Abstract
The main aim of the present study was to examine to what extent long-lasting subcortical actions of transcranial direct current stimulation (tDCS) may be related to its presynaptic actions. This was investigated in the red nucleus, where tDCS was recently demonstrated to facilitate transmission between interpositorubral and rubrospinal neurons. Changes in the excitability of preterminal axonal branches of interpositorubral neurons close to rubrospinal neurons were investigated during and after tDCS (0.2 mA) applied over the sensorimotor cortical area in deeply anaesthetized rats and cats. As a measure of the excitability, we used the probability of antidromic activation of individual interpositorubral neurons by electrical stimuli applied in the red nucleus. Our second aim was to compare effects of weak (≤1 μA) direct current applied within the red nucleus with effects of tDCS to allow the use of local depolarization in a further analysis of mechanisms of tDCS instead of widespread and more difficult to control depolarization evoked by distant electrodes. Local cathodal polarization was found to replicate all effects of cathodal tDCS hitherto demonstrated in the rat, including long-lasting facilitation of trans-synaptically evoked descending volleys and trisynaptically evoked EMG responses in neck muscles. It also replicated all effects of anodal tDCS in the cat. In both species, it increased the excitability of preterminal axonal branches of interpositorubral neurons up to 1 h post-tDCS. Local anodal polarization evoked opposite effects. We thus show that presynaptic actions of polarizing direct current may contribute to both immediate and prolonged effects of tDCS.
Key points
Transcranial direct current stimulation (tDCS) may affect both postsynaptic neurons and presynaptic axons providing input to these neurons.
Here, we show that presynaptic subcortical actions of tDCS outlast the duration of its application by up to 1 h and may contribute to long-lasting facilitation of activation of neurons in the red nucleus in experiments on deeply anaesthetized animals. This is demonstrated by increased axonal excitability of the interpositorubral neurons that, in turn, activate rubrospinal neurons.
We also show that effects of electric fields induced by tDCS may be reproduced by local cathodal polarization, by applying <1 μA direct current within the red nucleus. Such polarization replicates effects of anodal tDCS in the cat as well as cathodal tDCS in the rat and evokes similar long-lasting facilitation.
Introduction
Even though the benefits of transcranial direct current stimulation (tDCS) in clinical practice are well established (for recent reviews see Stagg & Nitsche, 2011; Brunoni et al. 2012; Edwardson et al. 2013), the mechanisms underlying these effects remain under investigation. In addition, the analysis of these mechanisms has primarily focused on effects of polarization of the cerebral cortex, and reduced animal preparations were used in only a small number of studies. With respect to the issue of postsynaptic and presynaptic effects of polarizing current in rat cortical slices in vitro, Rahman et al. (2013) demonstrated that the excitability of postsynaptic neurons and presynaptic fibres may be affected to a different extent, depending on the relative orientation of neural processes of individual neurons in the electric field. Similar conclusions were drawn by Bikson et al. (2004) and Kabakov et al. (2012) regarding effects of DC in hippocampal slice preparations, while Jefferys (1981) concluded that the predominant effect on the population spike in the hippocampus in his experimental conditions was evoked on the granule cells rather than on presynaptic axons. However, these studies concerned events occurring during tDCS or during application of uniform electric fields, but not the after-effects of tDCS. Long-lasting effects of DC polarization in a slice preparation were apparently examined only in association with long-term potentiation evoked by repetitive stimuli in the hippocampus by Fritsch et al. (2010).
The main aim of the present study was therefore to examine whether polarization of presynaptic terminal axonal branches providing input to postsynaptic neurons does or does not contribute to the prolonged effects of tDCS. This question was addressed with respect to effects of tDCS in the red nucleus (RN), where these effects may be as potent as at the cortical level (Bolzoni et al. 2013a,b). Rubrospinal neurons may be activated by electrical stimuli applied in the RN either directly or indirectly, i.e. transynaptically (Baldissera et al. 1972). Stronger actions of tDCS on trans-synaptic than on direct activation of rubrospinal neurons demonstrated by Bolzoni et al. (2013a)2013a indicate that these effects of tDCS might be related to changes in synaptic transmission or to changes at the level of presynaptic axons, rather than to changes in the excitability of the RN neurons themselves. However, the after-effects of tDCS might depend on changes induced within the postsynaptic neurons and/or the presynaptic nerve terminals (for review see Nitsche et al. 2012) in addition to changes in nerve fibres some distance from the terminals (see e.g. Bostock et al. 1998; Nodera & Kaji, 2006; Trevillion et al. 2007) as well as other factors, including release of transmitters and/or modulators from other neurons and from glia and changes analogous to long-term facilitation of synaptic transmission in the respiratory system (see e.g. Baker-Herman & Mitchell, 2002). The possibility that polarization of presynaptic axons in the RN is essential for the long-lasting facilitation of indirect activation of rubrospinal neurons by tDCS therefore remained to be verified. In the present study, this was undertaken by examining changes in excitability of axons of interpositorubral neurons, which constitute the main source of input to rubrospinal neurons (Davis, 1969; Toyama et al. 1970; Anderson, 1971; Baldissera et al. 1972; Eccles et al. 1975). As a measure of excitability of these axons, we used the probability of antidromic activation of single neurons in the contralateral nucleus interpositus (IN). Prolonged changes in the excitability would be compatible with presynaptic actions of tDCS, while an absence of such changes might restrict the sites of long-lasting effects of tDCS to synaptic transmission and postsynaptic neurons. The study was undertaken in the expectation that the results obtained would apply to mechanisms of tDCS not only in the RN but also more generally and that a better understanding of the mechanisms of tDCS would assist its clinical applications.
Previous experiments have indicated that subcortical effects of tDCS in both cats and rats may be attributed to spread of current within the brain, rather than being mediated by activation of cortical neurons or modulation of spontaneous activity of these neurons (Bolzoni et al. 2013a,b). However, the intensity of current at long distances from the source of the DC in electric fields in vivo is not directly measurable or easy to monitor. Direct measurements were possible in the in vitro slice preparations only where the distances between the neurons stimulated or recorded from and the sources of the polarizing current could be reduced to 0.1–1.0 mm (Jefferys, 1981) The distances between the sites of application of tDCS and the explored subcortical regions in vivo were much larger, ∼30 mm in the cat and 10 mm in the rat. Opposite effects of the anodal and cathodal polarizing current in these species (Bolzoni et al. 2013a,b) could therefore be related to the distribution of the electric fields at these distances. In order to be able to analyse effects of the polarizing current in more defined conditions, we tried to reduce these distances to a minimum by applying DC polarization at the stimulation site. To this end, we applied the direct current and the stimulus pulses through the same electrode (see Methods). However, prior to using this approach it had to be ascertained that effects of local polarization not only reproduce effects of tDCS during its application, as in cortical slices of Rahman et al. (2013) or hippocampal slices of Jefferys (1981), Bikson et al. (2004) and Kabakov et al. (2012), but also that these effects outlast the duration of the local polarization. In the first part of the present study, therefore, we compared the effects of tDCS with effects of locally applied polarization, prior to examining presynaptic effects of locally applied direct current.
Methods
Ethical approval
All experiments were approved by the Regional Ethics Committee for Animal Research (Göteborgs Djurförsöksetiska Nämnd) and comply with EU guidelines for animal care and with the ethical policies and regulations of The Journal of Physiology (Drummond, 2009) and of the National Institutes of Health (Bethesda, MD, USA; Animal Welfare Assurance no. A5036-01). The animals were bred and housed under veterinary supervision at the Laboratory of Experimental Biomedicine at Sahlgrenska Academy, where the experiments were carried out.
Preparation
The experiments were performed on 34 deeply anaesthetized adult rats of both sexes (Wistar, 210–420 g) and three cats (3.3–5 kg).
In rats, general anaesthesia was induced by inhalation of isoflurane (Baxter Medical AB, Kista, Sweden), followed by administration of α-chloralose (Rhône-Poulenc Santé, France; 80 mg kg−1, i.p.) supplemented with sodium pentobarbital (Apoteksbolaget, Göteborg, Sweden; 10–20 mg kg−1 i.p.) and with two or three additional doses of α-chloralose up to 140–160 mg kg−1 i.v. administered when increases in the continuously monitored blood pressure or heart rate were evoked by any experimental procedures or when muscle twitches were evoked by any stimuli. Following the initial jugular vein and tracheal cannulation, the head was fixed in a stereotactic frame, and the border zone between the first and second cervical segments was exposed over the left ventral funiculus and dorsal columns to allow recording of descending volleys evoked by supraspinal stimuli. The respiration was assisted by a high-frequency (60 Hz) and low-volume respiratory pump to maintain the CO2 level in the expired air at ∼3.5–4.2%. The neuromuscular transmission was intact, as in the study of Bolzoni et al. (2013a)2013a, or temporarily blocked by pancuronium bromide (Pavulon; Organon, Sollentuna, Sweden; 0.3 mg kg−1 i.p.). The core body temperature was kept at ∼38°C by servo-controlled heating lamps. In order to compensate for fluid loss, 10–15 ml of acetate buffer (Baxter Healthcare Ltd, Thetford, UK) was injected subcutaneously at the beginning of the experiments, followed by slow i.v. infusion of the buffer at a rate of 0.3–0.5 ml h−1. In some experiments, pentobarbital (3 mg ml−1; 1 mg kg−1 h−1) was added to the infusion solution to increase the depth of anaesthesia. The experiments were terminated by a lethal dose of pentobarbital i.v., transcardial formalin perfusion and subsequent removal of the brain for histological control.
The brain was exposed by craniotomy to allow stereotactic insertion of the stimulating or recording electrodes. A tungsten electrode was introduced into the right RN, aiming at the site 5.8 mm caudal to bregma, 1.0–1.2 mm from the mid-line and 7.2–7.8 mm from the surface of the brain, using a NeuroStar motorized, computer-steered stereotaxic system (Neurostar GmbH, Tubingen, Germany). A second electrode, usually a glass micropipette but in some experiments a tungsten electrode, was introduced into the left IN. The electrode was mounted in a step motor-driven electrode holder at an angle of 20 deg from vertical, with the tip directed rostrally. It was introduced from the site 2–3 mm caudal to lambda, aiming at the site 11.3 mm caudal to bregma, 2 mm from the mid-line and 6 mm from the surface of the skull (3.5–5.0 mm from the surface of the cerebellum), assisted by a previous selection of the optimal co-ordinates by the NeuroStar system. The explored areas were at locations where the largest antidromic field potentials were evoked by 15–30 μA stimuli applied in RN.
In cats, general anaesthesia was induced with sodium pentobarbital (40–44 mg kg−1, i.p.) and maintained with intermittent doses of α-chloralose (5 mg kg−1 administered every 1–3 h, up to 65 mg kg−1, i.v.). Following the initial cannulation of the cephalic vein, femoral artery and trachea, the head was fixed in a stereotactic frame, and a small laminectomy was made to expose the surface of the lateral funiculus between the first and second or between the second and third cervical segments as well as at the level of the 13th thoracic segment. During recordings, neuromuscular transmission was blocked by pancuronium bromide (0.3 mg kg−1 i.v., supplemented with ∼0.2 mg kg−1 h−1), and the animals were artificially ventilated. Mean blood pressure was kept at 100–130 mm g and end-tidal CO2 at 3.9–4.5% by adjusting the parameters of artificial ventilation and the rate of a continuous infusion of a bicarbonate buffer solution with 5% glucose (1–2 ml kg−1 h−1). The body temperature was kept at ∼37.5°C by servo-controlled heating lamps. The experiments were terminated by a lethal dose of pentobarbital i.v. followed by formalin perfusion.
The brain was exposed by a craniotomy to allow stereotactic insertion of the stimulating or recording tungsten electrodes in both the left and right RN and the left and right IN. The area in the RN was selected by recording antidromic field potentials evoked by stimulation of the contralateral lateral funiculus at the Th13 level and lower threshold descending volleys from the cervical segments, aiming at the site at Horsley–Clarke co-ordinates A3.5, R or L2.0 and H −3.5. After having located the RN on one side, stimuli applied in this nucleus were used to locate the IN on the opposite side. To this end, the electrodes were introduced into the cerebellum at an angle of 20 or 30 deg from the vertical, aiming at Horsley–Clarke co-ordinates P8, R5 or L5, H1, but exploring the areas from which most distinct and lowest threshold descending volleys were evoked by stimuli applied in the NR.
At the end of all experiments, the stimulation sites were marked with electrolytic lesions by passing direct current through tungsten electrodes. In order to mark the recording area, the last glass micropipette was cut off and left in situ or the recording sites were marked by electrolytic lesions through a tungsten electrode introduced to the same depth as the glass microelectrode in the last track. The locations of these sites were defined on 100-μm-thick transverse sections of the mesencephalon (Fig. 1AB) and sagittal sections of the cerebellum (Fig. 1CD), cut using a vibratome, mounted on slides and counterstained with Cresyl Violet.
Figure 1. Stimulation sites in the red nucleus (RN) and recording sites in the nucleus interpositus (IN) in rats.
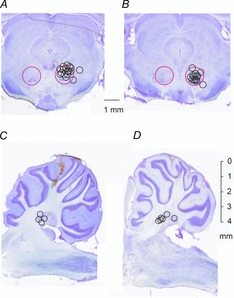
A and B, locations of the stimulating electrodes in the RN indicated on representative coronal sections through caudal and rostral parts, respectively, of the right RN nucleus. Large circles indicate the approximate borders of this nucleus. Small circles indicate the electrode locations defined by electrolytic lesions made at the end of the experiments; their diameters correspond to the distances of the estimated spread of current, within 0.2–0.3 mm from the electrode tip for 20–30 μA (see Fig. 11 of Gustafsson & Jankowska, 1976). C and D, recording sites in the nucleus interpositus (IN) in the cerebellum, indicated on parasagittal sections ∼2.0–2.5 and 1.5–2.0 mm from the mid-line. They include only the sites at which we found neurons that were antidromically activated by stimuli applied in RN.
Stimulation and recording
The right RN was stimulated with single, double or triple monopolar constant-current pulses of 10–50 μA and 0.2 ms long; stimuli of 50 μA were expected to act within ∼0.5 mm radius (Gustafsson & Jankowska, 1976). They were applied via tungsten electrodes (30–400 kΩ; manufactured from 0.2 mm wire, electrolytically sharpened and insulated except for the tip) against a larger electrode in contact with right paravertebral muscles at the level of the third cervical segment.
The effects of the stimuli were analysed on the following: (i) descending volleys recorded from the surface of the spinal cord at the C1/C2 segments, including both directly and trans-synaptically evoked volleys; (ii) short-latency EMG responses from a left paravertebral muscle at the level of the C2 segment (Bolzoni et al. 2013a), both recorded against a reference electrode in contact with a left neck muscle at the level of the C3/C4 segment; and (iii) antidromically evoked responses of single neurons recorded extracellularly in the IN contralateral to the stimulated RN. These were recorded through either a tungsten electrode (in rats and cats) or a glass micropipette filled with a 3 m solution of NaCl (impedance 1.5–5 MΩ; in rats). Responses evoked in an all-or-none fashion by near-threshold stimuli were accepted as being evoked by a single neuron. Antidromically evoked responses were differentiated from those evoked synaptically by their stable and short (0.4–0.9 versus 1.2–1.8 ms) latencies and, if possible, by collision with synaptically or spontaneously appearing spikes. Both single records and averages of 10–50 of these records were stored online (with the time resolution of 30 μs per address).
Transcranial DC stimulation
In rats, polarizing current was applied via a sponge soaked in 3% agar in saline (∼30 mm2; 0.2 mA resulting in ∼0.7 μA mm−2) attached to the skull over the major part of the right sensorimotor area as described by Bolzoni et al. (2013a)2013a. In cats, the polarizing current was applied via 3% agar in saline contained in a chamber attached to the skull over the pericruciate area with a contact area of ∼200 mm2 (0.2 mA resulting in ∼1 μA mm−2; Bolzoni et al. 2013b). The reference electrodes were in contact with saline-soaked cotton wool, covering an area twice the size of the stimulated area between the ipsilateral (left) lateral aspect of the skull and the temporal muscles in the cat or the ipsilateral ear lobe (rat). Transcranial DC stimulation was applied five to seven times for 5 min alternating with between-polarization periods of 5 min, preceded by a control period of several minutes and followed by several postpolarization periods of 5 min.
Local polarization
The local polarizing current was passed through the same tungsten electrode also used to activate rubrospinal or interpositorubral neurons and the same reference electrode (counter-electrode according to the terminology of Merrill et al. 2005). In order to ensure that the DC was sufficient, we applied it at intensities estimated to exceed the minimal effective intensity, e.g. 0.2 rather than 0.1 μA, monitored continuously on an inbuilt digital microamperometer.
While applying DC and stimulus pulses through the same electrode, we were aware of potential complications due to changes in the electrode–tissue interface or electrode polarization (for references, see Merrill et al. 2005). However, we were unable to use separate electrodes for stimulation and polarizing within the very small areas to be explored, especially in the rat. In addition, passing direct current within only a few micrometres distance would not obviate these problems, and we considered the risk of changes in the stimuli used for the excitability testing by polarization to be rather minimal in view of low intensities of both the intermittent (<50 μA, 0.2 ms) and constant stimuli (<1 μA) and because effects found during and following application of DC were generally similar. Given that no significant changes in electrode impedance were found to be caused by 0.75 mA applied for some 10–20 min in experiments of Ravid et al. (2011), the probability of serious changes to be caused by 0.1–0.3 μA might be negligible. Nevertheless, in order to verify that the passage of DC used in the present study did not interfere with the intermittent constant-current stimulus pulses delivered through this electrode, we compared the amplitude and the shape of these pulses before, during and after 30 min of the concurrent application of DC. As this kind of monitoring was not possible during the experiments, this was done while passing 0.2 μA DC through a tungsten electrode in Ringer solution. The current pulses were recorded to allow offline subtraction of those passed with and without DC. As shown in Fig. 2E, there were only marginal or non-measurable changes in these stimulus pulses.
Figure 2. Effects of local polarization on EMG responses evoked by stimuli applied in the RN.
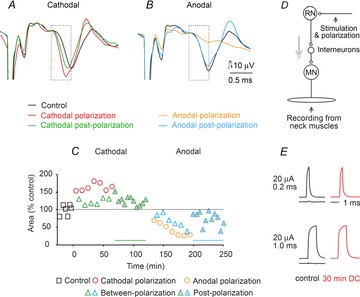
A and B, examples of EMG potentials recorded from neck muscles before, during and after cathodal or anodal polarization (1 μA) through the same electrodes via which a train of three 50 μA current pulses were applied to activate rubrospinal neurons. The illustrated records (averages of 20 single potentials) followed the third of these stimuli; the earlier parts of the records have been cropped off and the stimulus artefacts truncated. The records are aligned according to the onset of the stimulus artefacts (horizontally) and the onset of the EMG potentials (vertically). The dotted boxes indicate time windows during which their area was measured. C, time course of changes in the area of the EMG potentials expressed as a percentage of the area of control responses measured within time windows indicated by the dotted boxes in A and B. Continuous lines above the abscissa indicate the duration of the outlasting facilitatory and depressive effects. Significant differences were found when data from polarization, between-polarization and postpolarization groups were compared with control data. D, diagram of the stimulation and recording sites. The arrow indicates the direction of the neural traffic. E, comparison of constant-current 20 μA stimulus pulses delivered before (left) and during (right) passage of 0.2 μA cathodal direct current through a tungsten electrode (150 kΩ resistance against a reference electrode in Ringer solution). Current pulses were recorded 30 min from the beginning of application of the DC. When they were subtracted from each other, no differences or only marginal differences were found in the case of both 0.2 and 1.0 ms pulses; the longer-duration pulses were used to allow a better inspection of the shapes of these pulses. The difference traces are shown below control current pulses.
The local polarizing current was applied using the same experimental protocol as for tDCS, with repeated sequences of 5 min periods of polarization separated by 5 min between-polarization periods.
Analysis
Effects of polarizing current on antidromic field potentials, descending volleys and EMG responses were estimated from changes in the size and/or latencies of average potentials evoked during control periods and during or after application of tDCS or of local polarization. These changes were estimated by comparing the areas within selected time windows using software for sampling and analysis developed by E. Eide, T. Holmström and N. Pihlgren (University of Gothenburg). Changes in responses of single neurons were estimated from peristimulus time histograms and cumulative sums constructed online as described by Jankowska et al. (1997), allowing comparison of both the number of responses evoked by a certain number of stimuli (routinely five sequences of 20 stimuli, i.e. 100 stimuli) and of their latencies (see the Results).
Data were assigned into four main groups. The control group consisted of records made prior to the polarization, the polarization group consisted of records made during polarization periods, the between-polarization group consisted of records made between polarization periods and the postpolarization group consisted of records made following the last polarization. All data groups were tested for normal distribution (Shapiro–Wilk test) and for equal variance. Based on results of these tests, the differences between the data from various periods were assessed for statistical significance using one-way ANOVA or one-way ANOVA on ranks. When significant differences were found, multiple comparison post hoc tests (Holm–Sidak method for ANOVA and Dunn's method for ANOVA on ranks) against the control group were used. Additionally, when analysing the mean effects of local cathodal and anodal polarization, data sets from successive recording periods were compared with control data using one-way repeated-measures (RM) ANOVA or one-way RM ANOVA on ranks and adequate post hoc tests. An overall significance level for all ANOVA and multiple comparison tests was set at P < 0.05.
Results
Effects of locally applied cathodal and anodal polarizing current in comparison to effects of tDCS on activation of rubrospinal neurons
In preliminary experiments, in which 0.4–1.0 μA local cathodal polarization was used, no indications of detrimental effects of such current were found, because thresholds for effects evoked by RN stimuli were either reduced or remained unchanged during polarization. However, in some preliminary experiments the thresholds almost doubled when the cathodal polarization exceeded 1 μA, indicating an effect of ‘anodal surround’ (Katz & Miledi, 1965; see Ranck, 1975). In other experiments, the responses evoked by RN stimuli were facilitated during the first minutes of cathodal polarization but thereafter disappeared, indicating a possible cathodal block, although at a lower threshold than that found in other preparations, e.g. 6–125 μA for frog nerves (Bhadra & Kilgore, 2004). The increases in threshold occurred, in particular, when the polarizing current was applied at the sites from which interpositorubral neurons were activated at lowest stimulus intensities and were reminiscent of after-effects of cathodal direct current applied to peripheral nerves (Skoglund, 1945; Bhadra & Kilgore, 2004) except for their timing, because they occurred during minutes and not seconds. Accordingly, 0.1–0.2 μA was subsequently used when testing effects of local polarization on single neurons and 0.3–0.5 μA in other tests.
Figure 2A and B shows examples of records of EMG potentials evoked in neck muscles following RN stimuli in a rat before, during and after local application of 0.5 μA direct current for 5 min. The traces illustrate an increase in the area of the early parts of these potentials associated with shortened latency during and after cathodal polarization and the opposite effects of anodal polarization. The time course of these effects, plotted in Fig. 2C, shows that both the increases and the decreases occurred not only during application of the polarizing current but also during a period of 30–50 min after it had ceased. The changes were found to be significant when compared with control condtions (ANOVA, F(3,65) = 40.908, P ≤ 0.001; post hoc tests, P ≤ 0.001 for cathodal polarization, P = 0.004 for cathodal between-polarization and P < 0.032 for cathodal postpolarization; and ANOVA F(3,64) = 12.166, P ≤ 0.001; post hoc tests, P ≤ 0.001 for anodal polarization, P = 0.013 for anodal between-polarization and P < 0.011 for anodal postpolarization).
Overall, local cathodal polarization facilitated EMG responses in five of seven rats and local anodal polarization depressed them in all four rats. The mean degree of facilitation and depression evoked in this way is summarized in Table 1A and in Fig. 6A and B, where it is also compared with mean effects of tDCS (Bolzoni et al. 2013a). Both the individual and the pooled data show that the timing of effects of local polarization replicated the timing of effects of tDCS with the same polarity. Note that the mean sizes of EMG responses were doubled by local cathodal depolarization, although the effect was not as potent as of tDCS.
Table 1.
Summary of effects of local DC applied in the red nucleus on trisynaptically evoked EMG responses in rat neck muscles and descending volleys (A) and of effects of both local and transcranial DC on excitability of terminal axonal branches of interpositorubral neurons (B)
| A. Local DC | Control, no. of experiments | During polarization, mean ar ea (% control); ±SEM; no. of experiments | After polarization, mean area (% control); ±SBV; no. of experiments |
|---|---|---|---|
| EMG area, local cathodal | 202% | 236% | |
| ±9.72% | ±27.47% | ||
| 6 | 5 | 5 | |
| EMG area, local anodal | 45% | 60% | |
| ±3.08% | ±4.58% | ||
| 4 | 4 | 3 | |
| Descending volleys area, local cathodal | 154% | 165% | |
| ±6.90% | ±9.76% | ||
| 8 | 8 | 7 |
| B. Local and transcranial DC | Control, rate of activation; ±SEM; no. of cells | During polarization, rate of activation; ±SEM; no. of cells | After polarization, rate of activation; ±SBV; no. of cells |
|---|---|---|---|
| Antidromic IP activation rate, local cathodal (cats) | 46% | 81% | 64% |
| ±11% | ±16% | ±23% | |
| 4 | 4 | 3 | |
| Antidromic IP activation rate, local cathodal (rats) | 47% | 84% | 74% |
| ±20% | ±30% | ±10% | |
| 8 | 8 | 6 | |
| Antidromic IP activation rate, transcranial DC stimulation cathodal (rats) | 27% | 74% | 88% |
| ±20.19% | ±19.15% | ±16.33% | |
| 5 | 5 | 5 |
Data during polarization are for the last period of DC application; they are given as means and SEMs for the indicated numbers of experiments (A) or cells (B). Data after polarization are for effects of stimuli applied 40 min after the polarization had been stopped. The areas are expressed as a percentage of control areas. The activation rate is expressed as the number of stimuli in a series of 100 stimuli that were followed by antidromic activation of single interpositorubral (IP) neurons. The intensity of the stimuli was adjusted to be at threshold before application of the DC polarization.
Figure 6. Mean effects of local cathodal and anodal polarizing current in comparison to effects of tDCS in the rat.
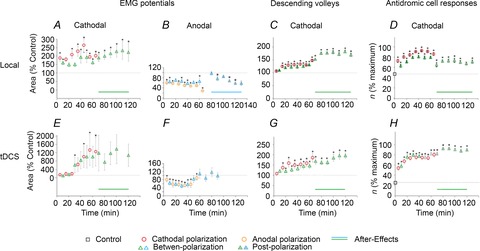
A and B, plots of changes in the area of EMG potentials evoked in neck muscles during and after application of local polarizing current, examined as in the experiment illustrated in Fig. 2A–C, but each data point being a mean of data for seven potentials found to be affected by polarization. C, similar pooled effects of local polarization on eight indirect descending volleys, including those illustrated in Fig. 3D, mean changes in excitability of terminal axonal branches of eight interpositorubral neurons monitored by changes in the number of antidromically evoked responses per 100 near-threshold stimuli as illustrated in Fig. 5E, F and G, as in A–C, but from experiments of Bolzoni et al. (2013a; see their Fig. 8CD), using the same experimental protocol except for the use of transcranially rather than locally applied polarizing current. In A–C and E–G, the data are expressed as a percentage of the areas of control responses before, during and after DC polarization (with standard error bars). In D and H are plotted numbers of responses (n) evoked by 100 stimuli before, during and after DC polarization, likewise with standard error bars. Horizontal dotted lines indicate 100% except for D and H, where they express the control rates of activation of interpositorubral neurons. Continuous lines above abscissa indicate the duration of the outlasting facilitatory and depressive effects. Notice similar patterns of changes evoked by locally and transcranially applied polarization. *Significant difference from control, P < 0.05. Statistical significance of difference between control and later periods was estimated using repeated-measures ANOVA or repeated-measures ANOVA on ranks with adequate post hoc comparisons (for details, see Table 2).
The effects of local cathodal polarization differed in the two remaining experiments, in which EMG responses were facilitated only at the beginning of the first period of cathodal polarization, after which the responses were reduced or disappeared altogether. In both these experiments, the thresholds for evoking EMG responses were lower than in the other experiments (40 and 30 μA compared with 50–70 μA), indicating that the stimulating RN electrode was positioned closer to the presynaptic nerve branches of the interpositorubral neurons so that the fibres were thereby more susceptible to the surround analectrotonus or to after-effects of catelectrotonus (Skoglund, 1945; see Methods). Whether 0.5 μA direct current applied within the terminal axonal projection area could have as strong damaging effects as more than 100 or 1000 times stronger current on peripheral nerves (Ravid et al. 2011) would remain an open question.
Whenever the indirect descending volleys were sufficiently distinct to be quantified, local cathodal depolarization facilitated them during and most often also following its application (Table 1A). In six rats, local cathodal polarization increased the area of the indirect volleys to >125%, in two rats to only 110–120% and in only two experiments was no facilitation detected. The examples of the strongest effects and their time course are shown in Fig. 3A–F and the average effects in Fig. 6C. Local cathodal polarization likewise increased indirect volleys evoked from RN in two cats (to 110–133%, the postpolarization facilitation lasting for 60 min).
Figure 3. Effects of local polarization on indirect descending volleys evoked by stimuli applied in the RN in three rats.
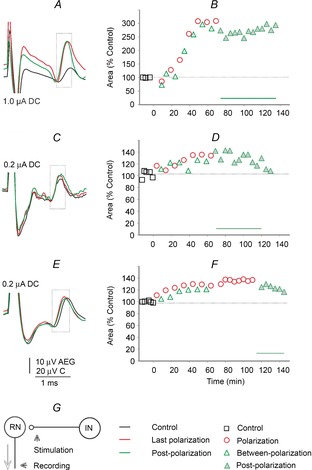
A, C and E, examples of indirect volleys recorded from the surface of the C2 segment before, during and after local cathodal polarization using 0.1, 0.2 and 0.2 μA direct current, respectively, through the same electrodes via which 30 μA double or triple current pulses were applied to activate rubrospinal neurons. Averages of 20 single potentials following the second of these stimuli are shown; the earlier and later parts of the records have been cropped off and the stimulus artefacts truncated. The records are aligned according to the onset of the stimulus artefacts (horizontally) and the positive peak of the descending volleys (vertically). The dotted boxes indicate time windows during which their area was measured. B, D and F, time course of changes in the area of volleys expressed as a percentage of the area of control volleys measured within time windows indicated by the dotted boxes. Significant differences were found for data from different periods for all comparisons in B, D and F. G, diagram of stimulation and recording sites
Taken together, the effects of local cathodal polarization on EMG responses and on indirect descending volleys evoked by stimuli applied in the RN showed the same features as effects of cathodal tDCS in rats and of anodal tDCS in cats. The changes were in the same direction and increased in a similar manner during subsequent periods of polarization. Both the facilitation and the depression outlasted the local polarization, even when the effects of local postpolarization were weaker than in the case of tDCS. Once the postpolarization effects reached a plateau, they remained practically unaltered during about 0.5 h and only started to decline thereafter. For these reasons, we used both tDCS and local DC polarization while examining the presynaptic effects described in the following sections.
Evidence for presynaptic actions of tDCS and of local polarization based on facilitation of antidromic activation of interpositorubral neurons by stimuli applied in the RN
Effects of tDCS and local polarization on terminal axonal branches of interpositorubral neurons in the RN were evaluated from changes in the probability of antidromic activation of single neurons, one of the measures routinely used to assess the excitability of primary afferents in the spinal cord (see e.g. Rudomin & Schmidt, 1999). The stimuli applied in the RN were near threshold prior to the polarization, being set to activate IN neurons in ≤50% of trials. Increased proportions of effective stimuli during or following local depolarization or tDCS were taken to indicate that the fibres on which they acted had become more excitable. The IN neurons were classified as antidromically activated based on the latencies of action potentials evoked in them (0.5–0.8 ms from the stimulus artefacts). As illustrated in Fig. 4E–N, these action potentials coincided with the antidromic field potentials following stimuli applied in the RN, and their latencies corresponded to latencies of antidromic activation of neurons in the IN found in the cat (0.7–0.9 ms; see Fig. 7 of Baldissera et al. 1972). Further criteria were the capability of action potentials to follow high-frequency stimuli (400 or even 600 Hz; Fig. 5G) and less than 0.05 ms jitter in latencies of potentials evoked by suprathreshold stimuli.
Figure 4. Examples of changes in the threshold of antidromic activation of single interpositorubral neurons by transcranial direct current stimulation (tDCS) in the rat.
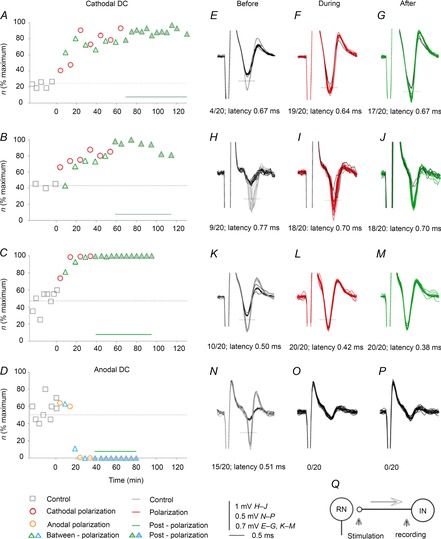
A–C, time course of facilitation of antidromic activation of three single interpositorubral neurons by near-threshold stimuli applied in the red nucleus (56, 23 and 25 μA, respectively) during and following cathodal tDCS. Ordinate, number of responses (n) evoked by 100 subsequent stimuli; abscissa, time from the last control records. Dotted horizontal lines indicate control level. Continuous horizontal lines above the abscissa indicate the duration of the outlasting facilitation. Significant differences were found for data from different periods for all comparisons in A–C. E–P, superimposed extracellular records of responses plotted in A–D, obtained before, during and after tDCS, with black traces representing field potentials visible when the neurons failed to be activated. Dotted horizontal lines indicate discrimination levels; each time when the responses of the neurons crossed them, they were counted online and used for computer-generated peristimulus time histograms and cumulative sums (Jankowska et al. 1997). Numbers below the records indicate the number of such responses evoked by 20 stimuli in a sequence and their minimal latencies. Q, diagram of the stimulation and recording sites.
Figure 5. Examples of decreases in the threshold of antidromic activation of single interpositorubral neurons by local cathodal polarization in the rat.
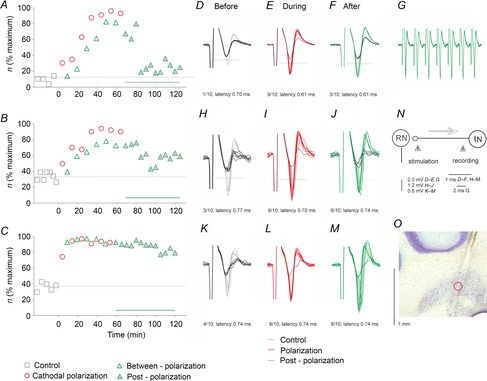
A–C, time course of facilitation of antidromic activation of single interpositorubral neurons by near-threshold stimuli applied in the red nucleus (23, 25 and 24 μA, respectively) during and following local cathodal polarization in three rats. The format is as in Fig. 4. D–M, superimposed extracellular records of responses plotted in A–C, obtained before (blue), during (red) and after (green) depolarization by 0.1 μA, with black traces representing field potentials visible when the neurons failed to be activated, as in Fig. 4. Numbers below the traces indicate the number of such responses evoked by 10 stimuli in a sequence and their minimal latencies. Significant differences were found for data from some periods in A and all periods in B and C. G, five superimposed single records illustrating minimal jitter and following of 400 Hz stimuli of responses evoked by suprathreshold stimuli evoked after records in F. N, diagram of the stimulation and recording sites. O, recording site of the third neuron in the left nucleus interpositus. The location of this site is indicated by a circle along the electrode track. It was estimated from the distance from the surface of the cerebellum. Note that responses evoked during and after the depolarization periods appeared at a rate that was much higher than originally, indicating a marked increase in the excitability of the stimulated fibres. The rate decreased during the postpolarization period but remained well above the control level for >1 h. Note also shortening of latencies of responses of the first two neurons.
Effects of cathodal tDCS were tested on antidromic activation of five interpositorubral neurons in rats (in five experiments). The facilitation was expressed as an increase in the number of responses evoked during five series of 20 subsequent stimuli, e.g. from a total of 10 to 40 responses per 100 stimuli prior to tDCS to a total of 40 to 90 per 100 stimuli during or after tDCS. Such changes will be referred to as changes in the rate of successful antidromic activation of the neurons. In all tests, the frequency of the stimuli remained constant, with the neurons responding with a single spike per stimulus or not responding, and not displaying spontaneous activity which might collide with antidromically conducted action potentials. As illustrated in Fig. 4A–M and summarized in Fig. 6H and Table 1B, the number of responses of all five neurons per 100 stimuli increased at least twofold. Plots in Fig. 4A–C show, however, that the degree of facilitation of activation of individual neurons and the timing of its development varied. The duration of postpolarization facilitation also varied, but the average rate of responses remained increased for at least 40 min following the last period of tDCS. In addition, the minimal latency of antidromic activation decreased in four of the five neurons by 0.1–0.2 ms, with respect both to the stimulus artefacts and to the onset of the antidromic field potentials, as illustrated in Fig. 4H–J and K–M. Significant differences were found for data in Fig. 4BDF when compared with control conditions (ANOVA on ranks, H = 68.467, d.f. = 3, P ≤ 0.001 and post hoc tests, P < 0.05 for all comparisons in Fig. 4A; ANOVA on ranks, H = 29.174, d.f. = 3, P ≤ 0.001 and post hoc tests, P < 0.05 for polarization and postpolarization but P ≥ 0.05 for between-polarization in Fig. 4B; and ANOVA on ranks, H = 57.364, d.f. = 3, P ≤ 0.001 and post hoc tests, P < 0.05 for all comparisons in Fig. 4C).
Anodal tDCS had on opposite effect on all three interpositorubral neurons tested in the rats. The decrease in the number of stimuli that evoked antidromic activation of two neurons was moderate (from ∼95 to 80% and from ∼80 to 60%) but was very strong in the third one (to nil; illustrated in Fig. 4D; ANOVA on ranks, H = 44.442, d.f. = 3, P ≤ 0.001 and post hoc test, P < 0.05 for postpolarization but not for polarization and between-polarization).
Effects of local cathodal polarizing current were examined on responses to stimuli applied in the RN of a total of 12 interpositorubral neurons; eight in rats and four in cats. Records in Fig. 5 show that local depolarization of only 0.1 μA replicated the effects of cathodal tDCS of 0.2 mA. Local depolarization increased the number of responses of these neurons from, for example, 10–20 to 80–90 per 100 stimuli or from 40–60 to 90–100 per 100 stimuli. The facilitation developed as slowly as that evoked by tDCS (Fig. 5A and B) or practically reached its maximum during the first period of depolarization (Fig. 5C). Following each period of depolarization, the rate of responses was retained at somewhat lower or similar levels, and most neurons continued to respond at increased rate for 10–15 min following the last polarization period, after which the rate decreased while still exceeding the original rate for 30–60 min. This was the case for six of eight neurons in rats (Fig. 6D and Table 1B) and three of four neurons in cats (Table 1B). It was also true for the population of rat neurons, with differences between the rates of after-polarization responses and the control responses being statistically significant for up to 60 min (see Table 2). The increases in the number of responses were associated with a shortening of the latency of antidromic activation of the neurons by 0.06–0.1 ms, i.e. of the same order as when evoked by tDCS. The range of effects was similar in the rats and in the cats. Significant differences were found for data in Fig. 5 when compared with control conditions in Fig. 5A (ANOVA on ranks, H = 60.490, d.f. = 3, P ≤ 0.001 and post hoc test, P < 0.05 for polarization and betweenpolarization, but not for postpolarization), for all data sets in Fig. 5B (ANOVA on ranks, H = 47.281, d.f. = 3, P ≤ 0.001 and post hoc tests, P < 0.05 for all comparisons) and all data sets in Fig. 5C (ANOVA on ranks, H = 42.409, d.f. = 3, P ≤ 0.001 and post hoc tests, P < 0.05 for all comparisons).
Table 2.
Analysis of pooled data for effects of local and transcranial polarization on EMG responses, descending volleys and antidromic cell responses, summarized in Fig. 6 and in Table 1
| Polarization | Between-polarization | Postpolarization | ||||
|---|---|---|---|---|---|---|
| EMG cathodal | ||||||
| Local polarization | RM ANOVA on ranks (n = 14) | χ2(7) = 53.476 P < 0.001 | RM ANOVA (n = 8) | F(7,7) = 4.713 P < 0.001 | RM ANOVA on ranks (n = 12) | χ2(6) = 27.893 P < 0.001 |
| tDCS | RM ANOVA on ranks (n = 12) | χ2(7) = 34.331 P < 0.001 | RM ANOVA on ranks (n = 10) | χ2(7) = 22.105 P = 0.002 | RM ANOVA (n = 5) | F(6,4) = 0.955 P = 0.476 |
| EMG anodal | ||||||
| Local polarization | RM ANOVA on ranks (n = 162) | χ2(7) = 77.570 P < 0.001 | RM ANOVA (n = 56) | F(6,6) = 2.873 P = 0.022 | RM ANOVA (n = 71) | F(6,10) = 11.296 P < 0.001 |
| tDCS | RM ANOVA (n = 9) | F(6,8) = 11.731 P < 0.001 | RM ANOVA on ranks (n = 9) | χ2(6) = 38.762 P < 0.001 | RM ANOVA (n = 5) | F(3,4) = 1.658 P = 0.288 |
| Indirect volleys | ||||||
| Local polarization | RM ANOVA on ranks (n = 41) | χ2(7) = 147.879 P < 0.001 | RM ANOVA on ranks (n = 21) | χ2(7) = 71.590 P < 0.001 | RM ANOVA on ranks (n = 27) | χ2(6) = 65.420 P < 0.001 |
| tDCS | RM ANOVA (n = 10) | F(7,9) = 10.171 P < 0.001 | RM ANOVA (n = 10) | F(7,9) = 8.844 P < 0.001 | RM ANOVA (n = 7) | F(5,6) = 8.454 P < 0.001 |
| Antidromic cell responses | ||||||
| Local polarization | RM ANOVA on ranks (n = 98) | χ2(7) = 242.435 P < 0.001 | RM ANOVA (n = 80) | F(7,79) = 25.244 P < 0.001 | RM ANOVA on ranks (n = 69) | χ2(6) = 114.11 P < 0.001 |
| tDCS | RM ANOVA on ranks (n = 49) | χ2(7) = 95.179 P < 0.001 | RM ANOVA on ranks (n = 35) | χ2(7) = 108.753 P < 0.001 | RM ANOVA on ranks (n = 30) | χ2(7) = 94.900 P < 0.001 |
The statistical significance of changes evoked by DC application when compared with control data was estimated using one-way repeated-measures (RM) ANOVA for data with normal distribution and equal variance (data presented as F value; P value), while Friedman RM ANOVA on ranks was used for data with non-normal distribution (χ2 value; P value); n is the number of recordings. Abbreviation: tDCS, transcranial direct current stimulation.
Discussion
Evidence for long-lasting presynaptic subcortical effects of local and transcranial DC polarization
The results of this study provide evidence that tDCS may induce long-lasting changes in the excitability of presynaptic terminal axonal branches within the interpositorubrospinal neuronal network. These increases were expressed by the much higher probability of antidromic activation of interpositorubral neurons by near-threshold stimuli applied in the RN and may be related to the depolarization of these axonal branches at the site of application of the stimuli used to excite them. As illustrated in the second part of the Results, these stimuli became more effective in activating interpositorubral neurons during cathodal tDCS in the rat, as well as during local depolarization of the terminal branches of interpositorubral neurons in both the rat and the cat, while local hyperpolarization and tDCS with opposite polarity decreased excitability of these terminal branches. Furthermore, presynaptic effects of both local polarization and tDCS occurred not only during the immediately following between-polarization periods but also during a period of at least 15 min after the last polarization and remained up to 1–2 h. The shortest-lasting after-effects of the local or transcranial polarization (about 15 min) may appear relatively brief, but they differ from after-effects of only a few seconds of a mere catelectrotonus or analectrotonus (Skoglund, 1945). Furthermore, as the degree of facilitation of antidromic activation of interpositorubral neurons increased during subsequent periods of polarization, the facilitation appeared to accumulate on top of the remaining effects of previously applied local polarization and tDCS.
The interpositorubrospinal network represents only one of the subcortical neuronal systems that are affected by tDCS, but the results are consistent with indications of presynaptic actions of tDCS previously found in other networks (for references see Introduction).
Polarity of the effective tDCS and of the local polarizing current
The facilitation of activation of human cortical neurons by anodal tDCS was explained in a similar way to the more potent activation of feline or primate cortical neurons by surface anodal than by surface cathodal short-pulse electrical stimuli (Phillips, 1956; Hern et al. 1962). The explanation was the relatively stronger hyperpolarization of apical dendrites associated with depolarization of deeper located initial segments of axons of pyramidal neurons, resulting in a reduced threshold for initiation of action potentials in these neurons (see Molaee-Ardekani et al. 2013; Rahman et al. 2013). Effects of anodal tDCS on subcortical neurons in the cat (Bolzoni et al. 2013b) might be interpreted by the same token, even though the facilitatory effects of cathodal rather than anodal tDCS in the rat (Bolzoni et al. 2013a) could not be accounted for in a similar manner. However, the present results show similar effects of cathodal polarization applied in the RN in both the cat and the rat; thereby, they indicate that the basic phenomena are the same in these species, or other species, even if the epiphenomena of tDCS related to the distribution of electrical fields within the brains of different sizes and shapes and to the arrangements of the stimulating electrodes may differ. Long-lasting effects of local polarization in experiments in animals may thus provide new tools for further examination of the mechanisms of tDCS.
On consequences of effects of polarization of presynaptic fibres for trans-synaptic activation of neurons and its analysis
Both the somata of cortical neurons and the terminal axonal networks providing input to them were found to be affected by polarization of the cerebral cortex, with the effects depending on their orientation with respect to the electric fields (Rahman et al. 2013). Similar conclusions were drawn regarding the sites of action of polarizing current applied in hippocampal slices, with a tendency for stronger synaptic or antidromic effects of fibres providing input to pyramidal tract neurons when they were stimulated within more negative parts of the uniform electric field than their target cells (Jefferys, 1981; Bikson et al. 2004; Kabakov et al. 2012). In the context of the present study, this would correspond to an increase in excitability of fibres running in the vicinity of the local cathodal polarization in the RN, or to a larger population of fibres within the whole RN with tDCS. Increases in excitability would allow action potentials to be initiated in a larger number of interpositorubral fibres close to the stimulating electrode as well as in fibres at somewhat greater distances from this electrode, thereby increasing the input to their target neurons and, in turn, the probability of activation of a greater proportion of these neurons.
Effects of both tDCS and locally applied DC were also expressed in shortening the latency of antidromic activation of interpositorubral neurons, allowing a new explanation of the shorter latencies of trans-synaptic activation of rubro-, reticulo-, cortico- or vestibulospinal neurons by tDCS. So far, we have attributed the earlier activation of these neurons mainly to a more effective initiation of action potentials (Bolzoni et al. 2013a,b). However, in view of the results of the present study, both tDCS and local depolarization might shorten the utilization time at the site of initiation of action potentials in presynaptic fibres by electrical stimuli and/or increase their conduction velocity.
The consequences of polarization of presynaptic fibres for the resulting release of transmitter and activation of their target cells following tDCS are less straightforward. We have found that when DC was applied locally, the depolarizing DC consistently facilitated the indirect activation of RN neurons. However, hyperpolarization rather than depolarization of terminals of interpositorubral fibres would be expected to enhance transmitter release in synapses between these fibres and rubrospinal neurons, as in the case of effects of locally applied DC close to the neuromuscular junction (Hubbard & Willis, 1962) or of current affecting afferents terminating on spinal motoneurons (Eccles et al. 1962).
In order to account for this discrepancy, it may be considered that even when the stimulating electrodes are close to some of the rubrospinal neurons, they are at a distance from many other neurons on which the depolarized interpositorubral fibres converge. This would be consistent with a high degree of divergence of interpositorubral projections within the RN, because a considerable number of interpositorubral neurons converge on individual rubrospinal neurons (Toyama et al. 1970; Baldissera et al. 1972), and the majority of rubrospinal neurons are activated by them (Baldissera et al. 1972). Given that anodal surround (Eccles et al. 1962; Katz & Miledi, 1965; Ranck, 1975) may be induced within very short distances from the sites of application of even very weak direct currents, depolarization of interpositorubral fibres at a certain location might be associated with hyperpolarization of terminals of the same fibres on rubrospinal neurons located further away. The increase in fibre excitability by the depolarizing local DC could, accordingly, be associated with facilitation of synaptic action of the same fibres by hyperpolarization of their terminals at a distance. If so, facilitatory effects of the local direct depolarizing current would not be at variance with the established effects of DC on synaptic transmission.
With respect to the prolonged effects of tDCS, our results show that changes in the excitability of presynaptic fibres evoked by either tDCS or local DC are sufficiently long lasting to account for them. Nevertheless, changes in the excitability of presynaptic fibres might be evoked in parallel with other effects of tDCS and may not be essential for the long-lasting facilitation of trans-synaptic activation.
Direct effects of tDCS on the subcortical postsynaptic neurons (rubro- or vestibulospinal; Bolzoni et al. 2013a,b) investigated so far were difficult to quantify, because responses induced by stimuli applied within either the RN or vestibular nuclei were, to a great extent, superimposed on stimulus artefacts. Nevertheless, direct activation of neurons in these nuclei appeared to be affected by tDCS to a much smaller extent than when they were activated trans-synaptically. For instance, in the cat, directly evoked responses of rubrospinal neurons were found to be facilitated in only two of seven experiments, while trans-synaptically evoked responses were enhanced in all of them (Bolzoni et al. 2013b). In addition, even when the directly evoked activation of rubrospinal neurons in the rat was facilitated during tDCS, the facilitation did not outlast the duration of the tDCS (see Fig. 3D of Bolzoni et al. 2013a).
The question of the relative impact of effects of tDCS on postsynaptic potentials evoked in target neurons of the depolarized presynaptic fibres, as well as direct actions of tDCS on these neurons, would therefore have to be addressed in more favourable experimental conditions.
It should be also taken into account that depolarization expressed as changes in the excitability of either the postsynaptic neurons or the presynaptic fibres would not be the only mechanism of facilitation evoked by cathodal polarization. As indicated repeatedly (see e.g. Lang et al. 2005; Stagg & Nitsche, 2011; Brunoni et al. 2012; Nitsche et al. 2012), effects of DC may also involve a number of other factors, such as release of neuromodulators and neurotransmitters by neurons and glia, changes in transmission of action potentials at axonal branch points or changes in invasion of the terminal axonal portions, postsynaptic membrane receptors or intraneuronal calcium concentration. They might also involve changes in synaptic transmission similar to those found in other cases of synaptic plasticity; in particular, in synapses on spinal phrenic motoneurons during long-term facilitation of their activation (see Baker-Herman & Mitchell, 2002).
Closing remarks
Irrespective of whether long-lasting facilitation of synaptic actions of locally depolarized presynaptic fibres turns out to be secondary to the depolarization or hyperpolarization of their terminals, the same mechanisms might be expected to be involved in other subcortical structures as in the red nucleus. The same basic mechanisms might also be hypothesized to operate within cortical neuronal networks and in the spinal cord. Further analysis of prolonged presynaptic actions of tDCS and of local depolarization together with an examination of the ensuing direct effects on postsynaptic neurons should thus provide more information on the mechanisms of tDCS. It might also be expected that conclusions based on such analysis would be applicable to any neuronal systems in view of the growing evidence that basic mechanisms of operation of the nervous system are shared across various animal species and various neuronal systems, including cortical networks in man.
Local polarization has the disadvantage of not being non-invasive and therefore of being applicable only in animals. However, when compared with polarization evoked by distant electrodes, in particular by tDCS, local polarization has the great advantage that its effects are much more spatially restricted and that the degree of polarization within the selected brain region may be easier to estimate and to control. Electrical field potentials evoked by tDCS are always very widespread, even in conditions of the most careful control of its strength and of the site of its application, and the resulting transcranial currents influence a variety of neurons that may contribute to the ultimate effects. Local polarization might therefore allow more specific questions on mechanisms of effects of electric fields to be addressed than when DC is applied via distant electrodes and may open new possibilities for further analysis of mechanisms of tDCS.
Acknowledgments
We wish to thank Dr Ingela Hammar for comments on a preliminary version of this paper and Jytte Grännsjö for excellent technical assistance during the experiments and with the histological control.
Glossary
- IN
nucleus interpositus
- RN
red nucleus
- tDCS
transcranial direct current stimulation
Additional information
Competing interests
None declared.
Author contributions
The experiments were performed at the Department of Physiology, University of Gothenburg. Both authors contributed to the design of the experiments as well as to the collection, analysis and interpretation of the data and to the drafting of the article; both approved the final version of the manuscript.
Funding
The work was supported by the National Institutes of Health (R01 NS040863 to E.J.) and University of Gothenburg.
References
- Anderson ME. Cerebellar and cerebral inputs to physiologically identified efferent cell groups in the red nucleus of the cat. Brain Res. 1971;30:49–66. doi: 10.1016/0006-8993(71)90005-9. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F, Lundberg A, Udo M. Stimulation of pre- and postsynaptic elements in the red nucleus. Exp Brain Res. 1972;15:151–167. doi: 10.1007/BF00235579. [DOI] [PubMed] [Google Scholar]
- Bhadra N, Kilgore KL. Direct current electrical conduction block of peripheral nerve. IEEE Trans Neural Syst Rehabil Eng. 2004;12:313–324. doi: 10.1109/TNSRE.2004.834205. [DOI] [PubMed] [Google Scholar]
- Bikson M, Inoue M, Akiyama H, Deans JK, Fox JE, Miyakawa H, Jefferys JG. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J Physiol. 2004;557:175–190. doi: 10.1113/jphysiol.2003.055772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolzoni F, Baczyk M, Jankowska E. Subcortical effects of transcranial direct current stimulation in the rat. J Physiol. 2013a;591:4027–4042. doi: 10.1113/jphysiol.2013.257063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolzoni F, Pettersson LG, Jankowska E. Evidence for long-lasting subcortical facilitation by transcranial direct current stimulation in the cat. J Physiol. 2013b;591:3381–3399. doi: 10.1113/jphysiol.2012.244764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H, Cikurel K, Burke D. Threshold tracking techniques in the study of human peripheral nerve. Muscle Nerve. 1998;21:137–158. doi: 10.1002/(sici)1097-4598(199802)21:2<137::aid-mus1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, Edwards DJ, Valero-Cabre A, Rotenberg A, Pascual-Leone A, Ferrucci R, Priori A, Boggio PS, Fregni F. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. 2012;5:175–195. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. Brachio-rubral and rubro-brachial activity in the cat. Brain Res. 1969;15:157–173. doi: 10.1016/0006-8993(69)90316-3. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Kostyuk PG, Schmidt RF. The effect of electric polarization of the spinal cord on central afferent fibres and on their excitatory synaptic action. J Physiol. 1962;162:138–150. doi: 10.1113/jphysiol.1962.sp006920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Scheid P, Taborikova H. Responses of red nucleus neurons to antidromic and synaptic activation. J Neurophysiol. 1975;38:947–964. doi: 10.1152/jn.1975.38.4.947. [DOI] [PubMed] [Google Scholar]
- Edwardson MA, Lucas TH, Carey JR, Fetz EE. New modalities of brain stimulation for stroke rehabilitation. Exp Brain Res. 2013;224:335–358. doi: 10.1007/s00221-012-3315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, Lu B. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66:198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B, Jankowska E. Direct and indirect activation of nerve cells by electrical pulses applied extracellularly. J Physiol. 1976;258:33–61. doi: 10.1113/jphysiol.1976.sp011405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hern JE, Landgren S, Phillips CG, Porter R. Selective excitation of corticofugal neurones by surface-anodal stimulation of the baboon's motor cortex. J Physiol. 1962;161:73–90. doi: 10.1113/jphysiol.1962.sp006874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard JI, Willis WD. Hyperpolarization of mammalian motor nerve terminals. J Physiol. 1962;163:115–137. doi: 10.1113/jphysiol.1962.sp006961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Djouhri L, Heden C, Szabo Lackberg Z, Yin XK. Modulation of responses of four types of feline ascending tract neurons by serotonin and noradrenaline. Eur J Neurosci. 1997;9:1375–1387. doi: 10.1111/j.1460-9568.1997.tb01492.x. [DOI] [PubMed] [Google Scholar]
- Jefferys JG. Influence of electric fields on the excitability of granule cells in guinea-pig hippocampal slices. J Physiol. 1981;319:143–152. doi: 10.1113/jphysiol.1981.sp013897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabakov AY, Muller PA, Pascual-Leone A, Jensen FE, Rotenberg A. Contribution of axonal orientation to pathway-dependent modulation of excitatory transmission by direct current stimulation in isolated rat hippocampus. J Neurophysiol. 2012;107:1881–1889. doi: 10.1152/jn.00715.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Miledi R. Propagation of electric activity in motor nerve terminals. Proc R Soc Lond B Biol Sci. 1965;161:453–482. doi: 10.1098/rspb.1965.0015. [DOI] [PubMed] [Google Scholar]
- Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, Rothwell JC, Lemon RN, Frackowiak RS. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain. Eur J Neurosci. 2005;22:495–504. doi: 10.1111/j.1460-9568.2005.04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill DR, Bikson M, Jefferys JG. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J Neurosci Methods. 2005;141:171–198. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Molaee-Ardekani B, Márquez-Ruiz J, Merlet I, Leal-Campanario R, Gruart A, Sánchez-Campusano R, Birot G, Ruffini G, Delgado-García JM, Wendling F. Effects of transcranial Direct Current Stimulation (tDCS) on cortical activity: a computational modeling study. Brain Stimul. 2013;6:25–39. doi: 10.1016/j.brs.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Müller-Dahlhaus F, Paulus W, Ziemann U. The pharmacology of neuroplasticity induced by non-invasive brain stimulation: building models for the clinical use of CNS active drugs. J Physiol. 2012;590:4641–4662. doi: 10.1113/jphysiol.2012.232975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodera H, Kaji R. Nerve excitability testing and its clinical application to neuromuscular diseases. Clin Neurophysiol. 2006;117:1902–1916. doi: 10.1016/j.clinph.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Phillips CG. Cortical motor threshold and the thresholds and distribution of excited Betz cells in the cat. Q J Exp Physiol Cogn Med Sci. 1956;41:70–84. doi: 10.1113/expphysiol.1956.sp001164. [DOI] [PubMed] [Google Scholar]
- Rahman A, Reato D, Arlotti M, Gasca F, Datta A, Parra LC, Bikson M. Cellular effects of acute direct current stimulation: somatic and synaptic terminal effects. J Physiol. 2013;591:2563–2578. doi: 10.1113/jphysiol.2012.247171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranck JB., Jr Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 1975;98:417–440. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- Ravid EN, Gan LS, Todd K, Prochazka A. Nerve lesioning with direct current. J Neural Eng. 2011;8:016005. doi: 10.1088/1741-2560/8/1/016005. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- Skoglund CR. Modification by electrotonus of the transmission in the artificial synapse formed by severed mammalian nerve. J Neurophysiol. 1945;8:377–386. doi: 10.1152/jn.1945.8.6.377. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Lin RL, Mezue M, Segerdahl A, Kong Y, Xie J, Tracey I. Widespread modulation of cerebral perfusion induced during and after transcranial direct current stimulation applied to the left dorsolateral prefrontal cortex. J Neurosci. 2013;33:11425–11431. doi: 10.1523/JNEUROSCI.3887-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- Toyama K, Tsukahara N, Kosaka K, Matsunami K. Synaptic excitation of red nucleus neurones by fibres from interpositus nucleus. Exp Brain Res. 1970;11:187–198. doi: 10.1007/BF00234322. [DOI] [PubMed] [Google Scholar]
- Trevillion L, Howells J, Burke D. Outwardly rectifying deflections in threshold electrotonus due to K+ conductances. J Physiol. 2007;580:685–696. doi: 10.1113/jphysiol.2006.126003. [DOI] [PMC free article] [PubMed] [Google Scholar]


