknown origin, estimated to affect 2–5% in all populations studied (Raspe, 1992; Wolfe et al., 1995; Assumpcao et al., 2009; Dhir et al., 2009). Fibromyalgia is characterized by widespread pain, fatigue, poor sleep, and dyscognition (Wolfe et al., 2010) and is often also associated with mood disorders such as depressive episodes and anxiety. Although the underlying pathophysiology is not entirely understood, fibromyalgia has been associated with augmented central nervous system (CNS) processing of nociceptive stimuli using both quantitative sensory testing (QST) and functional neuroimaging. (Gracely et al., 2002; Desmeules et al., 2003; Petzke et al., 2003; Smith et al., 2008). In this context the notion of dysfunctional endogenous pain inhibition has been proposed to play a pivotal role in the genesis of chronic widespread pain. This is supported by studies demonstrating impaired or absent conditioned pain modulation (CPM) in these patients (Lautenbacher and Rollman, 1997; Vierck et al., 2001; Julien et al., 2005; Chalaye et al., 2014).
In recent years, functional (Gracely et al., 2002; Giesecke et al., 2004; Jensen et al., 2012) and structural (Kuchinad et al., 2007; Schmidt-Wilcke et al., 2007; Ceko et al., 2013) brain imaging studies have shed some light on possible central mechanisms that might play a role in the genesis of chronic pain in fibromyalgia. One such approach is the investigation of fluctuations in blood oxygenation level dependent (BOLD) signals at rest, termed resting state functional connectivity (rs-fc). This method identifies and assesses the interaction between disparate cortical regions and networks. Only recently have changes in rs-fc been demonstrated in fibromyalgia, such as a hyper-connectivity between the default mode network (DMN), a constellation of brain regions involved in self-referential thought, and the insular cortex (IC), a brain region known to play a pivotal role in pain perception (Napadow et al., 2010). Interestingly this hyper-connectivity may be a marker for chronic pain intensity as two independent trials have shown that decreases in connectivity between the DMN and IC following treatment were associated with reductions in clinical pain (Napadow et al., 2012; Harris et al., 2013).
Treatment of fibromyalgia in terms of a clinically relevant reduction in widespread pain is often challenging and both pharmacological and non-pharmacological approaches (Bernardy et al., 2013). To date, three drugs have been approved by the U.S. Food and Drug Administration for the treatment of pain in fibromyalgia: pregabalin, a compound that binds to the α2δ subunit of a voltage dependent presynaptic calcium channel, and two selective serotonin (5-HT) and norepinephrine (NE) reuptake inhibitors, milnacipran (MLN) and duloxetine (Goldenberg et al., 2010; Schmidt-Wilcke and Clauw, 2010; Schmidt-Wilcke and Clauw, 2011). Other drugs, such as tricyclic compounds (TCA, e.g. amitryptiline), viewed as non-selective 5-HT and NE reuptake inhibitors, have also repeatedly been shown to be efficacious in the treatment of fibromyalgia and are frequently used in pharmacological treatment regimens. The way 5-HT and NE reuptake inhibitors act to reduce pain is still a matter of debate as spinal, subcortical, and cortical mechanisms have all been proposed. However the overall effect of any of these individual treatments has been modest (Häuser et al., 2013). Moreover a key limitation in the treatment of fibromyalgia, and chronic pain in general, is that there are no reliable tools to guide treatment assignment for individual patients. As such, clinical routine largely relies on trial and error.
Recently our group has shown that chemical and functional imaging in fibromyalgia can be used to predict treatment response to pregabalin in fibromyalgia (Harris et al., 2013). Here we sought to identify rs-fc patterns that might predict treatment response in fibromyalgia to the selective 5-HT and NE reuptake inhibitor MLN. Since preclinical studies have indicated that dual reuptake inhibitors are thought to have a favorable effect on endogenous pain inhibition which is believed to be dysfunctional in fibromyalgia (Lautenbacher and Rollman, 1997; Julien et al., 2005), we specifically focused on rs-fc to brain regions involved in antinociception and pain modulation such as: the periaqueductal gray (PAG), the rostral part of the anterior cingulate cortex (ACC), the dorsolateral prefrontal cortex (DLPFC) and the amygdala.
2. Material and methods
2.1. Subjects
We investigated 23 female patients diagnosed with fibromyalgia. Inclusion criteria were: (1) meeting 1990 American College of Rheumatology criteria for FM with chronic widespread pain for at least 6 months; (2) 18–70 years of age; (3) non-lactating and non-pregnant; (4) right handed; (5) score between 40 and 90 mm (inclusive) on a 100 mm pain Visual Analogue Scale (VAS); (6) willing to withdraw from CNS-active therapies marketed as antidepressants (monoamine oxidase inhibitors, tricyclics, tetracyclics, selective serotonin reuptake inhibitors, norepinephrine reuptake inhibitors, and SNRIs); (7) willing to withdraw from stimulant medications such as those used to treat attention deficit disorder and attention deficit hyperactivity disorder (e.g. mixed amphetamine salts, methylphenidate, dextroamphetamine) or fatigue associated sleep apnea or shift work (e.g. modafinil); (8) willing to withdraw from anorectic agents such as diethylpropion, sibutramine, and phentermine; and (9) if currently taking pregabalin and/or gabapentin, to remain on a stable dosage throughout the duration of the study. Major exclusion criteria were: (1) significant risk of suicide; (2) medical conditions including cardiac diseases, glaucoma, autoimmune disease, systemic infections (e.g. human immunodeficiency virus, hepatitis), active cancer, pulmonary disease or dysfunction, unstable endocrine disease (must be stable at least 3 months prior to study enrollment), unstable diabetes, unstable thyroid disease; (3) pregnant or lactating; (4) any other severe, acute, or chronic medical or psychiatric conditions that could increase risk or interfere with trial results; (5) body mass index greater than 36; (6) treatment with any experimental agent, including MLN, within 30 days before screening; and (7) contraindications with MRI procedures.
All study participants gave written informed consent. The study protocol and informed consent documents were approved by the University of Michigan Institutional Review Board (Ann Arbor, Michigan) and Forest Laboratories (New York, NY). All clinical data were verified for accuracy and the database was locked before analysis. All imaging data were stored, validated, analyzed, and assessed for quality at the University of Michigan independent of Forest personnel. Patient demographics, medications, and identification of inclusion for analysis are listed in Table 1.
Table 1.
Patients included in analysis, demographics, and medications/supplements.
| Patient | Age | Race | BMI | Medications and supplements |
|---|---|---|---|---|
| 1 | 54 | Caucasian | 33 | Tramadol, Vitamin D, Fish Oil |
| 2 | 26 | Caucasian | 36 | Lyrica, Vitamin D, Metaformin |
| 3 | 30 | Caucasian | 31 | Metronidazole, Benadryl, Motrin |
| 4 | 42 | Caucasian | 27 | Ibuprofen, Sudafed |
| 5 | 53 | Caucasian | 33 | Di-nox, Anacin, Aleve, Ibuprofen , Vitamin D, Vitamin B Complex, Pednisone, Mobic, Mucinex, Ventolin, Airborne |
| 6 | 36 | African American | 37 | Amlodipine Besylate, Lisinopril-HCTZ, Aleve |
| 7 | 40 | Caucasian | 24 | Nuvaring, Ibuprofen, Skelaxin, Vicodin, Tylenol, Quasense |
| 8 | 36 | Caucasian | 21 | Synthroid, Tylenol, Acetaminophen, Motrin, Ibuprofen |
| 9 | 39 | Caucasian | 21 | Nasonex, digestive enzyme, Calcium/Magnesium/Vitamin D |
| 10 | 50 | Caucasian | 29 | Lisinopril/hctz, Amoxicillin |
| 11 | 30 | Caucasian | 28 | Xanax, Cataphlam, Aleve |
| 12 | 40 | Caucasian | 23 | Motrin, Mega Dose Multivitamin/Mineral Complex, Probiotic |
| 13 | 53 | African American | 26 | B-complex 50, Vitamin D, Coq 10, Maca Herb, Omega 3 Fish oil, Hyurunate Acid, Motrin, Excedrin |
| 14 | 27 | Caucasian | 32 | Levothyroxine, Vitron-C, Singulair,Albuterol Sulfate, Vicodin, Multivitamin, hycosamine, Folic Acid, B-6, Rogaine, Zyrtec, Magnesium citrate, Cyclobenzabrine, Maxalt, Tylenol, Cortizone shots, Kelp, Vitamin D, Cephalexin |
| 15 | 55 | Caucasian | 35 | Avapro Norvasc, Aldactazyde, Detrol LA, Lyrica, Synthroid, Taclonex, Diprolene Gel, Aspirin, Vitamin D, Iron, Calcium, multivitamin, Tylenol, Motrin, Minocycline, methotrexate, Methotrexate |
BMI = Body Mass Index.
2.2. Treatment
All patients were randomized in a double-blind, two-period crossover study of MLN versus placebo (Fig. 1). Potential participants underwent an initial visit, prior to the first neuroimaging session, wherein they were evaluated for study criteria. After meeting inclusion/exclusion criteria, consenting patients were randomized to either MLN first or placebo first for Period 1, and which followed 1–4 week washout period to withdraw from all excluded medications that could interfere with efficacy and neuroimaging measures. This washout period included a 1-week single-blind placebo run-in period to reduce the possibility of placebo effects during the first double-blind treatment period. Following the placebo run-in period, all participants underwent their first neuroimaging scan (pretreatment for Period 1) which involved functional connectivity magnetic resonance imaging (fcMRI). Following this initial scan, subjects randomized to receive MLN in the first period, underwent dose escalation of MLN up to 200 mg/day over the course of 2 weeks, with a maintained fixed dose for 4 weeks, at which time an identical post-treatment fcMRI session was conducted. Those subjects randomized to placebo for Period 1 took matching placebo pills over the course of 6 weeks before undergoing an identical post-treatment fcMRI session. Results from post-treatment scans will be presented elsewhere. All participants then entered a 1 week taper and 2 weeks of a placebo washout, during which time a placebo sugar pill was consumed daily. Once the washout period was completed, all patients crossed over to the other study drug for Period 2 (i.e. those who had MLN for Period 1 received placebo for Period 2 and vice versa.). Neuroimaging sessions for Period 2 were identical to those in Period 1. Placebo data included in all analyses came from either Period 1 or Period 2 (depending on treatment order), and were included for all subjects regardless of treatment order. All subjects were informed that they would be dosed with MLN or placebo at various times throughout the study, but were not told when they were transferred from one treatment to the other. All investigators and research team members were blinded to study drug and placebo timing.
Fig. 1.
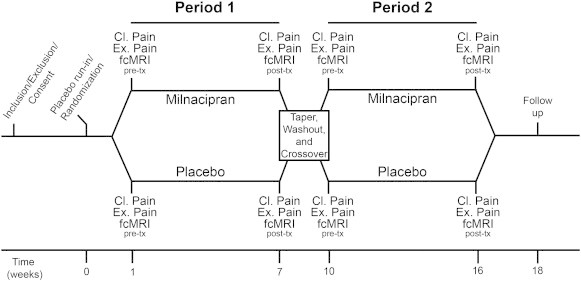
Overview of study design. Subjects underwent a baseline visit where they were assessed for study criteria. Those that met inclusion/exclusion criteria were consented and randomized into the milnacipran or placebo treatment arm. Both clinical pain and experimental pain were assessed at each neuroimaging visit. Prior to their first neuroimaging session, subjects began a 1 week placebo run-in period. Patients that entered the milnacipran period first were dose escalated over 2 weeks to a stable dose of 200 mg/day, and maintained this fixed daily dose for 4 weeks. Subjects randomized to placebo for the first period took matching placebo pills for 6 weeks. After the 6 total weeks for this period, subjects underwent a post-treatment neuroimaging session. Following the completion of period one, all subjects underwent a 1 week taper and 2 weeks of placebo washout. After the washout period, participants crossed over to the other study drug for the second period. MRI = magnetic resonance imaging; tx = treatment.
2.3. Pain assessment and analysis
We assessed treatment response for both clinical pain as well as evoked experimental pain in all participants prior to and following each study period. Clinical pain was assessed with the Short Form of the Brief Pain Inventory (BPI) which captures both pain severity (BPI Sev) and interference due to pain (BPI Int) (Cleeland and Ryan, 1994) measured over the course of the previous week. Changes in these components (post minus pre) were used as measures of treatment response for clinical pain. Changes in pain both following MLN and placebo were assessed in SPSS, version 20, by performing paired t-tests. Further, we specifically investigated whether changes following MLN treatment differed significantly from changes following placebo treatment by performing paired samples t-tests of the change scores for all behavioral measures, also in SPSS, version 20. When assessing for responders compared to non-responders to a treatment period, we defined a patient to be a responder if she had ≥30% improvement for a behavioral measure after a treatment period. Patients with <30% improvement after a treatment period were categorized as non-responders.
Evoked experimental pain data was also collected in and outside the MRI scanner before and after each treatment period. Pressure-pain was administered to the left thumbnail with three distinct conditions using the Multi-modal Automated Sensory Testing (MAST) system (Harte et al., 2013): 1) an equal pressure condition of 1.5 kg/cm2, 2) the amount of pressure required to elicit Pain50 on the 0–100 NRS, and 3) a faint touch rest condition. Both behavioral and BOLD fMRI response data will be analyzed and reported in a subsequent manuscript.
2.4. Resting state functional connectivity magnetic resonance imaging (fcMRI) acquisition and analysis
2.4.1. Data acquisition and pre-processing
In this investigation we were primarily interested in identifying baseline neuroimaging parameters that could predict changes in clinical and evoked pain specifically following treatment with MLN. Resting state fMRI data were acquired using a T2*-weighted spiral sequence (TR = 2.0 s, TE = 30 ms, FA = 90°, matrix size 64 × 64 with 43 slices, FOV = 20 cm and 3.12 × 3.12 × 3 mm voxels), using a 3 Tesla General Electric, Signa scanner 9.0, VH3 with 16 rod birdcage transmit–receive radio frequency coil. During the 6 min resting state fMRI acquisition period (180 scans), subjects were asked to remain awake with their eyes open and to stare at a motionless cross presented on the screen. Minimal cognitive tasks such as staring at a cross are thought not to disrupt resting state networks (Greicius et al., 2003). The first 6 images of the resting state scan were discarded from the data set and not analyzed in order to avoid equilibration effects. A T-1 weighted structural gradient echo data set (TR 1400 ms, TE 1.8 ms, flip angle 15°, FOV 256 × 256, yielding 124 sagittal slices with a defined voxel size of 1 × 1 × 1.2 mm) was also acquired for each subject.
Data were pre-processed and analyzed using FSL (http://www.fmrib.ox.ac.uk/fsl) and Statistical Parametric Mapping software packages (SPM, version 8, Functional Imaging Laboratories, London, UK), as well as the functional connectivity toolbox Conn (Cognitive and Affective Neuroscience Laboratory, Massachusetts Institute of Technology, Cambridge, USA) running under Matlab 7.5b (Mathworks, Sherborn, MA, USA). Upon collection of the functional data, cardiorespiratory artifacts were corrected for using the RETROICOR (Hu et al., 1995; Pfeuffer et al., 2002) algorithm in FSL. Pre-processing steps included motion correction (realignment to the first image of the time series), normalization to the standard SPM–EPI template (generating 2 × 2 × 2 mm resolution images) and smoothing (convolution with an 8 mm FWHM Gaussian Kernel). Subject head motion was assessed by evaluating three translations and three rotations for each scan. Translational thresholds were set to ±2 mm, while rotational thresholds were limited to ±1°. A subject was to be excluded from the analysis if head motion exceeded either of the thresholds in one of the six dimensions.
As SNRIs are thought to augment antinociceptive mechanisms, we were particularly interested in rs-fc to brain regions known to be involved in the descending antinociceptive/pain modulatory system. The following regions were chosen based on the current literature suggesting involvement in antinociception/pain modulation (Bingel, 2010): three seeds covering the rostral ACC, the ventral (vACC), pre-genual (pgACC) and subgenual (sgACC) regions, and bilateral seeds in: the dorsolateral prefrontal cortex (DLPFC), the amygdala, and the periaqueductal gray (PAG); seed regions were created using the SPM extension tool Marsbar. All seed regions were created as spheres. For further information including size and location, refer to Fig. 2.
Fig. 2.
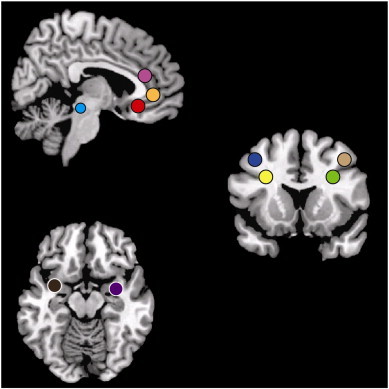
Region of interest seeds included in the rs-fc analysis. Fig. 2 displays a priori seed regions included in the resting state functional connectivity analysis. Three anterior cingulate cortex spheres with radii of 5 mm included the ventral ACC (x = −3, y = 32, z = 19), the pre-genual ACC (x = 0, y = 40, z = 0), subgenual ACC (x = −3, y = 32, z = –8), two periaqueductal gray spherical seeds with 4 mm radii (left: x = 6, y = −26, z = –14; right: x = −6, y = −26, z = –14), four dorsolateral prefrontal cortex spherical seeds of 5 mm radii (left medial-inferior: x = −30, y = 15, z = 29; left lateral-superior: x = −40, y = 12, z = 44; right medial-inferior: x = 30, y = 15, z = 29; right lateral-superior: x = 40, y = 12, z = 44), and two amygdala spherical seeds of 5 mm radii (left: x = −32, y = 2, z = –16; right: x = 32, y = 2, z = 16).
Within the Conn toolbox, seed regions' time-series were extracted; white matter, cerebrospinal fluid, and realignment parameters were entered into the analysis as covariates of no interest. A band-pass filter (frequency window: 0.01–0.1 Hz) was applied, thus removing linear drift artifacts and high frequency noise. First level analyses were performed correlating seed region signal with voxel signal throughout the whole brain, thereby creating seed region to voxel connectivity maps (one map per seed per individual).
2.4.2. Association of rs-fc with pain reduction (group level analysis)
For group analyses we focused on pre- MLN rs-fc as a predictor of subsequent change in pain resulting from the drug. Results from these regions were then compared to placebo. Our primary approach was to enter pre-treatment connectivity maps in a multiple regression analysis with changes in pain (post-MLN minus pre-MLN) in SPM. Results were deemed significant using a family wise error (FWE) cluster corrected threshold of p < 0.05. Within a priori brain regions, including: the IC, posterior cingulate cortex (PCC), precuneus, inferior parietal lobule (IPL), and DLPFC, a small volume correction using a sphere with a radius of 5 mm was performed, and results were deemed significant at a (FWE) cluster level corrected threshold of p < 0.05. These regions had been selected as a priori regions because we were specifically interested in rs-fc changes to regions known to be involved in pain processing, which allowed for small volume correction analysis based on previously published results. Significant results were then extracted and entered into SPSS, version 20, to assess for outliers. To determine if these results were specific to MLN, identical analyses were performed for the placebo period, using the significant regions identified as predicting response to MLN. In order to rule out chance differences by treatment period at baseline, we compared the MLN and placebo correlations for the two cross-over periods separately (i.e. those that had MLN first and those that had placebo first). Correlations were performed in SPSS, version 20, and results were found to be significant at p < 0.05.
Finally, we sought to incorporate multiple functional connectivity measures in a regression analysis to explore for collinearity amongst our rs-fc outcomes and to further improve prediction of treatment response, while simultaneously controlling for pre-treatment pain levels (Harris et al., 2013). To this end a multiple linear regression model was created with pre-treatment pain measures and rs-fc correlation values serving as independent variables and post-treatment pain as the dependent variable. A model was first constructed with each rs-fc measure individually and then in combination (when multiple rs-fc values predicted the same pain outcome) by way of forward selection. Significance was set at p < 0.05.
3. Results
Twenty-three female fibromyalgia patients were enrolled in the study. Eight were excluded from the analysis for the following reasons: six withdrew prior to completing all neuroimaging sessions (two prematurely stopped taking MLN, two had side effects from the drug that prevented participation in the trial, and two terminated due to personal reasons), and two additional participants were excluded because they did not reach the pre-specified dose of MLN before imaging. The drop-out rate due to adverse events of the medication was 9% (2 of 23 participants). Thus fifteen subjects (mean age: 40.7 ± 10.2) were included in the present analysis. Of these fifteen subjects, thirteen were Caucasian, while two were African American. The mean Body Mass Index of this sample was 29.1 ± 5.3. All subjects were confirmed to be right-handed. Additional details including medications and supplements for all patients are contained in Table 1.
3.1. Clinical pain assessment
Multiple dimensions of clinical pain were found to show significant decreases and trends towards decreased pain after treatment with MLN but not placebo (BPI Sev change; MLN: mean = −0.88 ± 1.8, p = 0.076; PBO: mean = −0.17 ± 2.3, p = 0.78; BPI Int change; MLN: mean = −1.1 ± 1.7, p = 0.03; PBO: mean = −0.56 ± 2.1, p = 0.31). When comparing the effects of treatment periods to each other, no significant differences between MLN and placebo treatment were observed (BPI Sev: p = 0.39, BPI Int: p = 0.50). According to our a priori definition of responders: 5 of 15 patients (33%) were responders to MLN and 3 of 15 patients (20%) were responders to placebo treatment for BPI Sev, whereas 7 of 15 patients (47%) were responders to MLN treatment and 4 of 15 patients (27%) were responders to placebo for BPI Int scores (Table 2).
Table 2.
Behavioral data.
| MLN Period |
PBO Period |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Age | Resp | pre BPI Sev | pre BPI Int | post BPI Sev | post BPI Int | Resp | pre BPI Sev | pre BPI Int | post BPI Sev | post BPI Int |
| FM01 | 36 | ΦѰ | 4.8 | 1.9 | 2.8 | 1.0 | Φ | 3.0 | 1.0 | 1.5 | 1.3 |
| FM02 | 40 | 7.8 | 8.1 | 6.8 | 9.0 | 6.3 | 7.9 | 5.0 | 8.4 | ||
| FM03 | 42 | ΦѰ | 4.3 | 0.9 | 1.0 | 0.4 | ΦѰ | 6.3 | 2.7 | 3.8 | 0.6 |
| FM04 | 54 | 7.0 | 6.9 | 7.0 | 5.7 | 7.3 | 6.6 | 7.0 | 6.6 | ||
| FM05 | 40 | 3.3 | 2.0 | 2.3 | 2.4 | Ѱ | 3.0 | 4.7 | 4.5 | 2.0 | |
| FM06 | 26 | 4.0 | 6.3 | 6.8 | 5.7 | 4.0 | 4.3 | 5.0 | 6.7 | ||
| FM07 | 55 | ΦѰ | 7.0 | 6.6 | 4.3 | 4.4 | 7.3 | 7.9 | 6.8 | 6.3 | |
| FM08 | 30 | 8.5 | 8.0 | 8.3 | 8.6 | 6.8 | 3.0 | 6.3 | 5.0 | ||
| FM09 | 39 | 5.5 | 6.3 | 6.3 | 5.1 | ΦѰ | 5.8 | 6.3 | 1.0 | 0.7 | |
| FM10 | 53 | Φ | 4.3 | 1.1 | 1.5 | 3.3 | 1.3 | 4.3 | 7.0 | 3.7 | |
| FM11 | 50 | ΦѰ | 5.3 | 6.0 | 3.3 | 2.3 | 3.5 | 3.0 | 5.0 | 5.1 | |
| FM12 | 53 | Ѱ | 7.0 | 5.9 | 4.8 | 3.4 | 6.3 | 5.0 | 5.0 | 4.3 | |
| FM13 | 27 | Ѱ | 8.0 | 9.4 | 6.0 | 6.0 | 7.3 | 9.0 | 8.0 | 8.6 | |
| FM14 | 30 | Ѱ | 5.5 | 6.9 | 6.5 | 3.3 | 5.8 | 2.1 | 5.5 | 2.0 | |
| FM15 | 36 | 4.5 | 4.1 | 6.0 | 3.6 | Ѱ | 4.5 | 5.1 | 4.3 | 3.1 | |
Table 2 includes behavioral data from all 15 patients that were analyzed in this study. Both pre- and post-treatment values are included from the MLN and placebo periods. A responder to a treatment period was defined as a 30% improvement from the pre-scan time point to the post-scan time point. Φ = responder for BPI Sev, Ѱ = responder for BPI Int. FM = fibromyalgia, BPI Sev = Brief Pain Inventory – Severity component score, BPI Int = Brief Pain Inventory – Interference component score, MLN = milnacipran, PBO = placebo.
3.2. Pre-treatment rs-fc outcomes predict response to MLN treatment and placebo
We found strong associations between baseline rs-fc values (i.e. rs-fc correlation values between our pre-specified antinociceptive brain regions and other brain regions involved in pain processing/modulation) and changes in clinical and experimental pain measures after treatment periods (Table 3). A significant association was found between rs-fc of the right PAG seed and the right mid-IC, and subsequent reduction in clinical pain severity (BPI Sev; MLN: r = 0.885, p < 0.001; placebo: r = –0.216, p = 0.440; Table 3 and Fig. 3A). In this region, less rs-fc activity was associated with greater reductions in clinical pain following MLN treatment but not placebo. We also observed a significant association between connectivity of the right DLPFC and the left IPL, and changes in BPI Sev during MLN but not placebo (MLN: r = 0.873, p < 0.001; placebo: r = 0.030, p = 0.917; Table 3 and Fig. 3B). With respect to the limbic system, connectivity between the left amygdala and the precuneus/posterior cingulate cortex (PCC) was found to have a negative correlation with change in BPI Sev in response to MLN but not placebo (MLN: r = –0.916, p < 0.001; placebo: r = –0.389, p = 0.152; Table 3 and Fig. 3C). For these regions greater rs-fc was associated with greater reductions in clinical pain following MLN but not placebo. Low levels of connectivity between the pgACC and the right posterior IC were found to be associated with a greater reduction in clinical pain interference (BPI Int) following MLN but not placebo (MLN: r = 0.900, p < 0.001; placebo: r = 0.082, p = 0.771; Table 3 and Fig. 4A).
Table 3.
Connectivity prediction of behavioral response results.
| Milnacipran period |
Placebo period |
|||||||
|---|---|---|---|---|---|---|---|---|
| Pre-tx connectivity (seed to region) | Size (voxels) | Coordinates (MNI) |
r value | p value | r value | p value | ||
| x | y | z | ||||||
| BPI Sev | ||||||||
| R PAG–R midIC | 21 | 34 | −2 | 16 | 0.885 | <0.001 | −0.216 | 0.440 |
| R DLPFC–L IPL | 152 | −40 | −44 | 46 | 0.873 | <0.001 | 0.030 | 0.917 |
| L amyg–PCC/precuneus | 232 | −4 | −64 | 10 | −0.916 | <0.001 | −0.389 | 0.152 |
| BPI Int | ||||||||
| pgACC–R IC | 133 | 36 | −6 | 10 | 0.900 | <0.001 | 0.082 | 0.771 |
| pgACC–L DLPFC | 95 | −52 | 12 | 34 | -0.050 | 0.859 | 0.869 | <0.001 |
Table 3 displays significant results from the pre-treatment scan connectivity prediction analyses for clinical and experimental pain changes to treatment by period. Both milnacipran and placebo period statistics are displayed, with results found to be significant in bold. Statistics from the placebo period are provided to demonstrate no significant effect. ACC = anterior cingulate cortex, pgACC = pregenual anterior cingulate cortex, BPI Int = Short Form of the Brief Pain Inventory–Interference score, BPI Sev = Short Form of the Brief Pain Inventory–Severity score; DLPFC = dorsolateral prefrontal cortex, IC = insular cortex, IPL = inferior parietal lobule, L = left, MNI = Montreal Neurological Institute, PAG = periaqueductal gray, Pre-tx = pre-treatment, R = right.
Fig. 3.
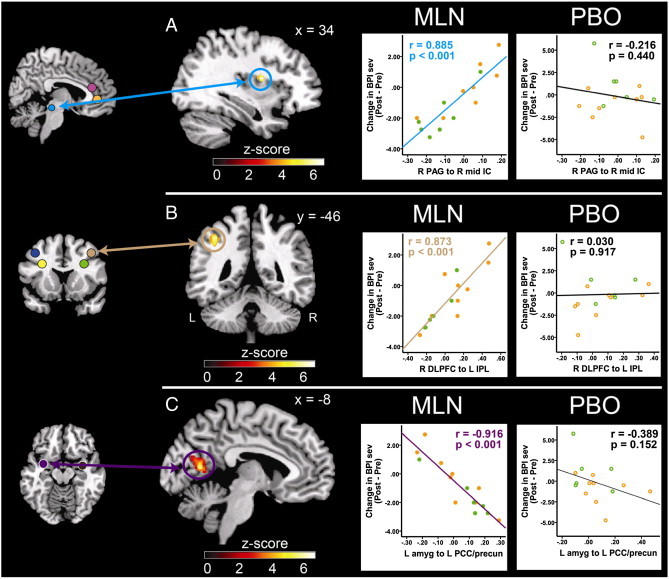
Pre-MLN resting state functional connectivity predicts pain severity reductions in response to MLN treatment. Fig. 3 displays pre-milnacipran (MLN) treatment connectivity as a predictor for clinical response to MLN. Results displayed contain seed-to-target connectivity (seed regions displayed on the left) and plots of significant regressions for the MLN treatment arm (A. Mid-IC; B. DLPFC; C. PCC/precuneus), and corresponding statistics for the placebo treatment period. Orange dots represent patients that had PBO treatment first, and MLN treatment second. Green dots represent patients that received MLN treatment first, and PBO second. ACC = anterior cingulate cortex, amyg = amygdala, BPI Sev = Brief Pain Inventory severity scores, DLPFC = dorsolateral prefrontal cortex, IC = insular cortex, IPL = inferior parietal lobule, L = left, MLN = milnacipran, PAG = periaqueductal gray, PBO = placebo, PCC = posterior cingulate cortex, pre-cun = precuneus, R = right.
Fig. 4.
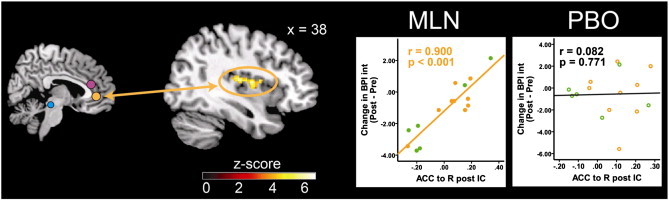
Pre-MLN resting state functional connectivity predicts decrease in pain interference reduction in response to MLN treatment. Fig. 4 displays pre-milnacipran (MLN) treatment connectivity as a predictor for pain response to MLN. Results displayed contain seed-to-target connectivity (seed regions displayed on left) and plots of significant regressions for the MLN treatment arm and corresponding statistics for the placebo treatment period. ACC = anterior cingulate cortex, BPI Int = Brief Pain Inventory interference scores, IC = insular cortex, IPL = inferior parietal lobule, L = left, MLN = milnacipran, PBO = placebo, R = right.
For placebo, lower levels of connectivity between the pgACC and the left DLPFC were associated with greater pain reduction, i.e. patients with less connectivity of these two structures at baseline would showed a greater response in clinical pain interference (BPI Int) during placebo treatment (placebo: r = 0.869, p < 0.001, MLN: r = –0.050, p = 0.859; Table 3, Fig. 5).
Fig. 5.
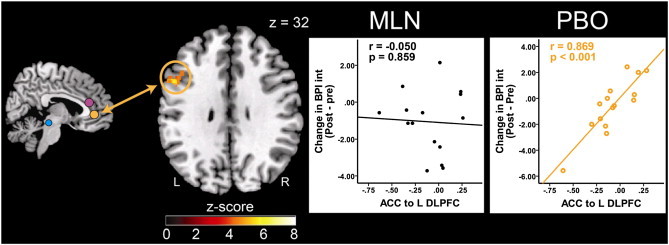
Pre-placebo resting state functional connectivity predicts clinical pain reduction in response to placebo treatment. Fig. 5 displays pre-placebo (PBO) treatment connectivity as a predictor for response to PBO pills. Results displayed contain seed-to-target connectivity (seed regions displayed on left) and plots of significant regressions for the PBO treatment arm, and corresponding statistics for the MLN treatment period. ACC = anterior cingulate cortex, BPI Int = Brief Pain Inventory interference scores, DLPFC = dorsolateral prefrontal cortex, L = left, MLN = milnacipran, PBO = placebo, R = right.
When assessing each treatment period separately, it was found that order of treatment (MLN or placebo administered in the first period) did not impact the results.
Two linear regression models predicting changes in BPI Sev during the MLN period were constructed. The first model for this measure included pre-MLN BPI Sev values (Adjusted R Square = 0.34, p = 0.014), right PAG to right mid-IC connectivity (Adjusted R Square = 0.50, p < 0.001), and right DLPFC to left IPL connectivity (Adjusted R Square = 0.10, p = 0.001) as independent predictors and explained 94% of the variance of post-MLN pain severity. A second model explained 95% of the variance of post-MLN BPI Sev scores which included pre-MLN BPI Sev values (Adjusted R Square = 0.34, p = 0.014), right PAG to right mid-IC connectivity (Adjusted R Square = 0.51, p < 0.001), and left amygdala to right PCC/precuneus (Adjusted R Square = 0.10, p < 0.001) as independent predictors (Table 4).
Table 4.
Pre-treatment connectivity outcomes predict subsequent clinical pain response to milnacipran.
| Dependent variable | Predictor | Standardized β | S.E. unstandardized | p value | R Square full model |
|---|---|---|---|---|---|
| Clinical pain | 0.94 | ||||
| Post-MLN BPI sev | |||||
| Clinical pain: pre-MLN BPI Sev | 0.62 | 0.30 | 0.014 | 0.34 | |
| R PAG to R mid-IC connectivity correlations | 0.70 | 1.64 | <0.001 | 0.84 | |
| R DLPFC to L IPL connectivity correlations | 0.42 | 0.85 | 0.001 | 0.94 | |
| Clinical pain | 0.95 | ||||
| Post-MLN BPI sev | |||||
| Clinical pain: pre-MLN BPI Sev | 0.62 | 0.30 | 0.014 | 0.34 | |
| R PAG to R mid-IC connectivity correlations | 0.70 | 1.64 | <0.001 | 0.85 | |
| L amygdala to PCC/precuneus connectivity correlations | −0.46 | 1.26 | <0.001 | 0.95 | |
| Clinical pain | 0.90 | ||||
| Post-MLN BPI Int | |||||
| Clinical pain: pre-MLN BPI Int | 0.78 | 0.16 | 0.001 | 0.58 | |
| pgACC to R posterior IC connectivity correlations | 0.69 | 1.37 | <0.001 | 0.90 |
Table 4 contains three separate linear regression models, two for post-milnacipran BPI Sev, and one for post-milnacipran BPI Int. These models were built using pre-milnacipran pain measures and pre-milnacipran connectivity correlations as predictors. The models explain 90–95% of the variance in post-milnacipran pain. pgACC = pregenual anterior cingulate cortex, vACC = ventral anterior cingulate cortex, amyg = amygdala, BPI Int = Short Form of the Brief Pain Inventory–Interference score, BPI Sev = Short Form of the Brief Pain Inventory–Severity score, DLPFC = dorsolateral prefrontal cortex, IC = insular cortex, IPL = inferior parietal lobule, L = left, MLN = milnacipran, PAG = periaqueductal gray, PCC = posterior cingulate cortex, R = right, S.E. = standard error. The R Square Full Model column displays overall explained variance in italics for each dependent variable. Below this value, the variance for each predictor, and then the contribution of the addition of subsequent predictors are displayed which total the overall variance.
One additional model was created to explain pain interference. In the first model, independent predictors including pre-MLN BPI Int scores (Adjusted R Square = 0.58, p = 0.001) and pre-MLN connectivity between the pgACC and right posterior IC (Adjusted R Square = 0.32, p < 0.001) explained 90% of the variance of post-MLN pain interference (Table 4).
4. Discussion
We investigated rs-fc of cortical and subcortical structures involved in pain modulation to determine parameters that would predict treatment response to treatment with MLN in patients with fibromyalgia. Importantly, we find that ACC–IC as well as PAG–IC connectivity at baseline were predictive of treatment response: patients that displayed lower pgACC–IC connectivity or PAG–IC connectivity, respectively, showed greater reductions in clinical pain following MLN treatment. Other rs-fc measures were also predictive of clinical response to MLN treatment, such as DLPFC–IPL and amygdala–precuneus/PCC connectivity, while less pgACC–DLPFC connectivity was predictive of placebo response. We hypothesize that a subgroup of fibromyalgia patients with poor connectivity between pro- and antinociceptive brain regions, profits from SNRI treatment, and this pattern reflects a dysfunctional endogenous antinociceptive system or can be viewed as a biomarker thereof.
A dysfunctional endogenous antinociceptive system has been suggested to be a contributor to the genesis of pain in FM (Lautenbacher and Rollman, 1997; Vierck et al., 2001; Julien et al., 2005). There is indeed evidence that CNS levels of the two key neurotransmitters within the antinociceptive system, 5-HT and NE, are lowered in FM as indicated by decreased levels of the corresponding metabolites in the cerebrospinal fluid (Russell et al., 1992; Legangneux et al., 2001). SNRIs are thought to support the antinociceptive system by increasing synaptic 5-HT and NE levels that in turn reduce the nociceptive input to the brain. SNRIs are well characterized with respect to their molecular mechanisms, targeting presynaptic transporter proteins (e.g. SERT), thereby raising synaptic 5-HT and NE levels. However, since 5-HT and NE can bind to different receptor subtypes with different effects and concentrations at different CNS sites, the overall effect of 5-HT and NE reuptake inhibition is highly complex. In the context of pain and pain modulation however predominantly spinal mechanisms are discussed to account for the analgesic effects (Yoshimura and Furue, 2006; Burnham and Dickenson, 2013). For 5-HT this is via binding to 5-HT1A, 5-HT1B and 5-HT1D receptors that lead to pre- and post-synaptic hyperpolarization of pain transmitting neurons. Furthermore 5-HT increases inhibitory transmitter release (e.g. GABA) from interneurons via 5-HT3 and possibly 5-HT2. NE, on the other hand is thought to act via presynaptic α1 receptors leading to a suppression of glutamate release from both Aδ and C afferent fibers, as well as via α2 receptors increasing inhibitory transmitter release from interneurons. Importantly these mechanisms seem to play a beneficial role in a variety of chronic pain conditions, such as diabetic neuropathy (Boyle et al., 2012), osteoarthritis (Chappell et al., 2009) as well as fibromyalgia (Goldenberg et al., 2010; Häuser et al., 2012b) and are not thought to be specific to one particular pain condition. Interestingly for fibromyalgia a recently published study investigating spinal effects of MLN via the assessment of the nociceptive flexion reflex (R III), came to the conclusion that MLN must also have supraspinal effects, based on the observation that there was a dose dependent analgesic effect in the absence of an MLN associated modulation of the nociceptive flexion reflex (Matthey et al., 2013). The only study to date that we are aware of to directly test whether diminished descending analgesic activity is predictive of treatment response with these classes of drugs is a study by Yarnitsky and colleagues showing that those neuropathic pain patients with impaired descending activity at baseline on QST were more likely to respond to duloxetine (Yarnitsky et al., 2012).
Our choice of seed regions for the rs-fc connectivity analyses was based on the current literature highlighting the role of some key structures in pain inhibition and pain modulation, i.e. the PAG, the rostral ACC, the DLPFC and the amygdala (Petrovic et al., 2002; Wager et al., 2004; Bingel et al., 2007). Our strongest findings, which are also well in line with our a priori hypotheses is, that ACC–IC connectivity and PAG–IC connectivity were predictive of treatment response. Within the endogenous antinociceptive system the PAG plays a central role coordinating via the rostroventromedial medulla (RVM) (Moreau and Fields, 1986; Heinricher et al., 2009) activity in the descending 5-HT and NE pathways that project to the spinal cord to decrease nociceptive routing from the periphery. The PAG itself receives input from both spinal nociceptive neurons a variety of cortical structures. It has been hypothesized that there are two cortical systems that mediate cortical top down modulation; one pathway involves descending input from the rostral ACC (to the prefrontal cortex and then to the PAG); a second pathway arrives at the PAG from the IC via the amygdala (Schweinhardt and Bushnell, 2010). That said, it needs to be acknowledged that most evidence stems from animal research (Fields, 2004) and that the precise mapping of these pathways in humans is currently unknown. However, the notion of the rostral ACC–PAG connectivity (Hardy and Leichnetz, 1981), interacting in this regard, has recently been supported by both functional (Petrovic et al., 2002; Bingel, 2010; Kong et al., 2010) and structural (Stein et al., 2012) imaging studies. The interaction of the rostral ACC and IC in antinociception and pain modulation on the other hand remains to be further elucidated. Both structures are part of a cortical opioidergic network (Petrovic et al., 2002), as such the rostral ACC might exert direct “antinociceptive activity” on the IC cortex, or both structures modulate the PAG either interactively or independently, and rs-fc reflects coordinated activity of the two systems.
When looking at resting state networks the bilateral IC together with the dorsal ACC, MCC and supplementary motor area make up the so called salience network and connectivity within this network influences perceptual decisions on pain. The vACC, pgACC and sgACC as such are not part of the salience network, but rather belong to the executive control network (vACC) and the DMN (pgACC and sgACC) (Beckmann et al., 2005). As such one would not expect highly correlated BOLD activity between the rostral ACC and IC even in healthy subjects; intriguingly a negative correlation in resting state activity between these two regions is predictive of MLN response. In a recently performed study we found an increased rostral ACC–IC rs-fc in young patients with temporomandibular disorder that we interpreted as an early compensatory mechanism in a group of young patients developing a chronic pain condition (Ichesco et al., 2012). Given that rostral ACC–IC connectivity reflects synchronized antinociceptive “effort”, it is tempting to hypothesize that a break down in this mechanism is then associated with further pain chronification, based on an increasingly dysfunctional antinociceptive system.
The other rs-fc patterns that predicted response to MLN treatment were the right DLPFC and the left IPL as well as amygdala and the posterior cingulate cortex. These connections are less well established in pain and antinociception research. The IPL as well as the posterior cingulate cortex are both part of the DMN. The role of the DMN in chronic pain is beginning to be investigated, as in our previous work in fibromyalgia suggesting that IC connectivity to the DMN is associated with increased pain (Napadow et al., 2010). Interestingly in this present work, we show that lowered DMN connectivity to antinociceptive regions, such as the DLPFC, are predictive of response to MLN. Other DMN regions such as the PCC have also been highlighted as being involved in pain in other functional and structural imaging studies (Erpelding et al., 2012).
Another interesting finding of our study is that pgACC–left DLPFC connectivity predicted response to placebo treatment. Fibromyalgia patients with a lower pgACC–left DLPFC connectivity showed greater pain reductions while undergoing placebo treatment. The placebo effect in fibromyalgia patients has recently been investigated in meta-analyses based on randomized, placebo-controlled trials. In these studies, 18–30% of patients have been shown to be placebo responders; as such the magnitude of placebo responders in drug trials of fibromyalgia is similar to that seen in other chronic pain conditions (Häuser et al., 2011; Häuser et al., 2012a). The neural mechanisms underlying the clinical observation that cognitive factors such as beliefs and expectations can modulate pain perception have been investigated using various brain imaging methods (Petrovic et al., 2002; Bingel, 2010), mostly using short term interventions. The role of the DLPFC cortex in placebo research has been underlined by both fMRI studies (Wager et al., 2004; Wager et al., 2011) as well as transcranial magnetic stimulation (TMS) studies describing a significant impairment of placebo analgesia associated with TMS induced functional lesions of the left DLPFC (Krummenacher et al., 2010). As such the DLPFC is viewed as key region for initiating placebo related changes in pain perception, the implementation of which then requires the interaction of other cortical and subcortical brain regions, such as the rostral ACC and the PAG. While increases in the rostral ACC activity and ACC–PAG connectivity during placebo analgesia are most likely to be mediated by opioidergic transmission (Petrovic et al., 2002), less is known about the neural correlates of ACC–DLPFC connectivity. However, our data not only support the notion of ACC–DLPFC playing a key role in placebo analgesia, they also extend it to a clinical setting, where baseline rs-fc predicts placebo effects visible 6 weeks after treatment initiation.
4.1. Individualized medicine
Despite the significant progress that has been made in understanding the pathophysiology of fibromyalgia, these advances have not yet been translated into pharmacological treatment. It is generally thought that the underlying pathophysiology of fibromyalgia is heterogeneous leading to a similar phenotype of chronic widespread pain, with only a subgroup of patients with diminished synaptic 5-HT and NE levels responding to drugs that augment this activity. Despite our small sample size the rates of both drug and placebo responders are well in line with larger, randomized studies. Consistent with this idea, the effect of any one of the United States Food and Drug Administration's approved drugs examined in isolation is modest with a 30% improvement in pain occurring in only 30–40% of patients (Häuser et al., 2012b), which is also the case in other chronic pain states; NSAIDs and opioids for example have modest efficacy in conditions such as osteoarthritis or chronic low back pain (Clauw, 2010). This stresses the need to develop tools that predict treatment response, in order to then design individually tailored therapies (Woolf, 2010). Only very recently have studies indicated that brain imaging might be suited to perform such predictions (Harris et al., 2013; Maarrawi et al., 2013). Resting state fMRI might be a particularly interesting tool, as it is easy and safe to perform with only little demands on patients' cognition and cooperation.
4.2. Limitations
There are some limitations to our study that need to be addressed. First of all our study is based on a rather small study sample, i.e. our findings might not be representative of the larger fibromyalgia population and may only apply to a subgroup of fibromyalgia patients. Also while our drop-out rates, as well as treatment response to MLN and placebo, were comparable to those seen in larger randomized, placebo-controlled trials, our results need to be reproduced in a larger study sample.
As rs-fc analyses allow no assumptions on causality, or on the directedness of influence, it is conceivable that connectivity between two regions could be driven by a third region, not identified in the analysis. Furthermore there is no necessity for a direct causal relationship between brain connectivity patterns identified in this study and clinical response. Dual reuptake inhibitors are likely to act first and foremost on the spinal level (in the context of pain inhibition), and the connectivity measures of forebrain structures might thus relate only indirectly to the site of action. In this scenario connectivity measures would need to be viewed as surrogate markers, however this would not detract from their potential clinical usefulness.
Finally, there are four SNRIs used in clinical practice, including venlafaxine, desvenlafaxine, duloxetine and MLN. MLN blocks 5-HT and NE reuptake to an equal extent whereas greater selectivity at 5-HT sites has been described for venlafaxine and duloxetine. In this regard it is uncertain whether our results can be generalized to other SNRIs or even TCAs. Besides confirmation by studies with larger sample sizes further research is needed to extent our findings to other SNRI and non-selective reuptake inhibitors (e.g. TCAs.).
4.3. Conclusion and outlook
Overall we were able to show that rs-fc patterns of brain structures involved in antinociception and pain modulation might be useful parameters for the prediction of treatment response to the SNRI MLN in fibromyalgia patients. As in clinical practice only a subset of patients respond to pharmacological treatment, such approaches might turn out useful tools to identify subgroups of patients likely to respond to one or the other approach moving towards an individualized medicine. Further research is needed to both confirm and extend our findings.
Acknowledgment
This was an investigator initiated study funded by Forest Laboratories (MD-SAV-09). Authors Ichesco, Hampson, Kairys, and Peltier, have no financial relationships to disclose. Dr. Clauw has consulted for Forest Laboratories, Pfizer, Inc., Cerephex Corporation, Eli Lilly and Company, Merck & Co., Nuvo Research Inc., Tonix Pharmaceuticals, Johnson & Johnson, Pierre Fabre, Cypress Biosciences, Wyeth Pharmaceuticals, UCB, AstraZeneca, Jazz Pharmaceuticals, Abbott Laboratories, and Iroko Pharmaceuticals. Dr. Harris has consulted for Pfizer, Inc. Dr. Harte has consulted for Pfizer, Inc. and analgesic Solutions. Dr. Schmidt-Wilcke was supported by a grant of the DFG (Deutsche Forschungsgemeinschaft, GZ: SchM 2665/1-1). The authors would like to thank Keith Newnham for his technical expertise with MRI acquisition.
References
- Assumpção A., Cavalcante A.B., Capela C.E., Sauer J.F., Chalot S.D., Pereira C.A. Prevalence of fibromyalgia in a low socioeconomic status population. BMC Musculoskeletal Disorders. 2009;10:64. doi: 10.1186/1471-2474-10-64. 19505321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F., DeLuca M., Devlin J.T., Smith S.M. Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society B: Biological Sciences. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardy K., Klose P., Busch A.J., Choy E.H., Häuser W. Cognitive behavioural therapies for fibromyalgia. Cochrane Database of Systematic Reviews. 2013;9:CD009796. doi: 10.1002/14651858.CD009796.pub2. 24018611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingel U. [Mechanisms of endogenous pain modulation illustrated by placebo analgesia: functional imaging findings] Schmerz (Berlin, Germany) 2010;24(2):122–129. doi: 10.1007/s00482-010-0901-7. 20376600 [DOI] [PubMed] [Google Scholar]
- Bingel U., Schoell E., Herken W., Büchel C., May A. Habituation to painful stimulation involves the antinociceptive system. Pain. 2007;131(1–2):21–30. doi: 10.1016/j.pain.2006.12.005. 17258858 [DOI] [PubMed] [Google Scholar]
- Boyle J., Eriksson M.E., Gribble L., Gouni R., Johnsen S., Coppini D.V. Randomized, placebo-controlled comparison of amitriptyline, duloxetine, and pregabalin in patients with chronic diabetic peripheral neuropathic pain: impact on pain, polysomnographic sleep, daytime functioning, and quality of life. Diabetes Care. 2012;35(12):2451–2458. doi: 10.2337/dc12-0656. 22991449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham L.J., Dickenson A.H. The antinociceptive effect of milnacipran in the monosodium iodoacetate model of osteoarthritis pain and its relation to changes in descending inhibition. Journal of Pharmacology and Experimental Therapeutics. 2013;344(3):696–707. doi: 10.1124/jpet.112.199489. 23297162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceko M., Bushnell M.C., Fitzcharles M.A., Schweinhardt P. Fibromyalgia interacts with age to change the brain. NeuroImage. Clinical. 2013;3:249–260. doi: 10.1016/j.nicl.2013.08.015. 24273710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalaye P., Lafrenaye S., Goffaux P., Marchand S. The role of cardiovascular activity in fibromyalgia and conditioned pain modulation. Pain. 2014;155:1064–1069. doi: 10.1016/j.pain.2013.12.023. 24345429 [DOI] [PubMed] [Google Scholar]
- Chappell A.S., Ossanna M.J., Liu-Seifert H., Iyengar S., Skljarevski V., Li L.C. Duloxetine, a centrally acting analgesic, in the treatment of patients with osteoarthritis knee pain: a 13-week, randomized, placebo-controlled trial. Pain. 2009;146(3):253–260. doi: 10.1016/j.pain.2009.06.024. 19625125 [DOI] [PubMed] [Google Scholar]
- Clauw D.J. Pain management: fibromyalgia drugs are ‘as good as it gets’ in chronic pain. Nature Reviews. Rheumatology. 2010;6(8):439–440. doi: 10.1038/nrrheum.2010.120. 20676122 [DOI] [PubMed] [Google Scholar]
- Cleeland C.S., Ryan K.M. Pain assessment: global use of the Brief Pain Inventory. Annals of the Academy of Medicine, Singapore. 1994;23(2):129–138. 8080219 [PubMed] [Google Scholar]
- Desmeules J.A., Cedraschi C., Rapiti E., Baumgartner E., Finckh A., Cohen P. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis and Rheumatism. 2003;48(5):1420–1429. doi: 10.1002/art.10893. 12746916 [DOI] [PubMed] [Google Scholar]
- Dhir V., Lawrence A., Aggarwal A., Misra R. Fibromyalgia is common and adversely affects pain and fatigue perception in north Indian patients with rheumatoid arthritis. Journal of Rheumatology. 2009;36(11):2443–2448. doi: 10.3899/jrheum.090157. 19833753 [DOI] [PubMed] [Google Scholar]
- Erpelding N., Moayedi M., Davis K.D. Cortical thickness correlates of pain and temperature sensitivity. Pain. 2012;153(8):1602–1609. doi: 10.1016/j.pain.2012.03.012. 22516588 [DOI] [PubMed] [Google Scholar]
- Fields H. State-dependent opioid control of pain. Nature Reviews. Neuroscience. 2004;5(7):565–575. doi: 10.1038/nrn1431. 15208698 [DOI] [PubMed] [Google Scholar]
- Giesecke T., Gracely R.H., Grant M.A., Nachemson A., Petzke F., Williams D.A. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis and Rheumatism. 2004;50(2):613–623. doi: 10.1002/art.20063. 14872506 [DOI] [PubMed] [Google Scholar]
- Goldenberg D.L., Clauw D.J., Palmer R.H., Mease P., Chen W., Gendreau R.M. Durability of therapeutic response to milnacipran treatment for fibromyalgia. results of a randomized, double-blind, monotherapy 6-month extension study. Pain Medicine (Malden, Mass.) 2010;11(2):180–194. doi: 10.1111/j.1526-4637.2009.00755.x. 20002596 [DOI] [PubMed] [Google Scholar]
- Gracely R.H., Petzke F., Wolf J.M., Clauw D.J. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis and Rheumatism. 2002;46(5):1333–1343. doi: 10.1002/art.10225. 12115241 [DOI] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. 12506194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S.G., Leichnetz G.R. Cortical projections to the periaqueductal gray in the monkey: a retrograde and orthograde horseradish peroxidase study. Neuroscience Letters. 1981;22(2):97–101. doi: 10.1016/0304-3940(81)90070-7. 6164962 [DOI] [PubMed] [Google Scholar]
- Harris R.E., Napadow V., Huggins J.P., Pauer L., Kim J., Hampson J. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology. 2013;119(6):1453–1464. doi: 10.1097/ALN.0000000000000017. 24343290 [DOI] [PubMed] [Google Scholar]
- Harte S.E., Mitra M., Ichesco E.A., Halvorson M.E., Clauw D.J., Shih A.J. Development and validation of a pressure-type automated quantitative sensory testing system for point-of-care pain assessment. Medical & Biological Engineering & Computing. 2013;51(6):633–644. doi: 10.1007/s11517-013-1033-x. 23381890 [DOI] [PubMed] [Google Scholar]
- Häuser W., Bartram-Wunn E., Bartram C., Tölle T.R. [Placebo responders in randomized controlled drug trials of fibromyalgia syndrome: systematic review and meta-analysis] Schmerz (Berlin, Germany) 2011;25(6):619–631. doi: 10.1007/s00482-011-1106-4. 22120916 [DOI] [PubMed] [Google Scholar]
- Häuser W., Sarzi-Puttini P., Tölle T.R., Wolfe F. Placebo and nocebo responses in randomised controlled trials of drugs applying for approval for fibromyalgia syndrome treatment: systematic review and meta-analysis. Clinical and Experimental Rheumatology. 2012;30(6 Suppl 74):78–87. 23137770 [PubMed] [Google Scholar]
- Häuser, Urrútia, Tort, Uçeyler, Walitt B. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia syndrome. Cochrane Database of Systematic Reviews. 2013;1:CD010292. doi: 10.1002/14651858.CD010292. 23440848 [DOI] [PubMed] [Google Scholar]
- Häuser W., Wolfe F., Tölle T., Uçeyler N., Sommer C. The role of antidepressants in the management of fibromyalgia syndrome: a systematic review and meta-analysis. CNS Drugs. 2012;26(4):297–307. doi: 10.2165/11598970-000000000-00000. 22452526 [DOI] [PubMed] [Google Scholar]
- Heinricher M.M., Tavares I., Leith J.L., Lumb B.M. Descending control of nociception: specificity, recruitment and plasticity. Brain Research Reviews. 2009;60(1):214–225. doi: 10.1016/j.brainresrev.2008.12.009. 19146877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Le T.H., Parrish T., Erhard P. Retrospective estimation and correction of physiological fluctuation in functional MRI. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1995;34(2):201–212. doi: 10.1002/mrm.1910340211. 7476079 [DOI] [PubMed] [Google Scholar]
- Ichesco E., Quintero A., Clauw D.J., Peltier S., Sundgren P.M., Gerstner G.E. Altered functional connectivity between the insula and the cingulate cortex in patients with temporomandibular disorder: a pilot study. Headache. 2012;52:441–454. doi: 10.1111/j.1526-4610.2011.01998.x. 21929661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K.B., Loitoile R., Kosek E., Petzke F., Carville S., Fransson P. Patients with fibromyalgia display less functional connectivity in the brain's pain inhibitory network. Molecular Pain. 2012;8:32. doi: 10.1186/1744-8069-8-32. 22537768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien N., Goffaux P., Arsenault P., Marchand S. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain. 2005;114(1–2):295–302. doi: 10.1016/j.pain.2004.12.032. 15733656 [DOI] [PubMed] [Google Scholar]
- Kong J., Tu P.C., Zyloney C., Su T.P. Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behavioural Brain Research. 2010;211(2):215–219. doi: 10.1016/j.bbr.2010.03.042. 20347878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummenacher P., Candia V., Folkers G., Schedlowski M., Schönbächler G. Prefrontal cortex modulates placebo analgesia. Pain. 2010;148(3):368–374. doi: 10.1016/j.pain.2009.09.033. 19875233 [DOI] [PubMed] [Google Scholar]
- Kuchinad A., Schweinhardt P., Seminowicz D.A., Wood P.B., Chizh B.A., Bushnell M.C. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2007;27(15):4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. 17428976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenbacher S., Rollman G.B. Possible deficiencies of pain modulation in fibromyalgia. Clinical Journal of Pain. 1997;13(3):189–196. doi: 10.1097/00002508-199709000-00003. 9303250 [DOI] [PubMed] [Google Scholar]
- Legangneux E., Mora J.J., Spreux-Varoquaux O., Thorin I., Herrou M., Alvado G. Cerebrospinal fluid biogenic amine metabolites, plasma-rich platelet serotonin and [3H]imipramine reuptake in the primary fibromyalgia syndrome. Rheumatology. 2001;40(3):290–296. doi: 10.1093/rheumatology/40.3.290. [DOI] [PubMed] [Google Scholar]
- Maarrawi J., Peyron R., Mertens P., Costes N., Magnin M., Sindou M. Brain opioid receptor density predicts motor cortex stimulation efficacy for chronic pain. Pain. 2013;154(11):2563–2568. doi: 10.1016/j.pain.2013.07.042. 23900133 [DOI] [PubMed] [Google Scholar]
- Matthey A., Cedraschi C., Piguet V., Besson M., Chabert J., Daali Y. Dual reuptake inhibitor milnacipran and spinal pain pathways in fibromyalgia patients: a randomized, double-blind, placebo-controlled trial. Pain Physician. 2013;16(5):E553–E562. 24077206 [PubMed] [Google Scholar]
- Moreau J.L., Fields H.L. Evidence for GABA involvement in midbrain control of medullary neurons that modulate nociceptive transmission. Brain Research. 1986;397(1):37–46. doi: 10.1016/0006-8993(86)91367-3. 3801864 [DOI] [PubMed] [Google Scholar]
- Napadow V., Kim J., Clauw D.J., Harris R.E. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis and Rheumatism. 2012;64(7):2398–2403. doi: 10.1002/art.34412. 22294427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V., LaCount L., Park K., As-Sanie S., Clauw D.J., Harris R.E. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis and Rheumatism. 2010;62(8):2545–2555. doi: 10.1002/art.27497. 20506181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P., Kalso E., Petersson K.M., Ingvar M. Placebo and opioid analgesia — imaging a shared neuronal network. Science (New York, N.Y.) 2002;295(5560):1737–1740. doi: 10.1126/science.1067176. 11834781 [DOI] [PubMed] [Google Scholar]
- Petzke F., Clauw D.J., Ambrose K., Khine A., Gracely R.H. Increased pain sensitivity in fibromyalgia: effects of stimulus type and mode of presentation. Pain. 2003;105(3):403–413. doi: 10.1016/S0304-3959(03)00204-5. 14527701 [DOI] [PubMed] [Google Scholar]
- Pfeuffer J., Van de Moortele P.F., Ugurbil K., Hu X., Glover G.H. Correction of physiologically induced global off-resonance effects in dynamic echo-planar and spiral functional imaging. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2002;47(2):344–353. doi: 10.1002/mrm.10065. 11810679 [DOI] [PubMed] [Google Scholar]
- Raspe H. [Rheumatism epidemiology in Europe] Sozial- und Präventivmedizin. 1992;37(4):168–178. doi: 10.1007/BF01624572. 1414018 [DOI] [PubMed] [Google Scholar]
- Russell I.J., Vaeroy H., Javors M., Nyberg F. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis and Rheumatism. 1992;35(5):550–556. doi: 10.1002/art.1780350509. 1374252 [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T., Clauw D.J. Pharmacotherapy in fibromyalgia (FM) — implications for the underlying pathophysiology. Pharmacology & Therapeutics. 2010;127(3):283–294. doi: 10.1016/j.pharmthera.2010.03.002. 20388527 [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T., Clauw D.J. Fibromyalgia: from pathophysiology to therapy. Nature Reviews. Rheumatology. 2011;7(9):518–527. doi: 10.1038/nrrheum.2011.98. 21769128 [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T., Luerding R., Weigand T., Jürgens T., Schuierer G., Leinisch E. Striatal grey matter increase in patients suffering from fibromyalgia — a voxel-based morphometry study. Pain. 2007;132(Suppl. 1):S109–S116. doi: 10.1016/j.pain.2007.05.010. 17587497 [DOI] [PubMed] [Google Scholar]
- Schweinhardt P., Bushnell M.C. Pain imaging in health and disease — how far have we come? Journal of Clinical Investigation. 2010;120(11):3788–3797. doi: 10.1172/JCI43498. 21041961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B.W., Tooley E.M., Montague E.Q., Robinson A.E., Cosper C.J., Mullins P.G. Habituation and sensitization to heat and cold pain in women with fibromyalgia and healthy controls. Pain. 2008;140(3):420–428. doi: 10.1016/j.pain.2008.09.018. 18947923 [DOI] [PubMed] [Google Scholar]
- Stein N., Sprenger C., Scholz J., Wiech K., Bingel U. White matter integrity of the descending pain modulatory system is associated with interindividual differences in placebo analgesia. Pain. 2012;153(11):2210–2217. doi: 10.1016/j.pain.2012.07.010. 22959599 [DOI] [PubMed] [Google Scholar]
- Vierck C.J., Jr., Staud R., Price D.D., Cannon R.L., Mauderli A.P., Martin A.D. The effect of maximal exercise on temporal summation of second pain (windup) in patients with fibromyalgia syndrome. Journal of Pain: Official Journal of the American Pain Society. 2001;2(6):334–344. doi: 10.1054/jpai.2001.25533. 14622813 [DOI] [PubMed] [Google Scholar]
- Wager T.D., Atlas L.Y., Leotti L.A., Rilling J.K. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2011;31(2):439–452. doi: 10.1523/JNEUROSCI.3420-10.2011. 21228154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Rilling J.K., Smith E.E., Sokolik A., Casey K.L., Davidson R.J. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science (New York, N.Y.) 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. 14976306 [DOI] [PubMed] [Google Scholar]
- Wolfe F., Clauw D.J., Fitzcharles M.A., Goldenberg D.L., Katz R.S., Mease P. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care & Research. 2010;62(5):600–610. doi: 10.1002/acr.20140. 20461783 [DOI] [PubMed] [Google Scholar]
- Wolfe F., Ross K., Anderson J., Russell I.J., Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis and Rheumatism. 1995;38(1):19–28. doi: 10.1002/art.1780380104. 7818567 [DOI] [PubMed] [Google Scholar]
- Woolf C.J. Overcoming obstacles to developing new analgesics. Nature Medicine. 2010;16(11):1241–1247. doi: 10.1038/nm.2230. 20948534 [DOI] [PubMed] [Google Scholar]
- Yarnitsky D., Granot M., Nahman-Averbuch H., Khamaisi M., Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain. 2012;153(6):1193–1198. doi: 10.1016/j.pain.2012.02.021. 22480803 [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Furue H. Mechanisms for the anti-nociceptive actions of the descending noradrenergic and serotonergic systems in the spinal cord. Journal of Pharmacological Sciences. 2006;101(2):107–117. doi: 10.1254/jphs.crj06008x. 16766858 [DOI] [PubMed] [Google Scholar]


