Abstract
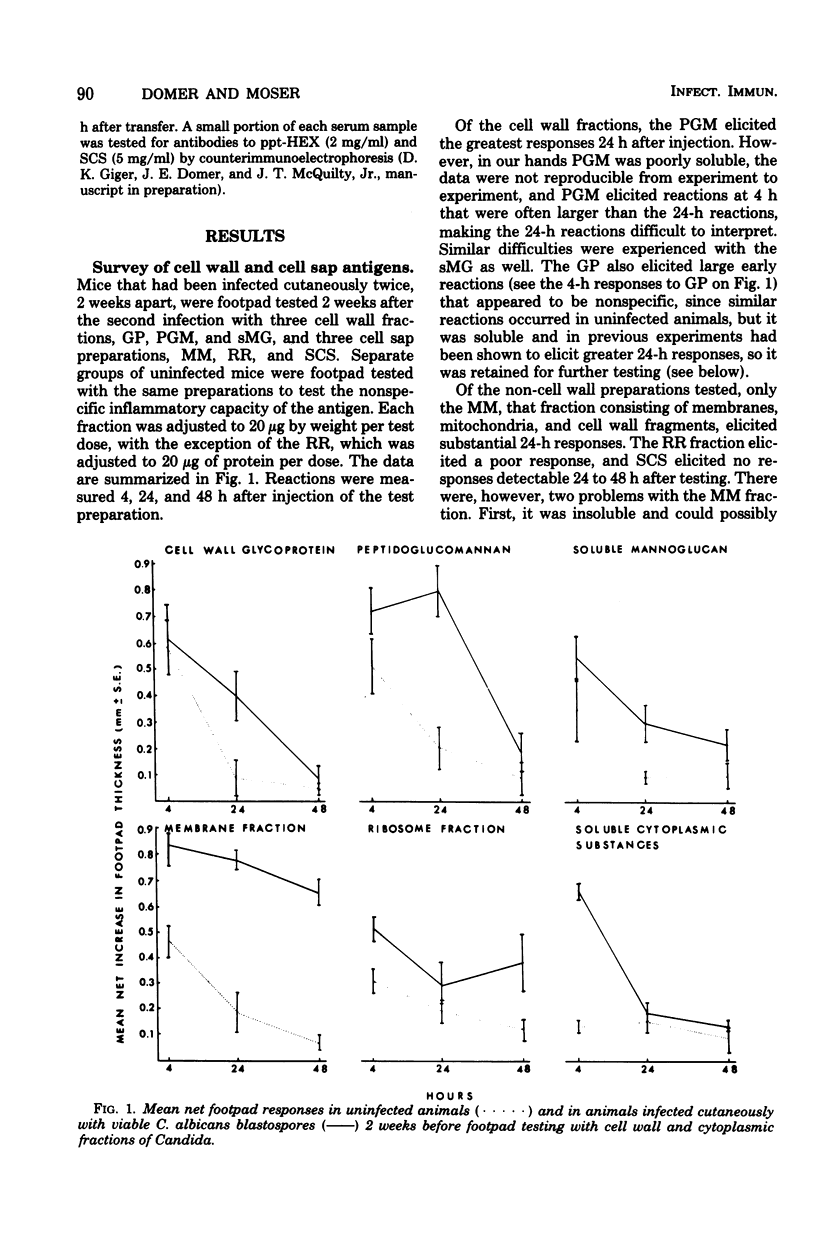
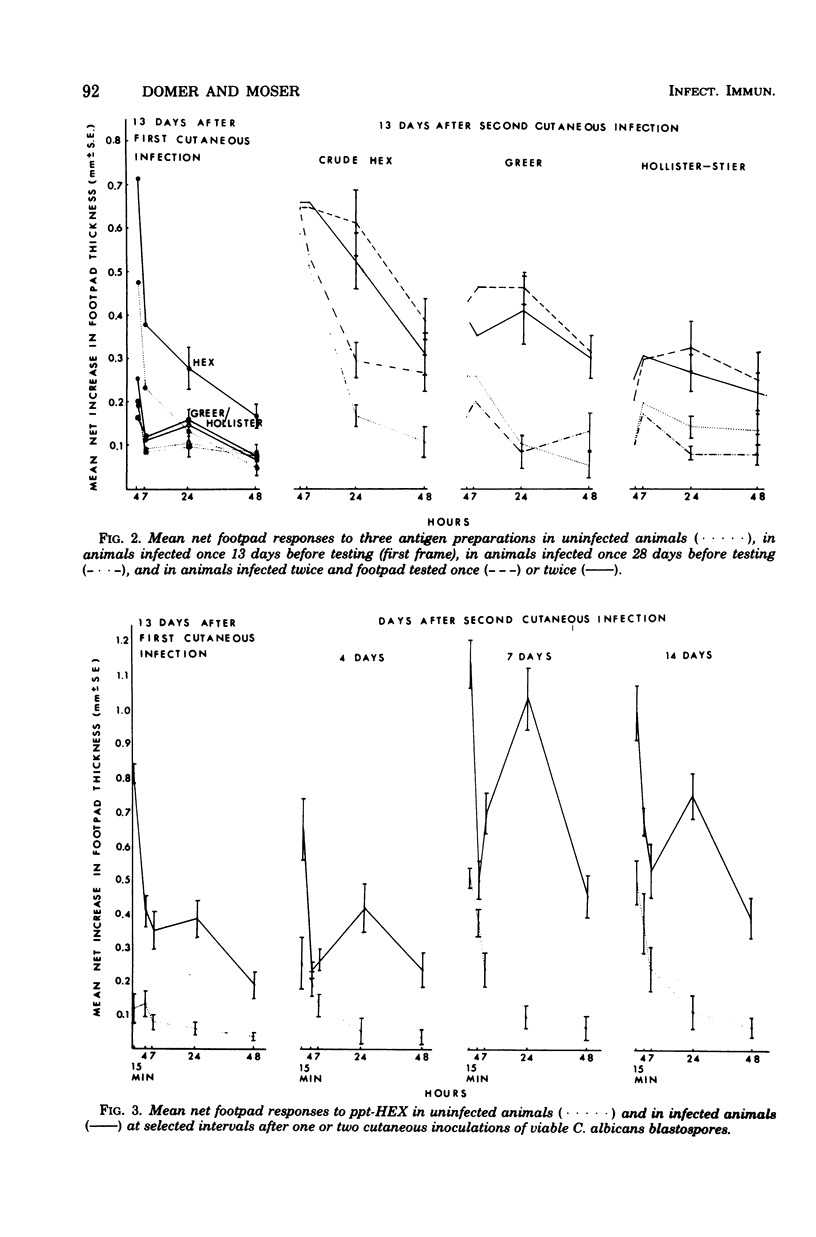
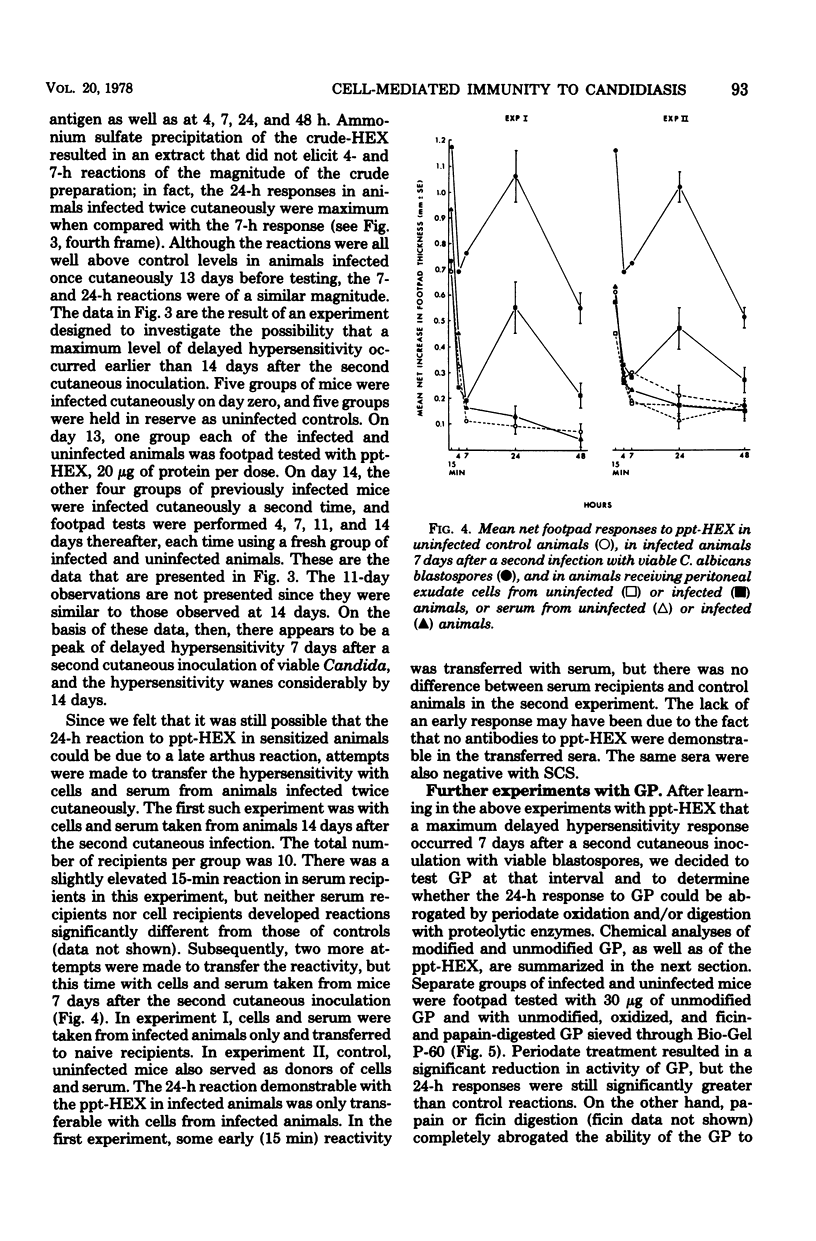
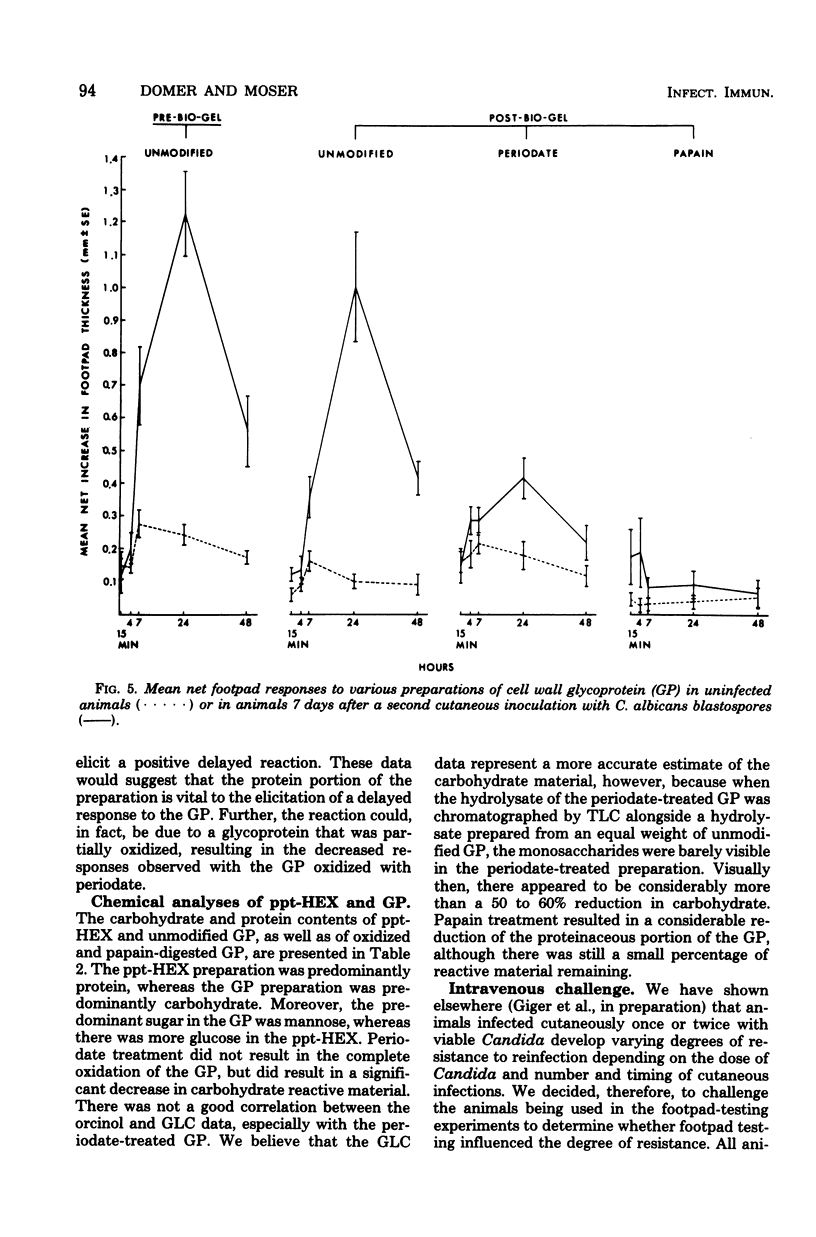
Attempts were made to isolate an antigen(s) from Candida albicans suitable for detecting hypersensitivity in a murine model of candidiasis. Using footpad reactivity in cutaneously infected animals as the assay, comparisons were made of two commercial extracts and cell wall and cytoplasmic preparations made in the laboratory. An extract of the cell wall, a glycoprotein (GP) removed with ethylenediamine, and an extract prepared from the membrane fraction of disrupted C. albicans blastospores proved most useful in demonstrating delayed hypersensitivity in the murine model. The activity of the GP fraction was considerably reduced by oxidation with periodate and was abrogated entirely by digestion with proteolytic enzymes. The extract from the membrane fraction was obtained by incubating the insoluble membrane fraction with phosphate-buffered saline, pH 7.4, at 50 degrees C, and the proteins in the extract were subsequently precipitated with ammonium sulfate to yield a test preparation that was approximately 75% protein and 25% carbohydrate. The precipitated extract was designated ppt-HEX. Footpad reactivity to ppt-HEX could be transferred with cells and not with serum if the cells were taken from animals at the appropriate time after sensitization. Since the membrane and GP fractions appear to elicit true delayed hypersensitivity reactions, further investigations into their specificity and biochemistry seem warranted.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford R. H., Des Prez R. M. Tranformation of lymphocytes of normal and hospitalized adults by Candida albicans extract. Proc Soc Exp Biol Med. 1973 Dec;144(3):826–829. doi: 10.3181/00379727-144-37691. [DOI] [PubMed] [Google Scholar]
- BARKER S. A., CRUICKSHANK C. N., MORRIS J. H., WOOD S. R. The isolation of trichophytin glycopeptide and its structure in relation to the immediate and delayed reactions. Immunology. 1962 Nov;5:627–632. [PMC free article] [PubMed] [Google Scholar]
- Bice D. E., Lopez M., Rothschild H., Salvaggio J. Comparison of Candida-delayed hypersensitivity skin test size with lymphocyte transformation, migration inhibitory factor production and antibody titer. Int Arch Allergy Appl Immunol. 1974;47(1):54–62. doi: 10.1159/000231200. [DOI] [PubMed] [Google Scholar]
- Budtz-Jorgensen E. Delayed hypersensitivity to Candida albicans in man demonstrated in vitro: the capillary tube migration test. Acta Allergol. 1972 Feb;27(1):41–49. doi: 10.1111/j.1398-9995.1972.tb01641.x. [DOI] [PubMed] [Google Scholar]
- CUTLER S. J., EDERER F. Maximum utilization of the life table method in analyzing survival. J Chronic Dis. 1958 Dec;8(6):699–712. doi: 10.1016/0021-9681(58)90126-7. [DOI] [PubMed] [Google Scholar]
- Cooper M. G. Delayed-type hypersensitivity in the mouse. I. Induction and elicitation by Salmonella adelaide flagellin and its derivatives. Scand J Immunol. 1972;1(2):167–178. doi: 10.1111/j.1365-3083.1972.tb00596.x. [DOI] [PubMed] [Google Scholar]
- Cooper M. G. Delayed-type hypersensitivity in the mouse. II. Transfer by thymus-derived (T) cells. Scand J Immunol. 1972;1(3):237–245. doi: 10.1111/j.1365-3083.1972.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Crowle A. J. Delayed hypersensitivity in the mouse. Adv Immunol. 1975;20:197–264. doi: 10.1016/s0065-2776(08)60209-6. [DOI] [PubMed] [Google Scholar]
- Davis T. E., Jr, Domer J. E. Glycohydrolase contamination of commercial enzymes frequently used in the preparation of fungal cell walls. Anal Biochem. 1977 Jun;80(2):593–600. doi: 10.1016/0003-2697(77)90683-2. [DOI] [PubMed] [Google Scholar]
- Domer J. E., Hamilton J. G., Harkin J. C. Comparative study of the cell walls of the yeastlike and mycelial phases of Histoplasma capsulatum. J Bacteriol. 1967 Aug;94(2):466–474. doi: 10.1128/jb.94.2.466-474.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domer J. E. In vivo and in virto cellular responses to cytoplasmic and cell wall antigens of Histoplasma capsulatum in artificially immunized or infected guinea pigs. Infect Immun. 1976 Mar;13(3):790–799. doi: 10.1128/iai.13.3.790-799.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinov N. P., Kashkina M. A., Kashkin A. P., Iljina V. P. The immune response to polysaccharides from yeast-like fungi in sensitized experimental animals. Mykosen. 1975 Oct;18(10):407–415. doi: 10.1111/j.1439-0507.1975.tb03517.x. [DOI] [PubMed] [Google Scholar]
- Ellsworth J. H., Reiss E., Bradley R. L., Chmel H., Armstrong D. Comparative serological and cutaneous reactivity of candidal cytoplasmic proteins and mannan separated by affinity for concanavalin A. J Clin Microbiol. 1977 Jan;5(1):91–99. doi: 10.1128/jcm.5.1.91-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foroozanfar N., Yamamura Y., Hobbs J. R. Standardization of lymphocyte transformation to Candida immunogen. Clin Exp Immunol. 1974 Feb;16(2):301–309. [PMC free article] [PubMed] [Google Scholar]
- Frisk A., Wasserman J. Lymphocyte stimulation with Candida albicans antigens. Antonie Van Leeuwenhoek. 1969 Jun;35:E13–E13. [PubMed] [Google Scholar]
- Frisk A., von Stedingk L. V., Wasserman J. Lymphocyte stimulation in Candida albicans infections. Sabouraudia. 1974 Mar;12(1):87–94. [PubMed] [Google Scholar]
- Kabe J., Aoki K., Ishizaki T., Miyamoto T., Nakazawa H., Tomaru M. Relationship of dermal and pulmonary sensitivity to extracts of Candida albicans. Am Rev Respir Dis. 1971 Sep;104(3):348–357. doi: 10.1164/arrd.1971.104.3.348. [DOI] [PubMed] [Google Scholar]
- Kabe J., Aoki Y., Miyamoto T. Antigenicity of fractions from extracts of Candida albicans. The immediate and delayed-type respiratory responses in guinea pigs. J Allergy. 1971 Feb;47(2):59–75. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- North R. J., Spitalny G. Inflammatory lymphocyte in cell-mediated antibacterial immunity: factors governing the accumulation of mediator T cells in peritoneal exudates. Infect Immun. 1974 Sep;10(3):489–498. doi: 10.1128/iai.10.3.489-498.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa Y., Uesaka H., Suzuki H., Ito Y. An evaluation of methanolysis for thin-layer chromatographic analysis of the monosaccharides in an immunologically active polysaccharide-peptide complex. J Chromatogr. 1969 Sep 23;43(4):528–530. doi: 10.1016/s0021-9673(00)99243-4. [DOI] [PubMed] [Google Scholar]
- Reiss E., Stone S. H., Hasenclever H. F. Serological and cellular immune activity of peptidoglucomannan fractions of Candida albicans cell walls. Infect Immun. 1974 May;9(5):881–890. doi: 10.1128/iai.9.5.881-890.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo-Moreno A., Schneidau J. D., Jr Nature of the skin-reactive principle in culture filtrates prepared from Paracoccidioides brasiliensis. J Bacteriol. 1967 Jun;93(6):1741–1748. doi: 10.1128/jb.93.6.1741-1748.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkind D., Frey J. A., Davis J. R., Petersen E. A., Dinowitz M. Delayed hypersensitivity to fungal antigens in mice. I. Use of the intradermal skin and footpad swelling tests as assays of active and passive sensitization. J Infect Dis. 1976 Jan;133(1):50–56. doi: 10.1093/infdis/133.1.50. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. The quantitative estimation of cerebrosides in nervous tissue. J Neurochem. 1956 May;1(1):42–53. doi: 10.1111/j.1471-4159.1956.tb12053.x. [DOI] [PubMed] [Google Scholar]
- Shannon D. C., Johnson G., Rosen F. S., Austen K. F. Cellular reactivity to Candida albicans antigen. N Engl J Med. 1966 Sep 29;275(13):690–693. doi: 10.1056/NEJM196609292751302. [DOI] [PubMed] [Google Scholar]
- Sikl D., Masler L., Bauer S., Sandula J., Tomsíková A., Zavázal V. Allergenaktivität der Polysaccharid-Proteinkomplexe von Candida. Z Immunitatsforsch Allerg Klin Immunol. 1969 Jul;138(3):207–216. [PubMed] [Google Scholar]
- Suzuki M., Hayashi Y. Skin reaction and macrophage migration inhibition tests for polysaccharides from Aspergillus fumigatus and Candida albicans. Jpn J Microbiol. 1975 Oct;19(5):355–362. doi: 10.1111/j.1348-0421.1975.tb00892.x. [DOI] [PubMed] [Google Scholar]
- VOGEL R. A., KREHL W. Experimental sensitization of guinea pigs with Candida albicans and adjuvants. Am Rev Tuberc. 1957 Oct;76(4):692–696. doi: 10.1164/artpd.1957.76.4.692. [DOI] [PubMed] [Google Scholar]


