Abstract
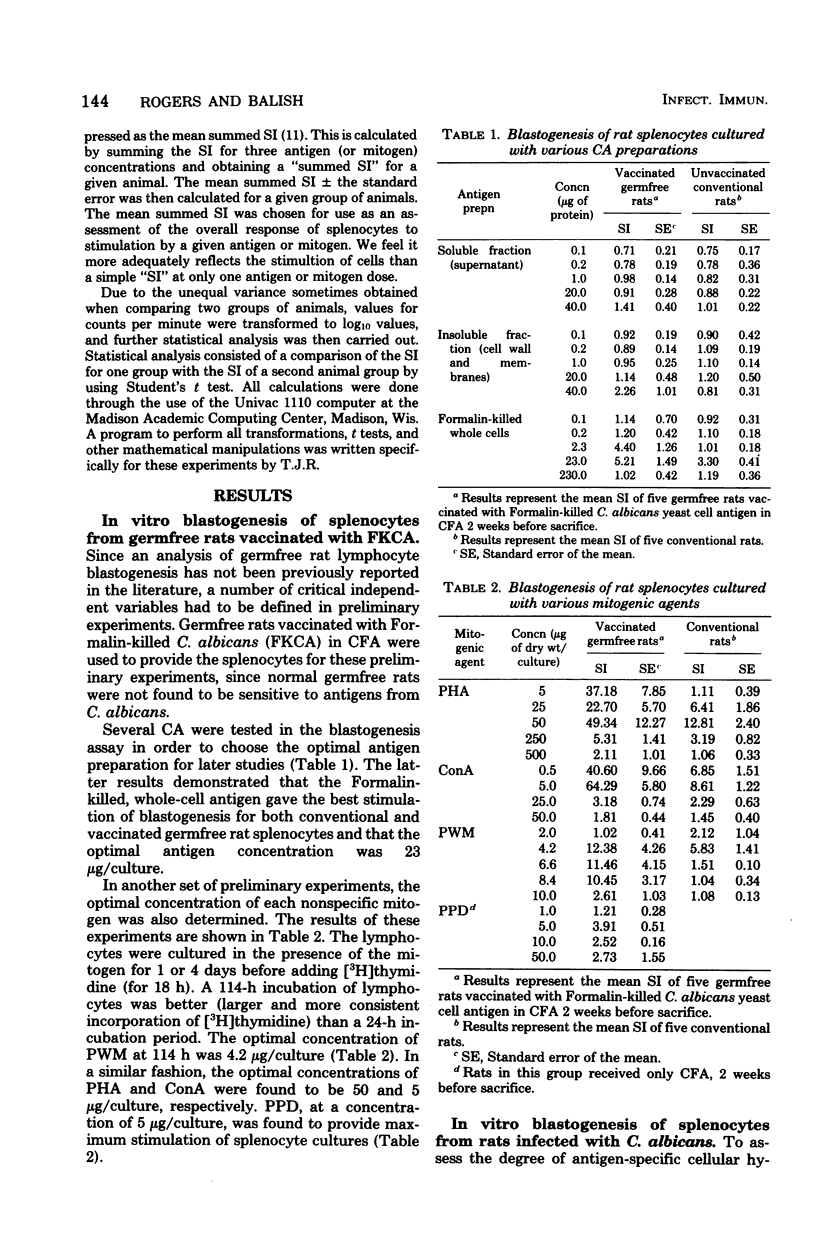
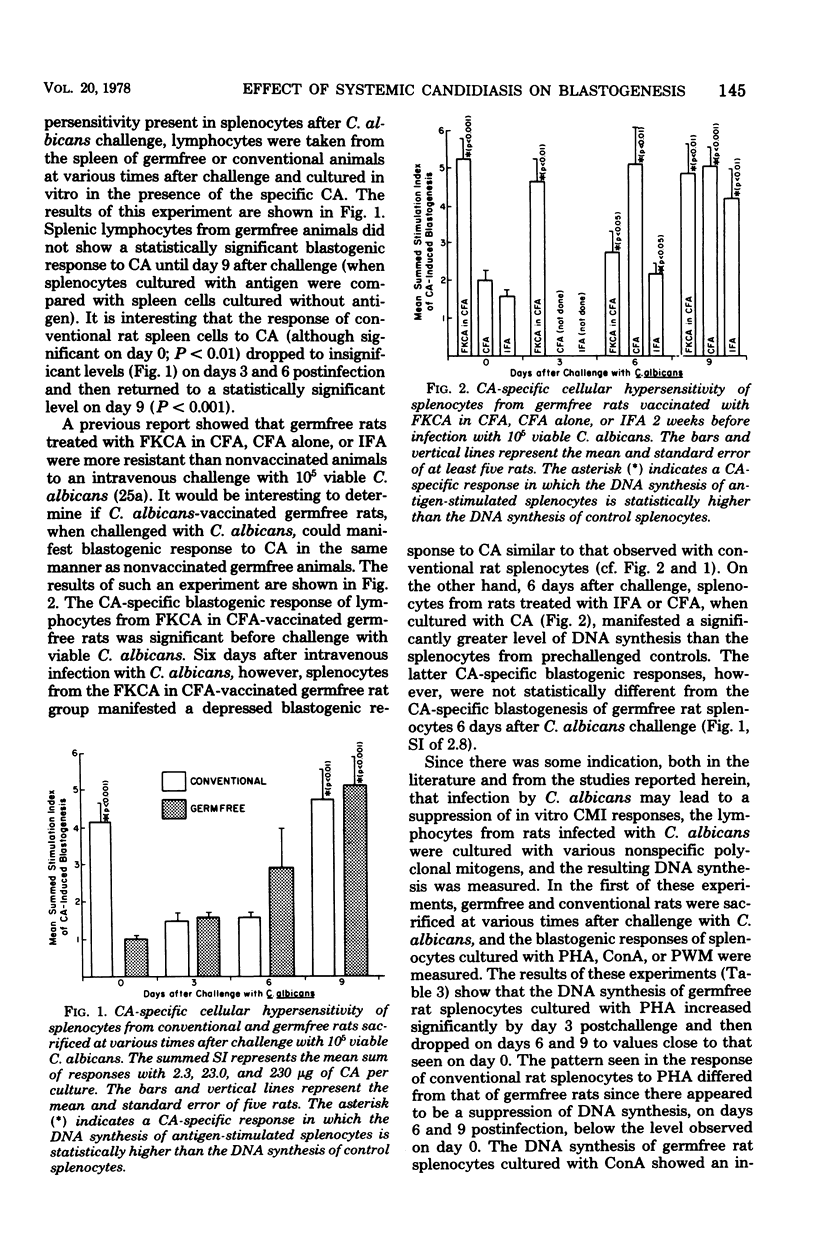
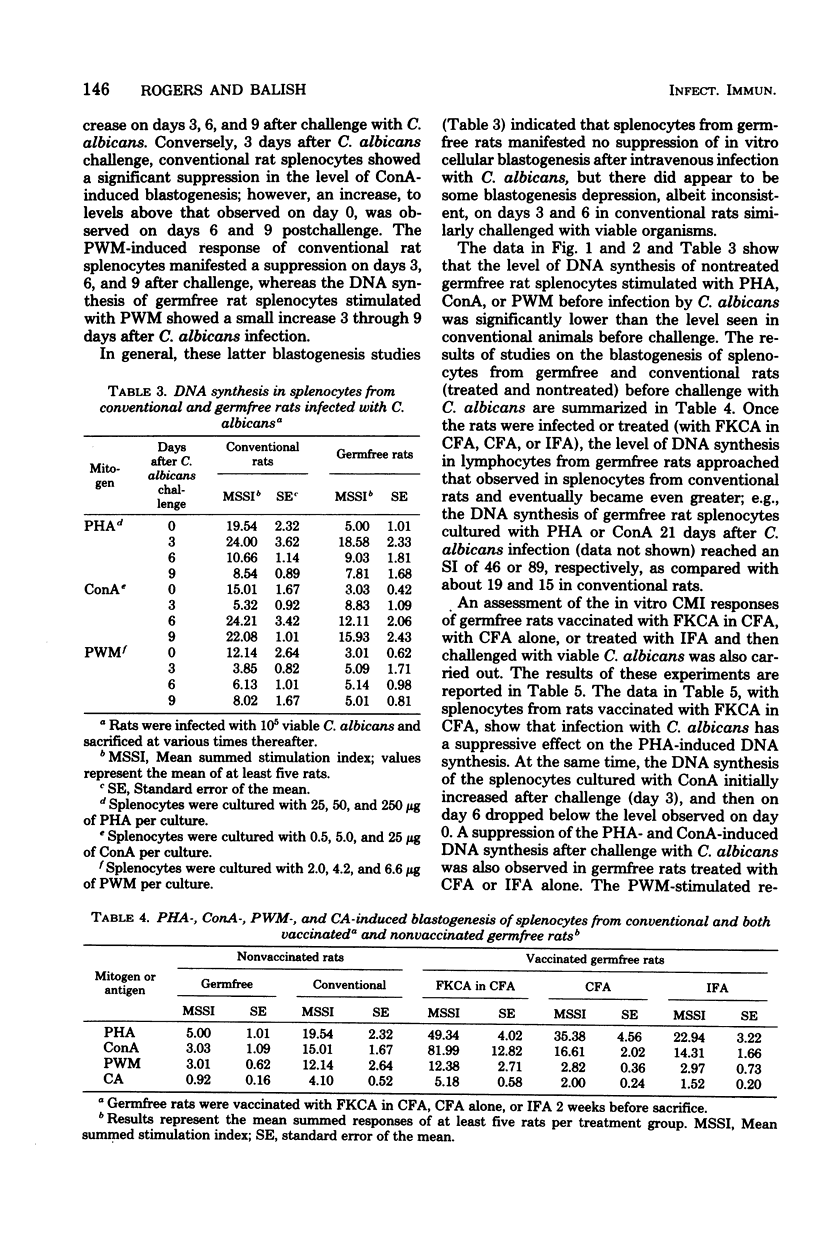
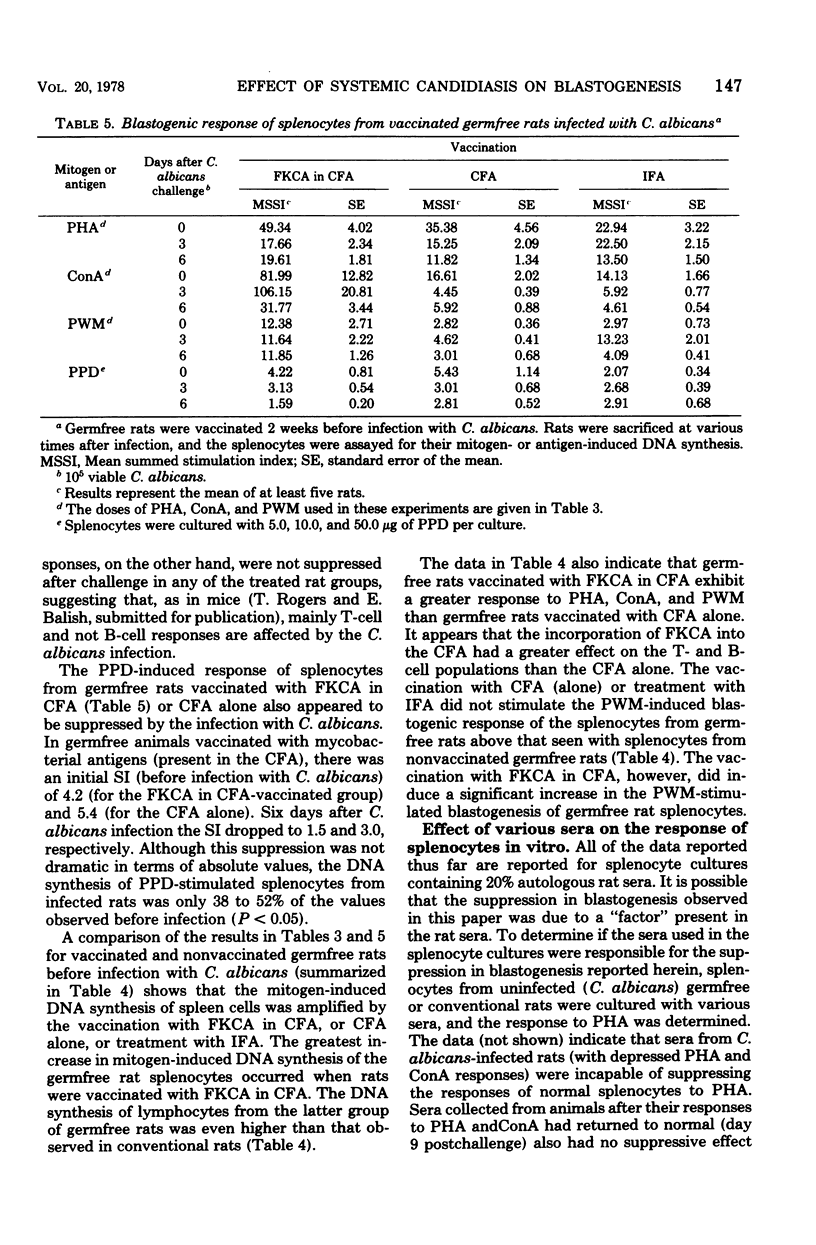
Germfree and conventional rats were challenged (intravenously) with Candida albicans and sacrificed at various times after infection, and their spleen cells were harvested to examine the effect of disseminated candidiasis on in vitro lymphocyte hypersensitivity to Candida antigens (CA). Results showed that conventional rat splenocytes, initially responsive in vitro to stimulation by CA, manifested a depression in CA-specific responsiveness after challenge with viable C. albicans (days 3 to 6 postchallenge). In addition, the latter splenocyte response to phytohemagglutinin (PHA) and concanavalin A (ConA) was suppressed by 3 to 6 days after challenge with Candida. In contrast to conventional rats, the response of germfree rat splenocytes to CA was insignificant before challenge with C. albicans, and it was increased at 9 days after infection. The response of uninfected germfree rat splenocytes to PHA and ConA was significantly lower than that of unchallenged conventional rats. Challenge with viable C. albicans did not result in a suppression of gnotobiotic rat splenocyte responses to PHA and ConA, but rather, the disseminated infection resulted in as much as fivefold increases in PHA or ConA-induced blastogenesis. These findings suggest that disseminated candidiasis is capable of suppressing blastogenesis in immunologically mature conventional rats and of improving lymphocyte blastogenesis from immunologically immature germfree rats.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUER H., HOROWITZ R. E., LEVENSON S. M., POPPER H. The response of the lymphatic tissue to the microbial flora. Studies on germfree mice. Am J Pathol. 1963 Apr;42:471–483. [PMC free article] [PubMed] [Google Scholar]
- Bash J. A., Durkin H. G., Waksman B. H. Kinetic study of T lymphocytes after sensitization against soluble antigen. III. Potentiation and suppression of the PHA response by antigen-activated lymphocytes of low density. J Exp Med. 1975 Oct 1;142(4):1017–1022. doi: 10.1084/jem.142.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bash J. A., Waksman B. H. The suppressive effect of immunization on the proliferative responses of rat T cells in vitro. J Immunol. 1975 Feb;114(2 Pt 2):782–787. [PubMed] [Google Scholar]
- Boros D. L., Pelley R. P., Warren K. S. Spontaneous modulation of granulomatous hypersensitivity in schistosomiasis mansoni. J Immunol. 1975 May;114(5):1437–1441. [PubMed] [Google Scholar]
- Budtz-Jörgensen E. Cellular immunity in acquired candidiasis of the palate. Scand J Dent Res. 1973;81(5):372–382. doi: 10.1111/j.1600-0722.1973.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Colley D. G. Immune responses to a soluble schistosomal egg antigen preparation during chronic primary infection with Schistosoma mansoni. J Immunol. 1975 Jul;115(1):150–156. [PubMed] [Google Scholar]
- Cutler J. E. Acute systemic candidiasis in normal and congenitally thymic-deficient (nude) mice. J Reticuloendothel Soc. 1976 Feb;19(2):121–124. [PubMed] [Google Scholar]
- Fulton A. M., Dustoor M. M., Kasinski J. E., Blazkovec A. A. Blastogenesis as an in vitro correlate of delayed hypersensitivity in guinea pigs infected with Listeria monocytogenes. Infect Immun. 1975 Sep;12(3):647–655. doi: 10.1128/iai.12.3.647-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly R., Cusumano C. L., Waldman R. H. Suppression of cell-mediated immunity after infection with attenuated rubella virus. Infect Immun. 1976 Feb;13(2):464–469. doi: 10.1128/iai.13.2.464-469.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Gery I., Waksman B. H. Suppressive effects of in vivo immunization on PHA responses in vitro. J Immunol. 1974 Jan;112(1):215–221. [PubMed] [Google Scholar]
- HOROWITZ R. E., BAUER H., PARONETTO F., ABRAMS G. D., WATKINS K. C., POPPER H. THE RESPONSE OF THE LYMPHATIC TISSUE TO BACTERIAL ANTIGEN. STUDIES IN GERMFREE MICE. Am J Pathol. 1964 May;44:747–761. [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick C. H., Chandler J. W., Schimke R. N. Chronic mucocutaneous moniliasis with impaired delayed hypersensitivity. Clin Exp Immunol. 1970 Mar;6(3):375–385. [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick C. H., Rich R. R., Graw R. G., Jr, Smith T. K., Mickenberg I., Rogentine G. N. Treatment of chronic mucocutaneous moniliasis by immunologic reconstitution. Clin Exp Immunol. 1971 Dec;9(6):733–748. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANKIEWICZ E., LIVAK M. Effect of Candida albicans on the evolution of experimental tuberculosis. Nature. 1960 Jul 16;187:250–251. doi: 10.1038/187250a0. [DOI] [PubMed] [Google Scholar]
- Mardon D. N., Balish E. Modifications in a French pressure cell-laboratory press system. Appl Microbiol. 1969 May;17(5):777–777. doi: 10.1128/am.17.5.777-777.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musher D. M., Schell R. F., Jones R. H., Jones A. M. Lymphocyte transformation in syphilis: an in vitro correlate of immune suppression in vivo? Infect Immun. 1975 Jun;11(6):1261–1264. doi: 10.1128/iai.11.6.1261-1264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima I., Kobayashi T., Kato N. Alterations in the antibody response to bovine serum albumin by capsular polysaccharide of Klebsiella pneumoniae. J Immunol. 1971 Oct;107(4):1112–1121. [PubMed] [Google Scholar]
- Olson G. B., Wostmann B. S. Lymphocytopoiesis, plasmacytopoiesis and cellular proliferation in nonantigenically stimulated germfree mice. J Immunol. 1966 Aug;97(2):267–274. [PubMed] [Google Scholar]
- Pelley R. P., Ruffier J. J., Warren K. S. Suppressive effect of a chronic helminth infection, schistosomiasis mansoni, on the in vitro responses of spleen and lymph node cells to the T cell mitogens phytohemagglutinin and concanavalin A. Infect Immun. 1976 Apr;13(4):1176–1183. doi: 10.1128/iai.13.4.1176-1183.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard M., Sharon N. Responses of the Peyer's Patches in Germ-Free Mice to Antigenic Stimulation. Infect Immun. 1970 Jul;2(1):96–100. doi: 10.1128/iai.2.1.96-100.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. J., Balish E. Immunity to experimental renal candidiasis in rats. Infect Immun. 1978 Feb;19(2):737–740. doi: 10.1128/iai.19.2.737-740.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. J., Balish E., Manning D. D. The role of thymus-dependent cell-mediated immunity in resistance to experimental disseminated candidiasis. J Reticuloendothel Soc. 1976 Oct;20(4):291–298. [PubMed] [Google Scholar]
- Schwab J. H. Suppression of the immune response by microorganisms. Bacteriol Rev. 1975 Jun;39(2):121–143. doi: 10.1128/br.39.2.121-143.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher R., Holm G., Kok S. H., Koornhof H. J., Glover A. T and CR+ lymphocyte profile in leprosy and the effect of treatment. Infect Immun. 1976 Jan;13(1):31–35. doi: 10.1128/iai.13.1.31-35.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORBECKE G. J., BENACERRAF B. Some histological and functional aspects of lymphoid tissue in germfree animals. II. Studies on phagocytosis in vivo. Ann N Y Acad Sci. 1959 May 8;78:247–253. doi: 10.1111/j.1749-6632.1959.tb53107.x. [DOI] [PubMed] [Google Scholar]
- THORBECKE G. J. Some histological and functional aspects of lymphoid tissue in germfree animals. I. Morphological studies. Ann N Y Acad Sci. 1959 May 8;78:237–246. doi: 10.1111/j.1749-6632.1959.tb53106.x. [DOI] [PubMed] [Google Scholar]
- Ueda K., Yamazaki S., Someya S. Hyporeactivity to tuberculin in germ-free mice. J Reticuloendothel Soc. 1975 Aug;18(2):107–117. [PubMed] [Google Scholar]
- Ueda K., Yamazaki S., Someya S. Impairment and restoration of the delayed type hypersensitivity in germ-free mice. Jpn J Microbiol. 1973 Nov;17(6):533–536. doi: 10.1111/j.1348-0421.1973.tb00942.x. [DOI] [PubMed] [Google Scholar]
- Valdimarsson H., Wood C. B., Hobbs J. R., Holt P. J. Immunological features in a case of chronic granulomatous candidiasis and its treatment with transfer factor. Clin Exp Immunol. 1972 Jun;11(2):151–163. [PMC free article] [PubMed] [Google Scholar]


