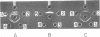Abstract
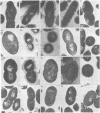
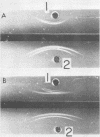
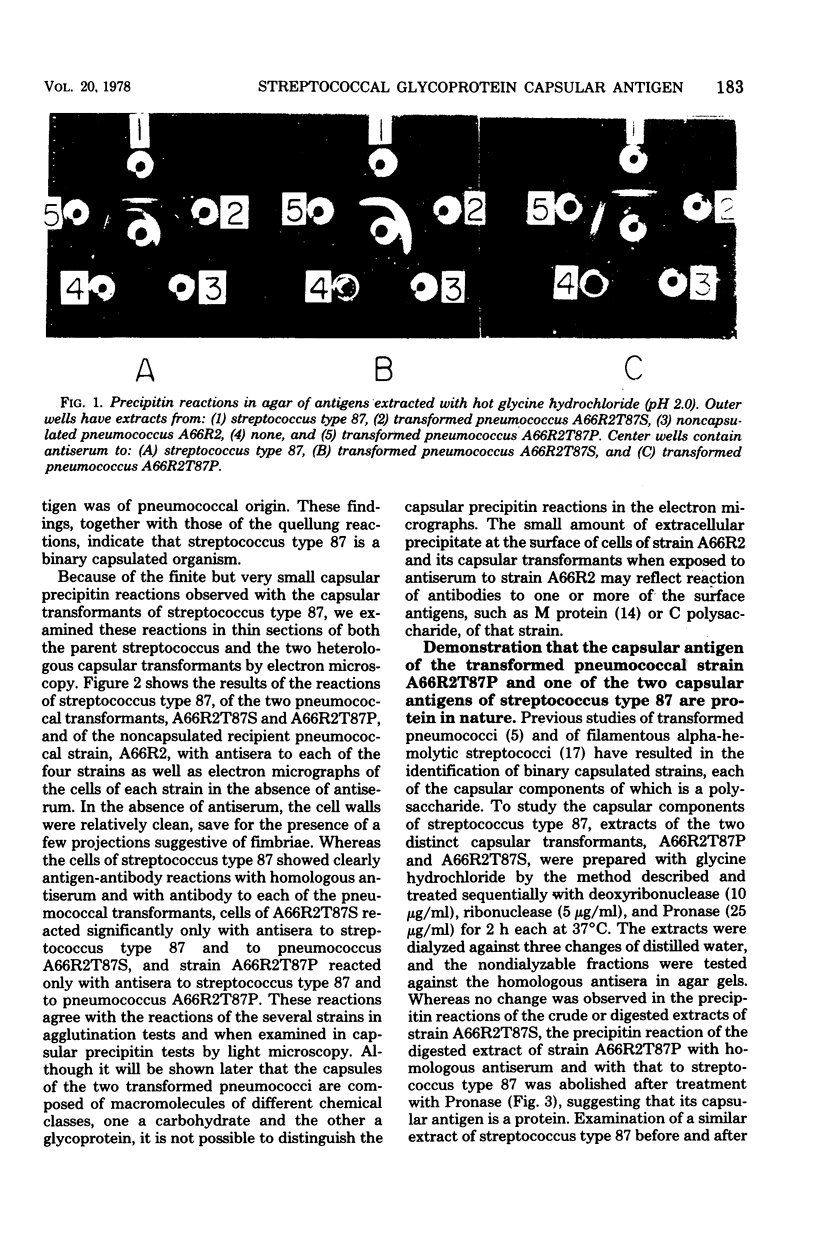
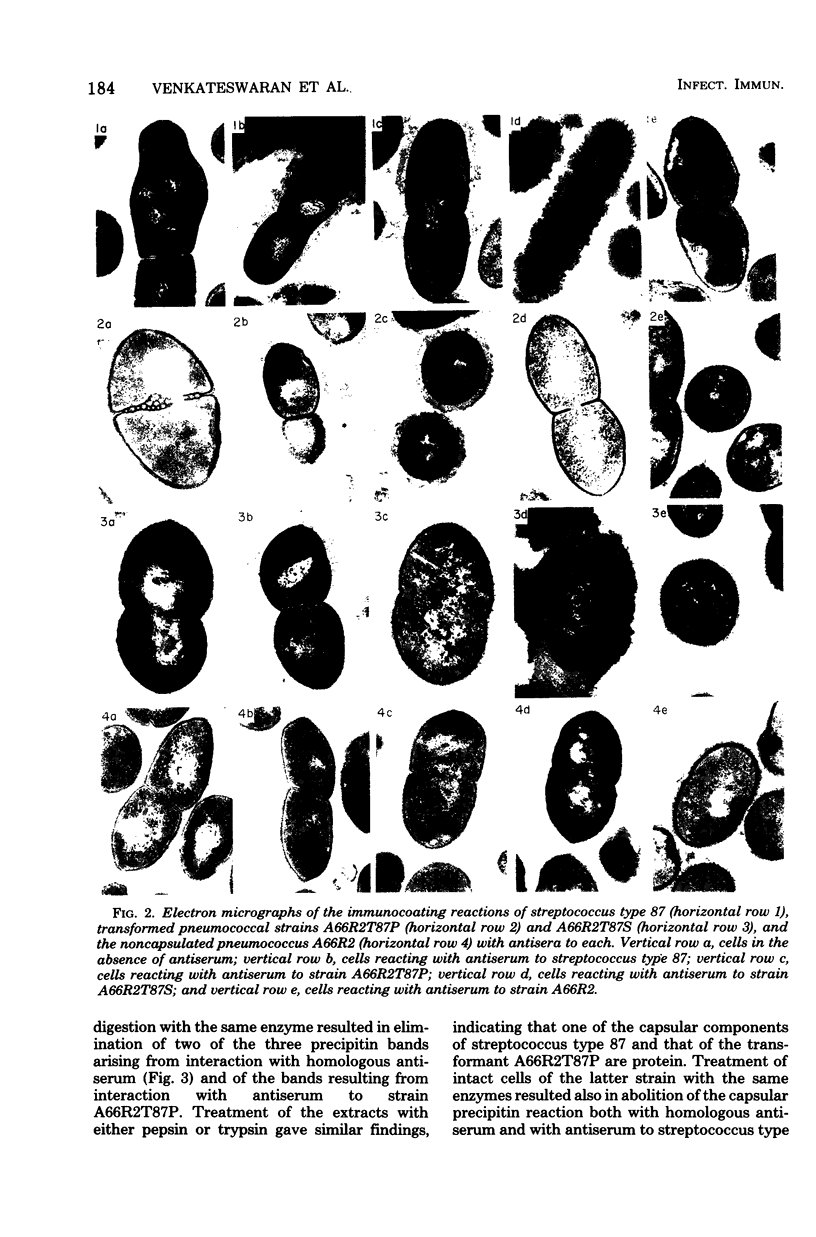
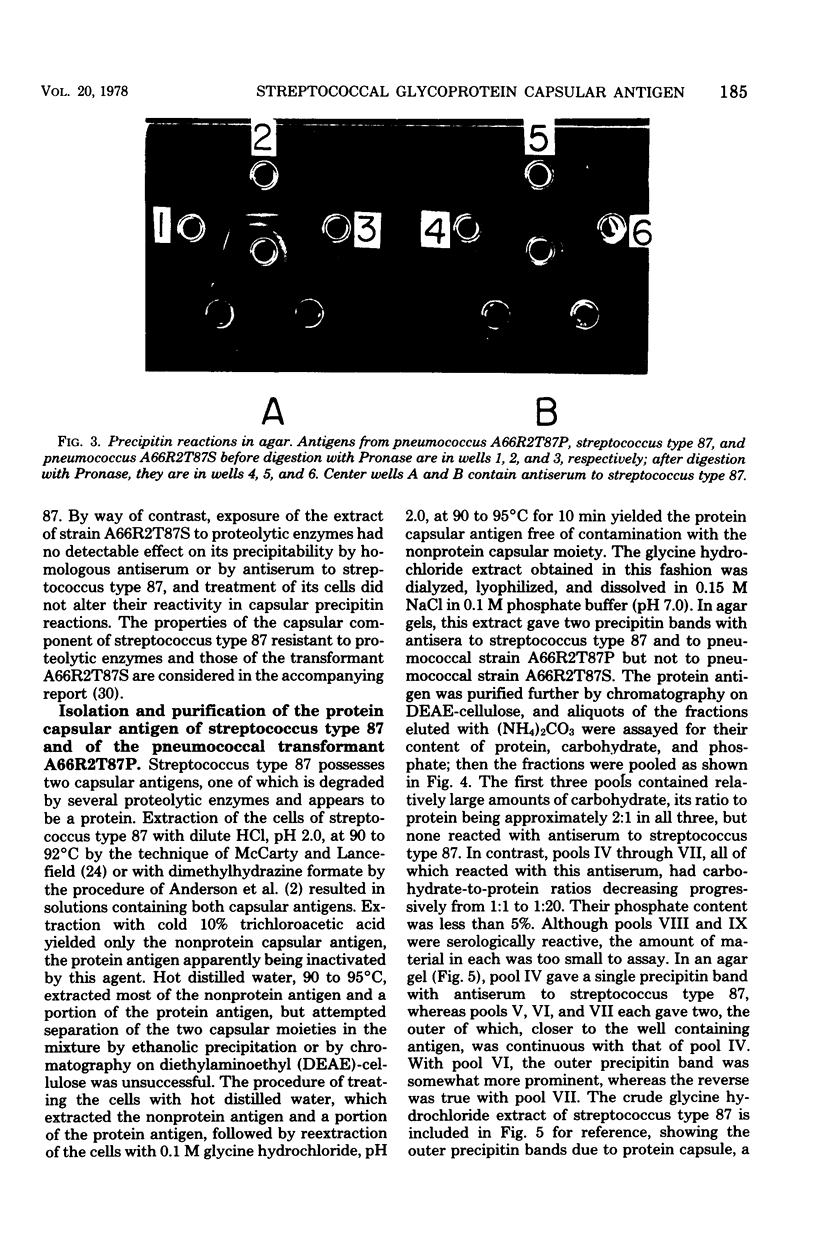
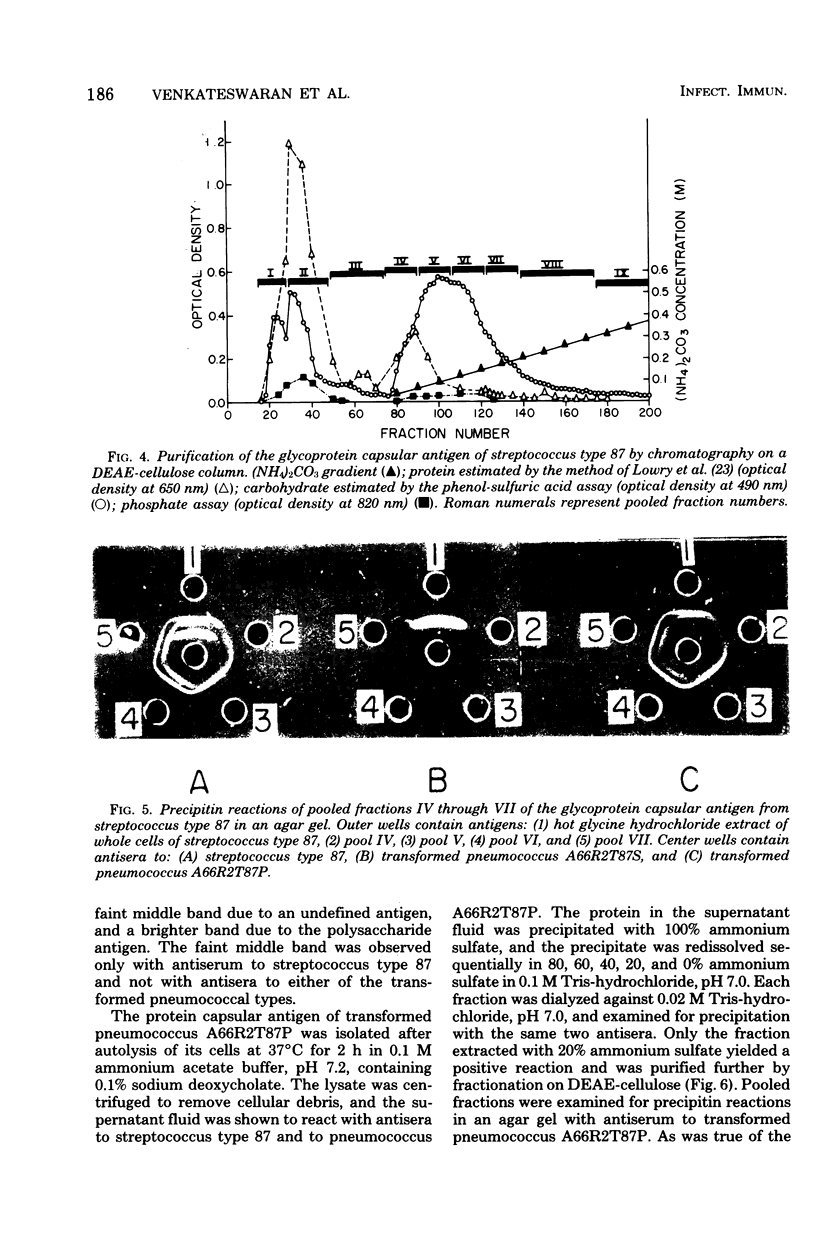
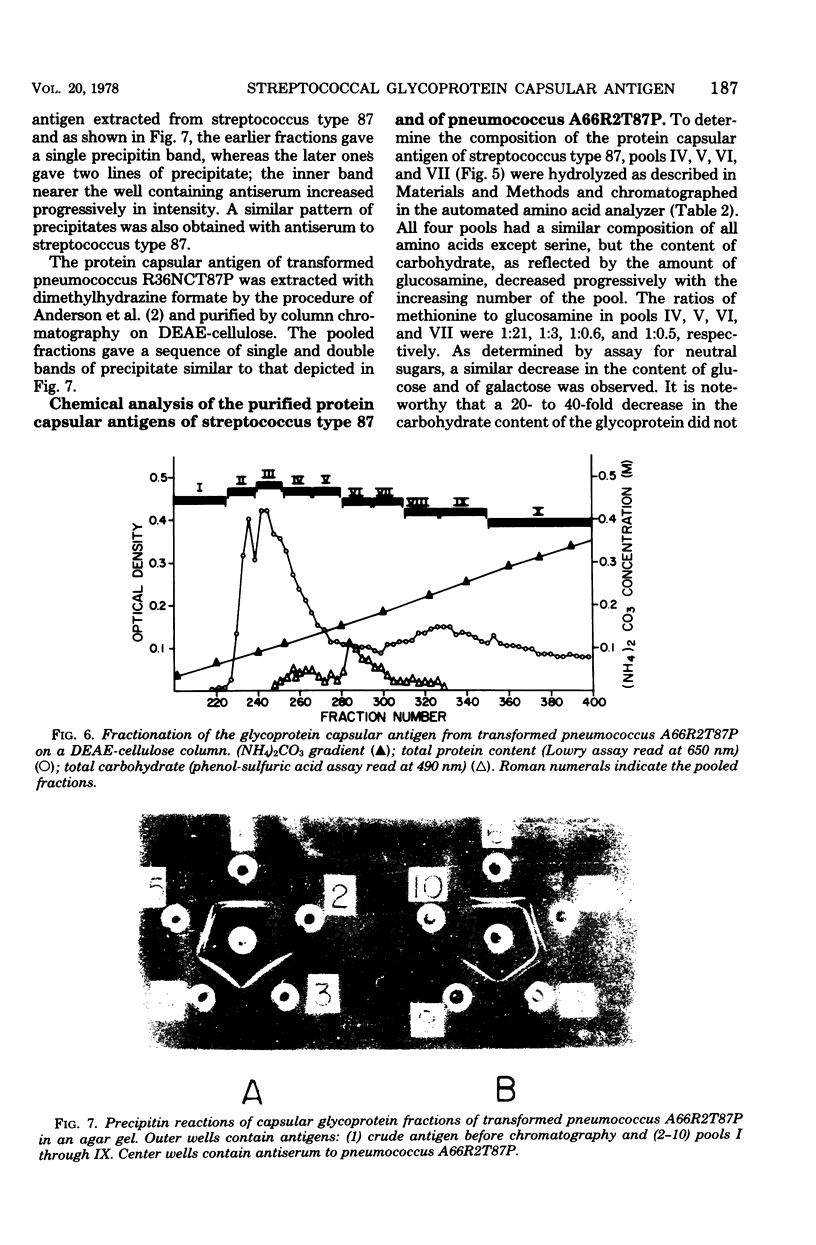
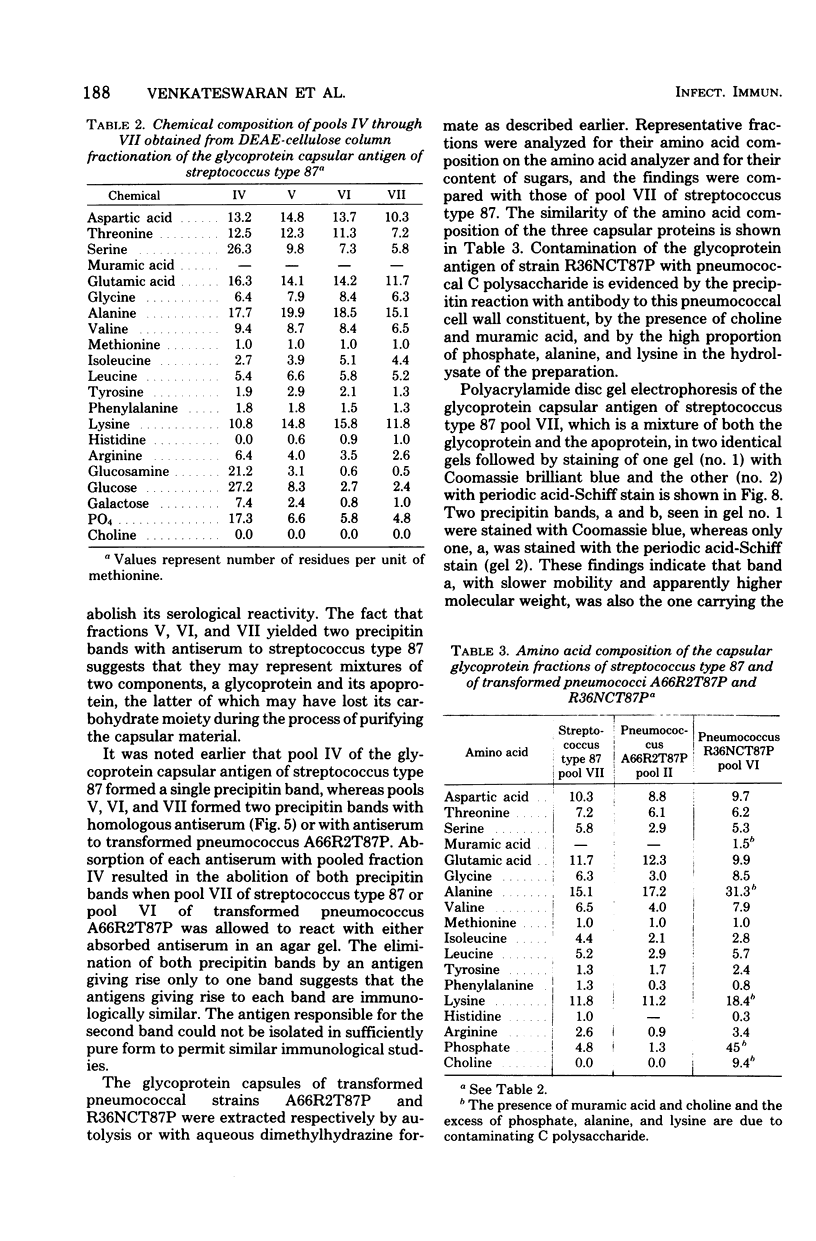
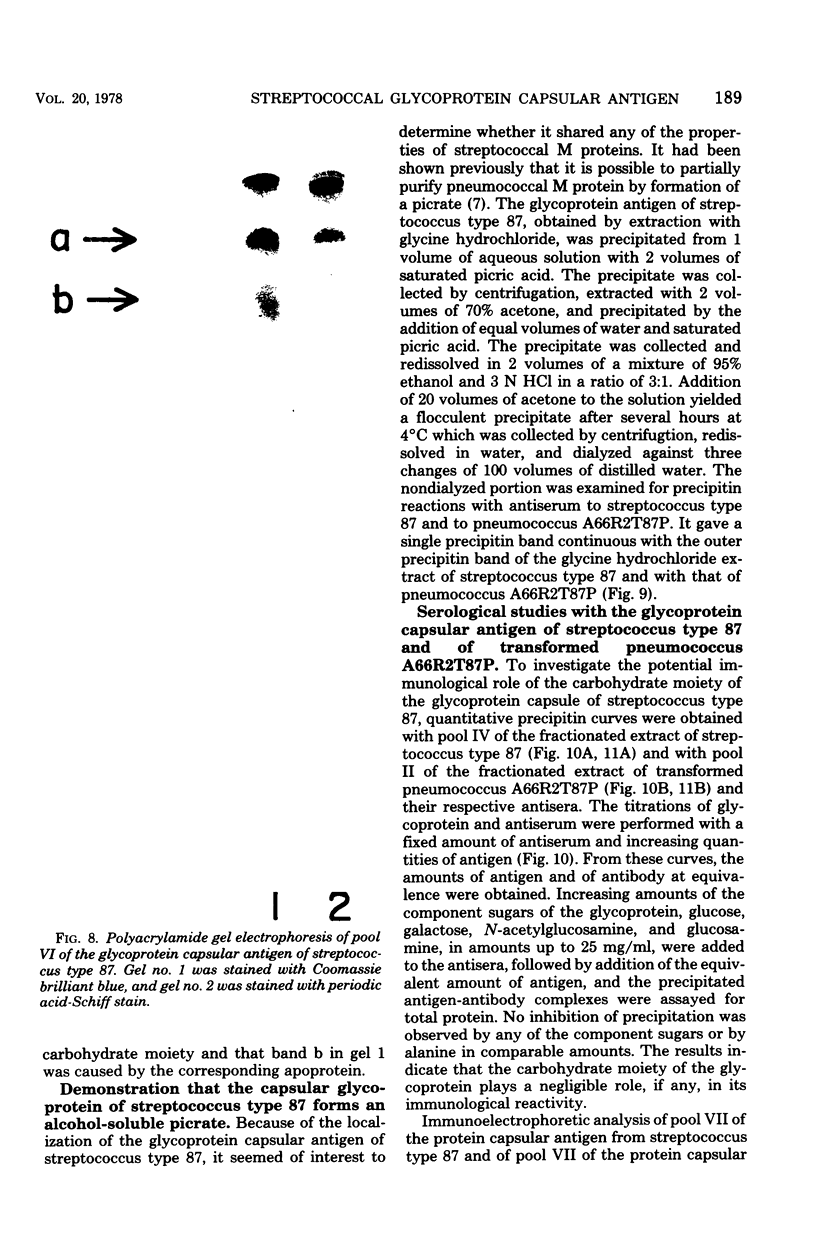
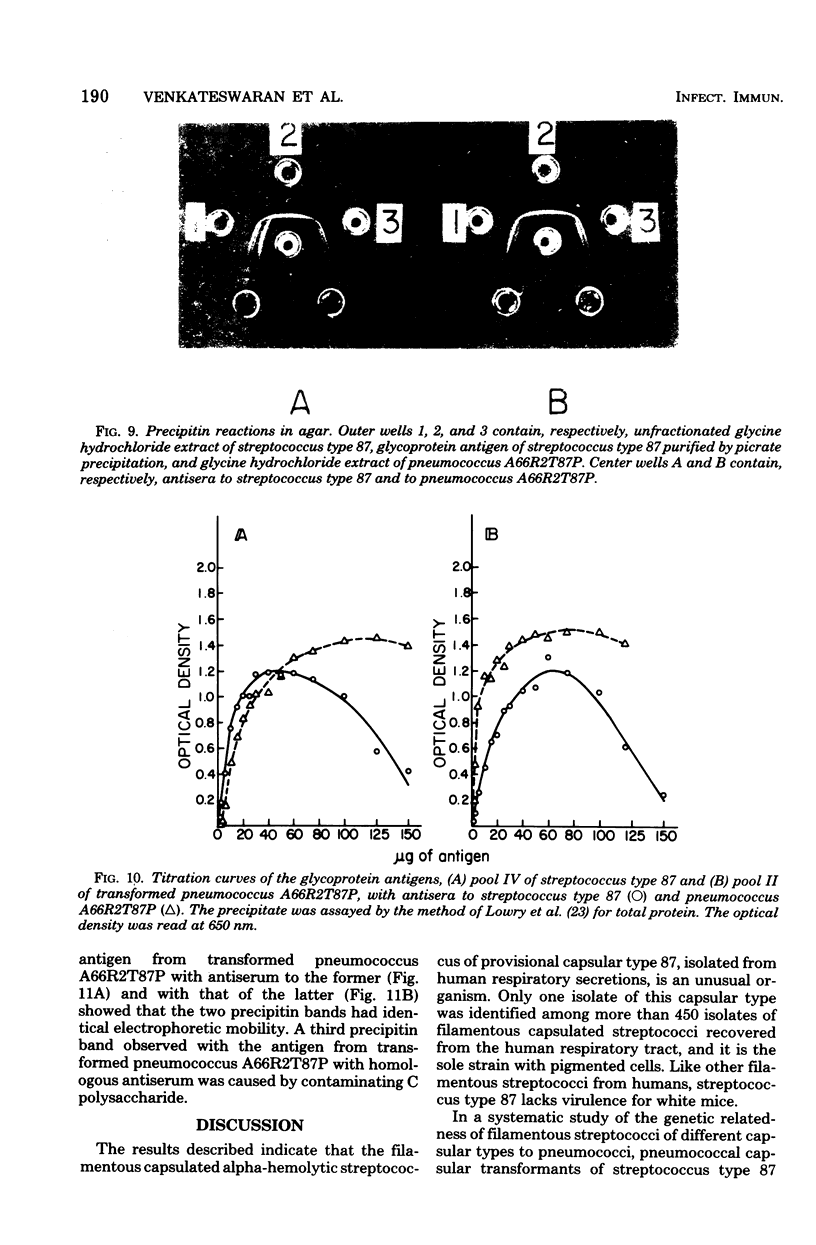
A filamentous alpha-hemolytic streptococcus of provisional capsular type 87 isolated from the human respiratory tract has been shown to be binary capsulated. One of the capsular antigens appears to be a glycoprotein; the other appears to be a polysaccharide. Transformation reactions with deoxyribonucleic acid from streptococcus type 87 and a number of noncapsulated pneumococci yielded transformed pneumococci with either a glycoprotein capsule or a polysaccharide capsule, but not with both. Capsular precipitin (quellung) reactions were observed when streptococcus type 87 was treated with homologous antiserum or with antisera to either of the two distinct capsular transformants. Each of the transformed pneumococci gave a quellung reaction with its homologous antiserum or with antiserum to streptococcus type 87, but neither reacted with antiserum to the heterologous transformant. Chemical analysis showed the glycoprotein antigen of streptococcus type 87 to contain, in addition to amino acids, glucose, galactose, glucosamine, and phosphate. The amino acid composition of the glycoprotein capsular antigens from streptococcus type 87 and of those from transformed pneumococci were similar, showing only minor differences. The glycoprotein capsular antigen from streptococcus type 87 gave two closely associated precipitin bands with homologous antiserum or antisera to transformed pneumococci with the glycoprotein capsule. That the two precipitin bands represent two unrelated proteins is precluded largely on the basis of the unlikely probability of 100% cotransformation of the genes coding for both proteins in the pneumococcal transformants that were isolated. Chemical analyses of the various fractions of the glycoprotein indicate that the two precipitin bands may represent a glycoprotein and its corresponding apoprotein.
Full text
PDF


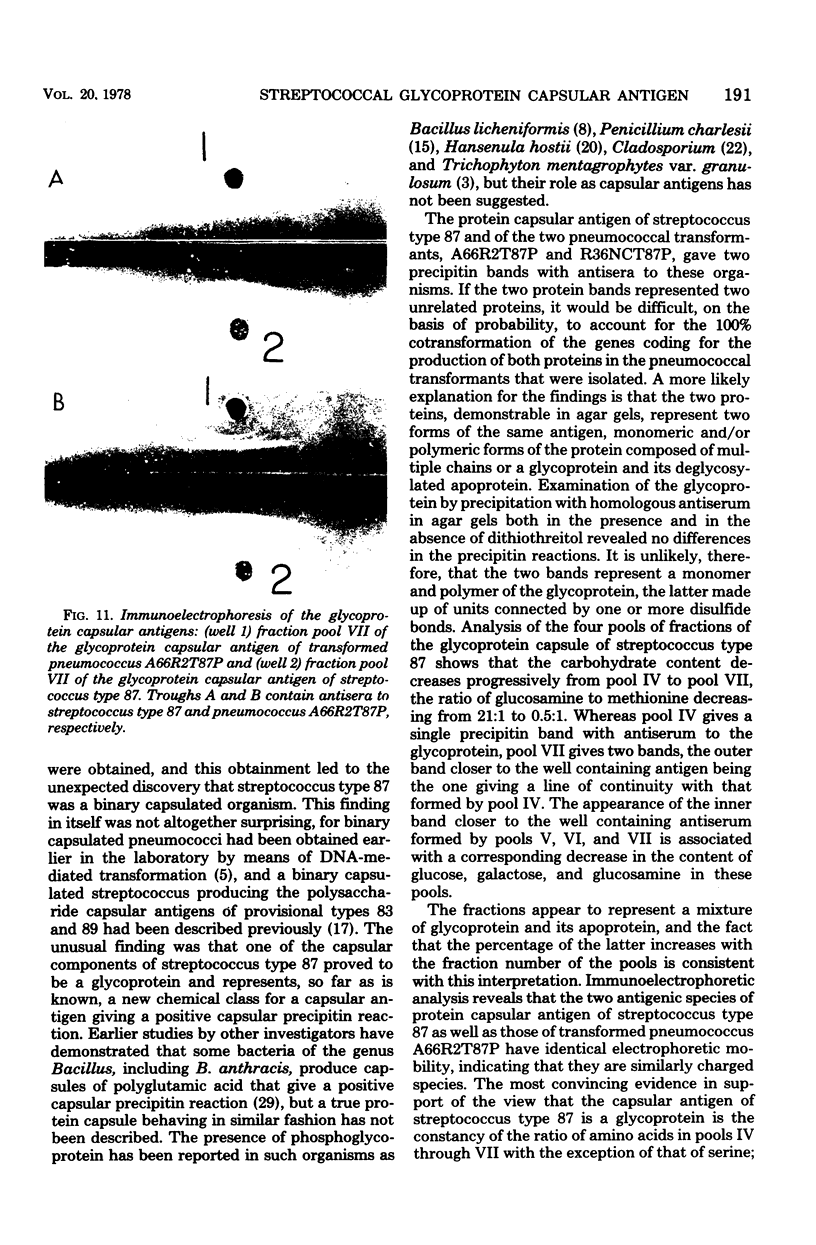


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUSTRIAN R., BERNHEIMER H. P. Simultaneous production of two capsular polysaccharides by pneumococcus. I. Properties of a pneumococcus manifesting binary capsulation. J Exp Med. 1959 Oct 1;110:571–584. doi: 10.1084/jem.110.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. C., Archibald A. R., Baddiley J., Curtis M. J., Davey N. B. The action of dilute aqueous NN-dimethylhydrazine on bacterial cell walls. Biochem J. 1969 Jun;113(1):183–189. doi: 10.1042/bj1130183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold M. T., Grappel S. F., Lerro A. V., Blank F. Peptido polysaccharide antigens from Trichophyton mentagrophytes var. granulosum. Infect Immun. 1976 Aug;14(2):376–382. doi: 10.1128/iai.14.2.376-382.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer H. P., Wermundsen I. E., Austrian R. Qualitative differences in the behavior of pneumoncoccal deoxyribonucleic acids transforming to the same capsular type. J Bacteriol. 1967 Jan;93(1):320–333. doi: 10.1128/jb.93.1.320-333.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fox E. N. M proteins of group A streptococci. Bacteriol Rev. 1974 Mar;38(1):57–86. doi: 10.1128/br.38.1.57-86.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gander J. E., Jentoft N. H., Drewes L. R., Rick P. D. The 5-O-beta-D-galactofuranosyl-containing exocellular glycopeptide of Penicillium charlesii. Characterization of the phosphogalactomannan. J Biol Chem. 1974 Apr 10;249(7):2063–2072. [PubMed] [Google Scholar]
- HAYASHI M., UNEMOTO T., MIYAKI K. Improvement on the colorimetric determination of choline with iodine. Chem Pharm Bull (Tokyo) 1962 Jun;10:533–535. doi: 10.1248/cpb.10.533. [DOI] [PubMed] [Google Scholar]
- Karakawa W. W., Kane J. A., Austrian R. Filamentous capsulated streptococci from the human respiratory tract. 3. Immunochemical studies of the cross-reactivity between the cell wall antigens of a filamentous streptococcus and of pneumococcus. Infect Immun. 1973 Dec;8(6):969–976. doi: 10.1128/iai.8.6.969-976.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakawa W. W., Kane J. A., Austrian R. Filamentous capsulated streptococci from the human respiratory tract. II. Antigenic structure of provisional capsular types 89 and 83-89. Infect Immun. 1973 Dec;8(6):962–968. doi: 10.1128/iai.8.6.962-968.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakawa W. W., Kane J. A., Buettger C., Austrian R. Filamentous capsulated streptococci from the human respiratory tract. I. Antigenic attributes of provisional capsular type 83 and its relationship to streptococci of so-called group M. Infect Immun. 1973 Dec;8(6):952–961. doi: 10.1128/iai.8.6.952-961.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak L. P., Bretthauer R. K. Studies on the biosynthesis of Hansenula holstii mannans from guanosine diphosphate mannose. Biochemistry. 1970 Mar 3;9(5):1115–1122. doi: 10.1021/bi00807a010. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lai C., Listgarten M., Rosan B. Serology of Streptococcus sanguis: localization of antigens with unlabeled antisera. Infect Immun. 1973 Sep;8(3):475–481. doi: 10.1128/iai.8.3.475-481.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd K. O. Molecular organization of a covalent peptido-phospho-polysaccharide complex from the yeast form of Cladosporium werneckii. Biochemistry. 1972 Oct 10;11(21):3884–3890. doi: 10.1021/bi00771a008. [DOI] [PubMed] [Google Scholar]
- McCARTY M., LANCEFIELD R. C. Variation in the group-specific carbohydrate of group A streptococci. I. Immunochemical studies on the carbohydrates of variant strains. J Exp Med. 1955 Jul 1;102(1):11–28. doi: 10.1084/jem.102.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter W. H. Application of nitrous acid deamination of hexosamines to the simultaneous GLC determination of neutral and amino sugars in glycoproteins. Anal Biochem. 1975 Jan;63(1):27–43. doi: 10.1016/0003-2697(75)90186-4. [DOI] [PubMed] [Google Scholar]
- Rosan B. Antigens of Streptococcus sanguis. Infect Immun. 1973 Feb;7(2):205–211. doi: 10.1128/iai.7.2.205-211.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosan B. Determination of muramic acid in automated amino acid analysis. Anal Biochem. 1972 Aug;48(2):624–628. doi: 10.1016/0003-2697(72)90119-4. [DOI] [PubMed] [Google Scholar]
- Venkateswaran P. S., Stanton N. Filamentous capsulated streptococci from the human respiratory tract: chemical and immunochemical characterization of the polysaccharide capsular antigen of provisional binary capsular type 87. Infect Immun. 1978 Apr;20(1):194–199. doi: 10.1128/iai.20.1.194-199.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosti K. L., Johnson R. H., Dillon M. F. Further characterization of purified fractions of M protein from a strain of group A, type 12 Streptococcus. J Immunol. 1971 Jul;107(1):104–114. [PubMed] [Google Scholar]