Abstract
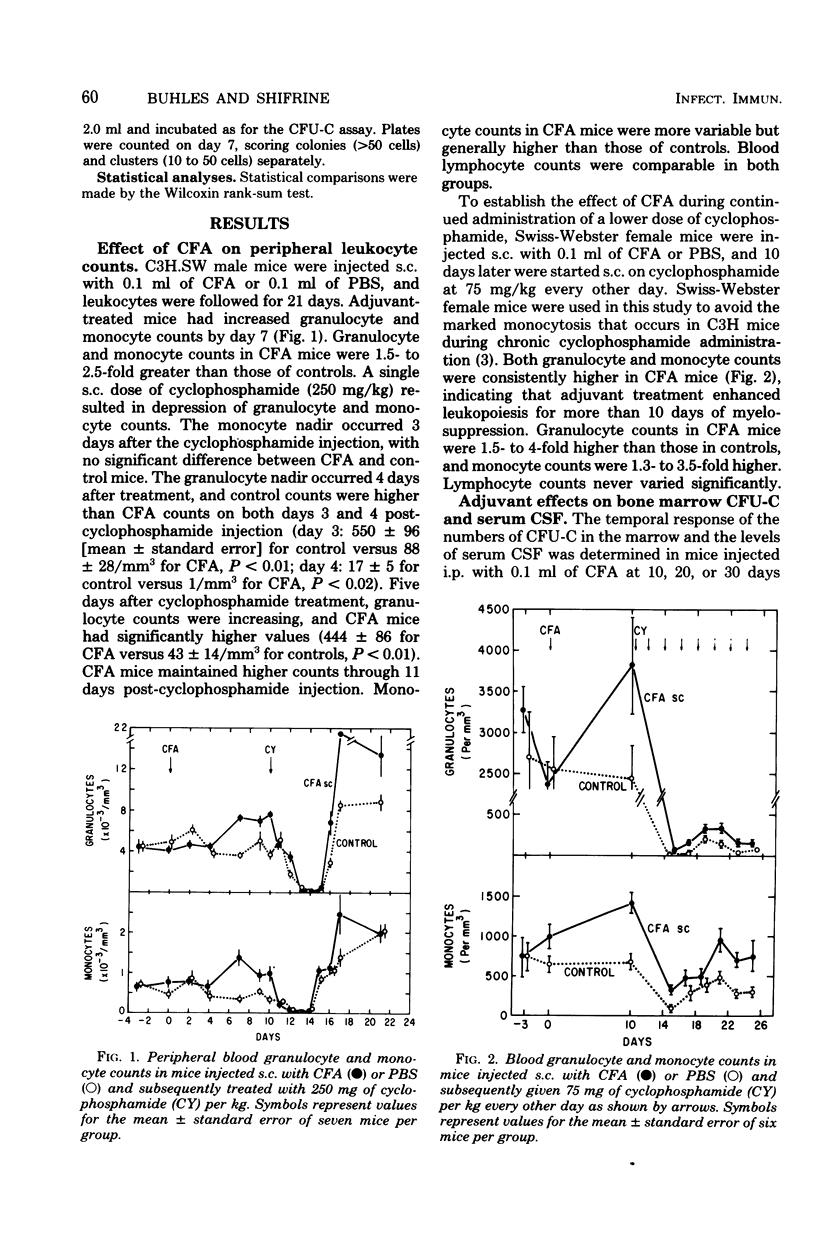
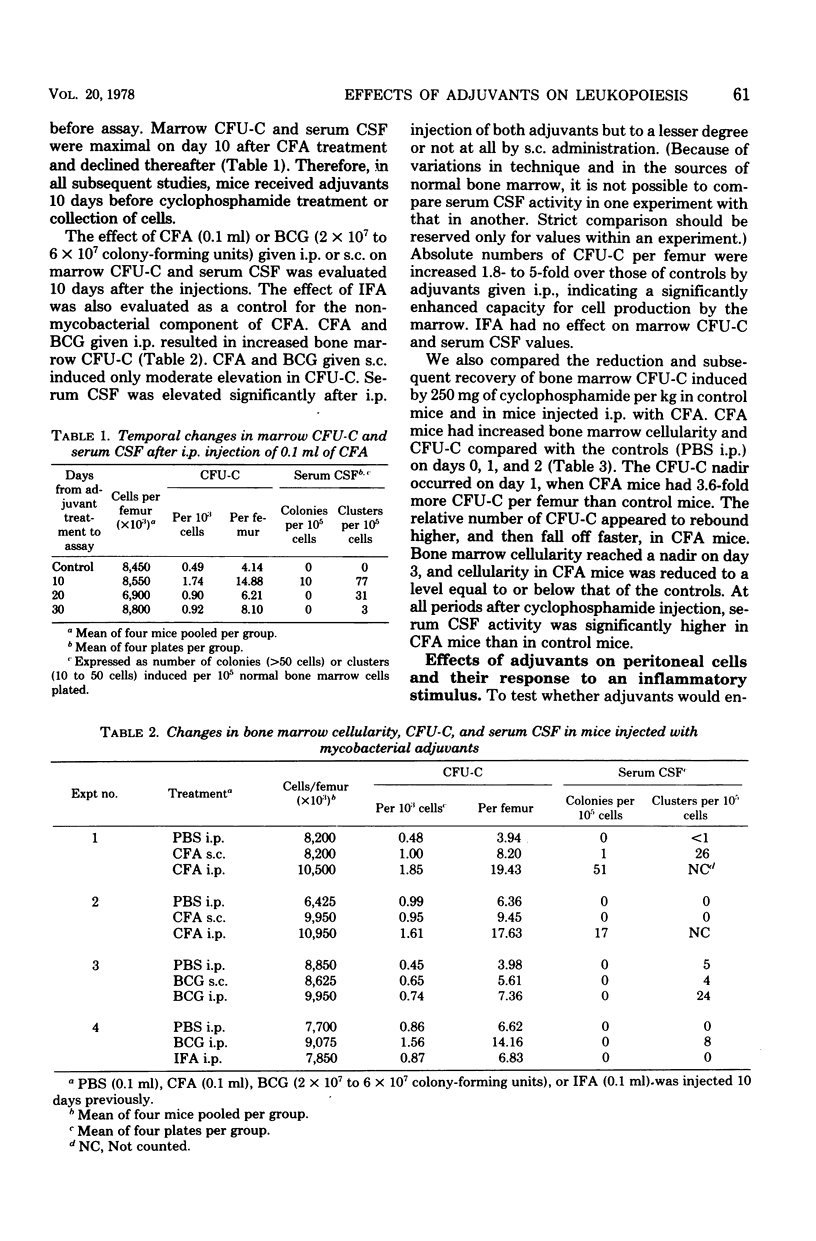
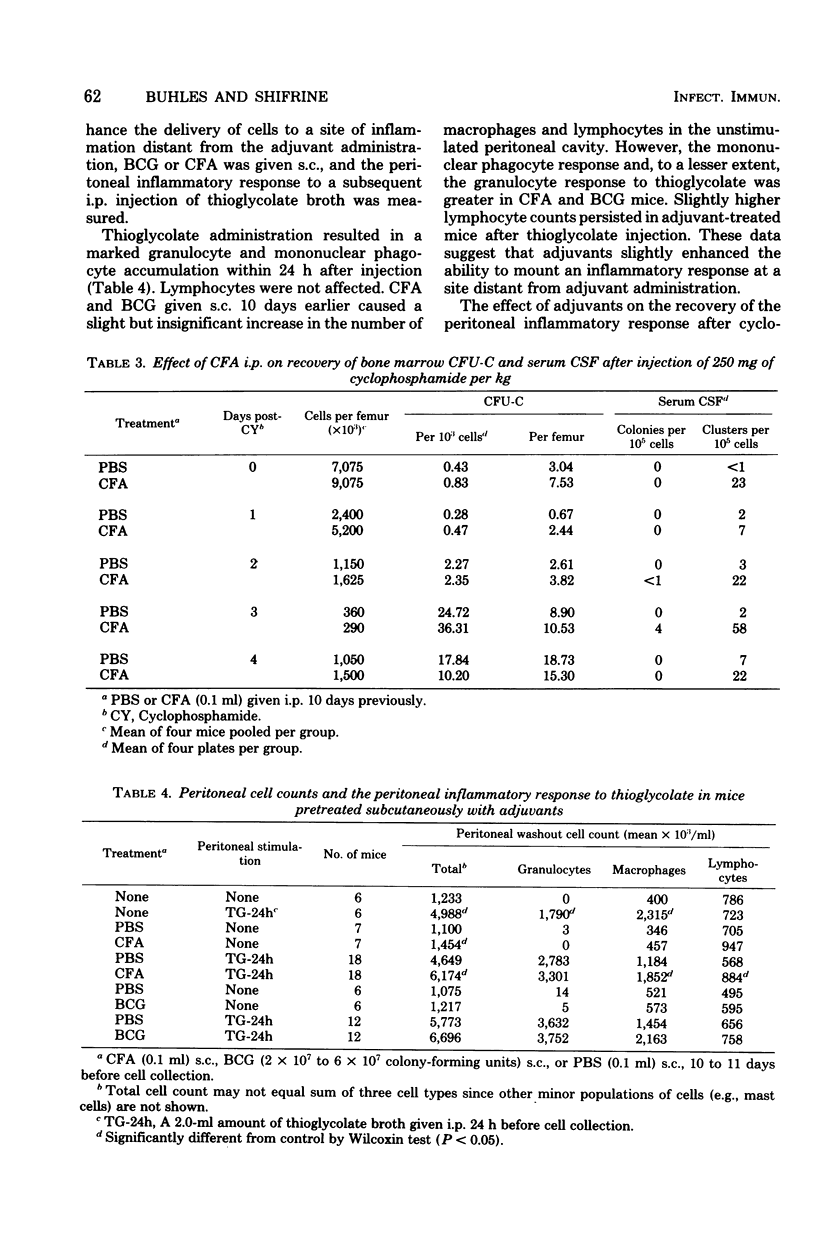
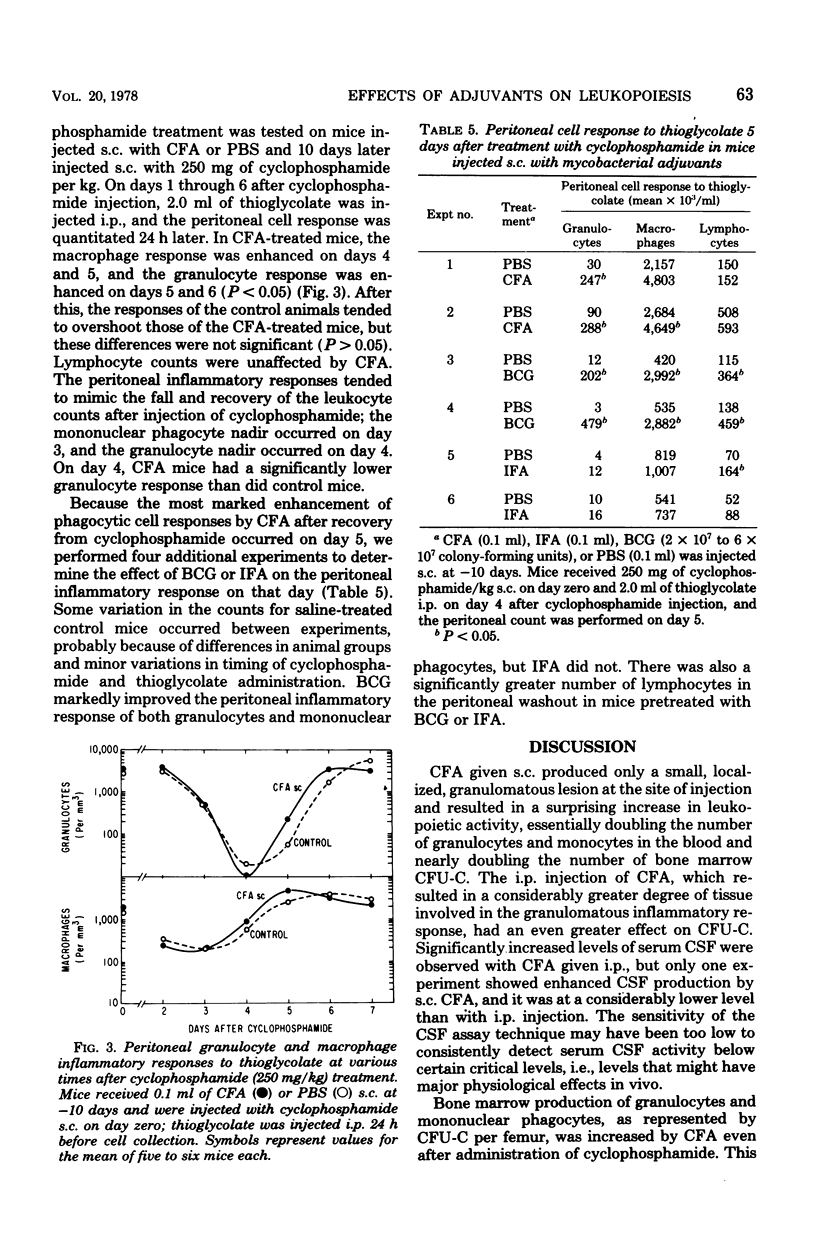
The effects of complete Freund adjuvant (CFA) or Mycobacterium bovis BCG on leukopoiesis and on leukopoietic recovery from cyclophosphamide treatment in mice was studied. CFA injected subcutaneously or intraperitoneally resulted in increased blood granulocyte and monocyte counts, increased numbers of bone marrow granulocyte and mononuclear phagocyte progenitors, and increased hematopoietic colony-stimulating factor in the serum. Furthermore, the quantitative cellular response within 24 h to an induced sterile intraperitoneal inflammation (thioglycolate) was augmented by subcutaneous CFA. In mice given CFA subcutaneously, blood granulocyte counts, as well as the peritoneal granulocyte and macrophage response to intraperitoneal thioglycolate, recovered more quickly than did those of the controls after a 250-mg/kg dose of cyclophosphamide. CFA-treated mice consistently maintained blood granulocyte and monocyte counts 1.3-to 4-fold higher than those of the controls for 2 weeks while receiving 75 mg of cyclophosphamide per kg every other day. Mice pretreated with CFA intraperitoneally had higher numbers of bone marrow colony-forming units in culture and higher levels of serum colony-stimulating factor after 250-mg/kg injections of cyclophosphamide. Similarly, BCG resulted in increased bone marrow colony-forming units in culture, increased serum colony-stimulating factor, and a faster return of the peritoneal inflammatory response after cyclophosphamide injection. These results show that mycobacterial adjuvants accelerate recovery of leukopoietic functions after cyclophosphamide treatment and suggest a mechanism whereby such adjuvants afford nonspecific protection against infection in immunosuppressed mice.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin P. E., McCulloch E. A., Till J. E. Characterization of the factor in L-cell conditioned medium capable of stimulating colony formation by mouse marrow cells in culture. J Cell Physiol. 1971 Apr;77(2):121–134. doi: 10.1002/jcp.1040770202. [DOI] [PubMed] [Google Scholar]
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Buhles W. C., Jr, Shifrine M. Adjuvant protection against bacterial infection in granulocytopenic mice. J Infect Dis. 1977 Jul;136(1):90–95. doi: 10.1093/infdis/136.1.90. [DOI] [PubMed] [Google Scholar]
- Buhles W. C., Jr, Shifrine M. Effects of cyclophosphamide on macrophage numbers, functions and progenitor cells. J Reticuloendothel Soc. 1977 May;21(5):285–297. [PubMed] [Google Scholar]
- Cline M. J., Rothman B., Golde D. W. Effect of endotoxin on the production of colony-stimulating factor by human monocytes and macrophages. J Cell Physiol. 1974 Oct;84(2):193–196. doi: 10.1002/jcp.1040840205. [DOI] [PubMed] [Google Scholar]
- Dimitrov N. V., Andre S., Eliopoulos G., Halpern B. Effect of corynebacterium parvum on bone marrow cell cultures (38557). Proc Soc Exp Biol Med. 1975 Feb;148(2):440–442. doi: 10.3181/00379727-148-38557. [DOI] [PubMed] [Google Scholar]
- Eaves A. C., Bruce W. R. In vitro production of colony-stimulating activity. I. Exposure of mouse peritoneal cells to endotoxin. Cell Tissue Kinet. 1974 Jan;7(1):19–30. doi: 10.1111/j.1365-2184.1974.tb00395.x. [DOI] [PubMed] [Google Scholar]
- Golde D. W., Finley T. N., Cline M. J. Production of colony-stimulating factor by human macrophages. Lancet. 1972 Dec 30;2(7792):1397–1399. doi: 10.1016/s0140-6736(72)92966-2. [DOI] [PubMed] [Google Scholar]
- McNeill T. A. Antigenic stimulation of bone marrow colony forming cells. 3. Effect in vivo. Immunology. 1970 Jan;18(1):61–72. [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. Studies on colony formation in vitro by mouse bone marrow cells. II. Action of colony stimulating factor. J Cell Physiol. 1970 Aug;76(1):89–99. doi: 10.1002/jcp.1040760113. [DOI] [PubMed] [Google Scholar]
- SMITH W. W., ALDERMAN I. M., GILLESPIE R. E. Hematopoietic recovery induced by bacterial endotoxin in irradiated mice. Am J Physiol. 1958 Mar;192(3):549–556. doi: 10.1152/ajplegacy.1958.192.3.549. [DOI] [PubMed] [Google Scholar]
- SMITH W. W., ALDERMAN I. M., GILLESPIE R. E. Increased survival in irradiated animals treated with bacterial endotoxins. Am J Physiol. 1957 Oct;191(1):124–130. doi: 10.1152/ajplegacy.1957.191.1.124. [DOI] [PubMed] [Google Scholar]
- SMITH W. W., ALDERMAN I. M., GILLESPIE R. E. Resistance to experimental infection and mobilization of granulocytes in irradiated mice treated with bacterial endotoxin. Am J Physiol. 1958 Feb;192(2):263–267. doi: 10.1152/ajplegacy.1958.192.2.263. [DOI] [PubMed] [Google Scholar]
- Sher N. A., Chaparas S. D., Greenberg L. E., Bernard S. Effects of BCG, Corynebacterium parvum, and methanol-extration residue in the reduction of mortality from Staphylococcus aureus and Candida albicans infections in immunosuppressed mice. Infect Immun. 1975 Dec;12(6):1325–1330. doi: 10.1128/iai.12.6.1325-1330.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan J. W., Stanley E. R. Tissue sources of bone marrow colony stimulating factor. J Cell Physiol. 1971 Dec;78(3):451–460. doi: 10.1002/jcp.1040780314. [DOI] [PubMed] [Google Scholar]
- Smith W. W., Brecher G., Fred S., Budd R. A. Effect of endotoxin on the kinetics of hemopoietic colony-forming cells in irradiated mice. Radiat Res. 1966 Apr;27(4):710–717. [PubMed] [Google Scholar]
- Wolmark N., Fisher B. The effect of a single and repeated administration of Corynebacterium parvum on bone marrow macrophage colony production in syngeneic tumor-bearing mice. Cancer Res. 1974 Nov;34(11):2869–2872. [PubMed] [Google Scholar]
- Wolmark N., Levine M., Fisher B. The effect of a single and repeated administration of Corynebacterium parvum on bone marrow macrophage colony production in normal mice. J Reticuloendothel Soc. 1974 Oct;16(4):252–257. [PubMed] [Google Scholar]


