Abstract
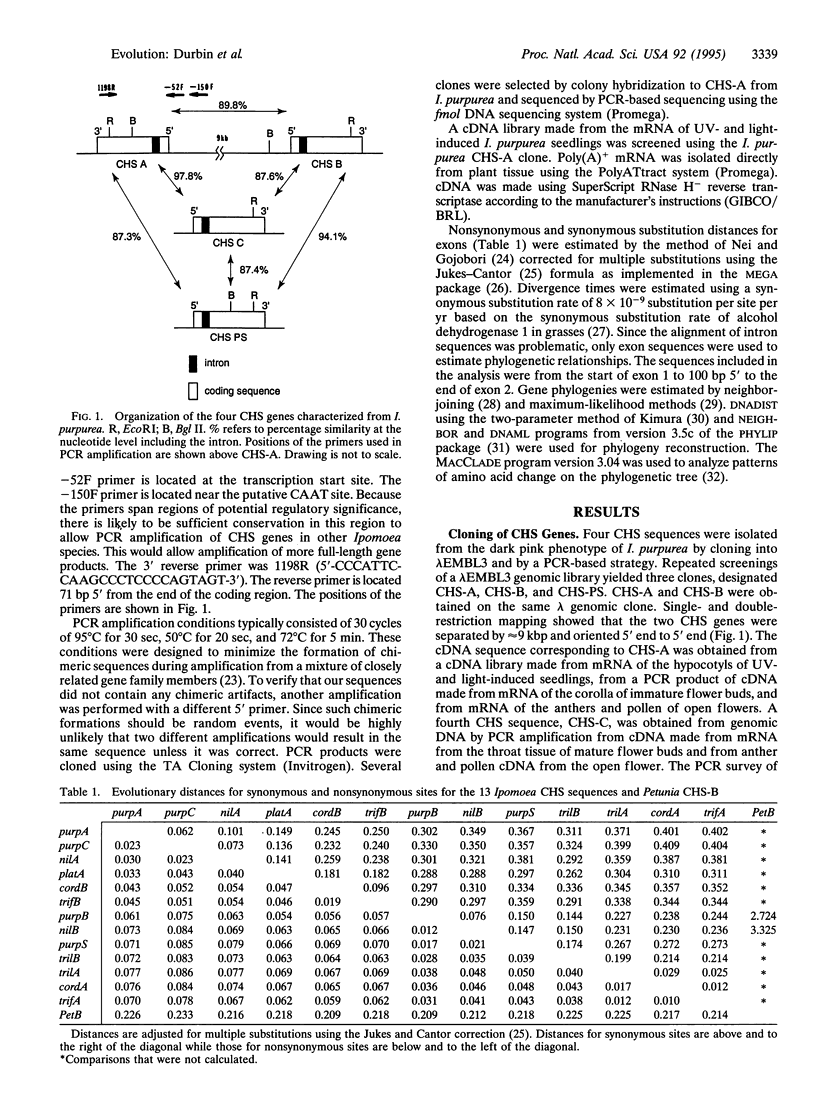
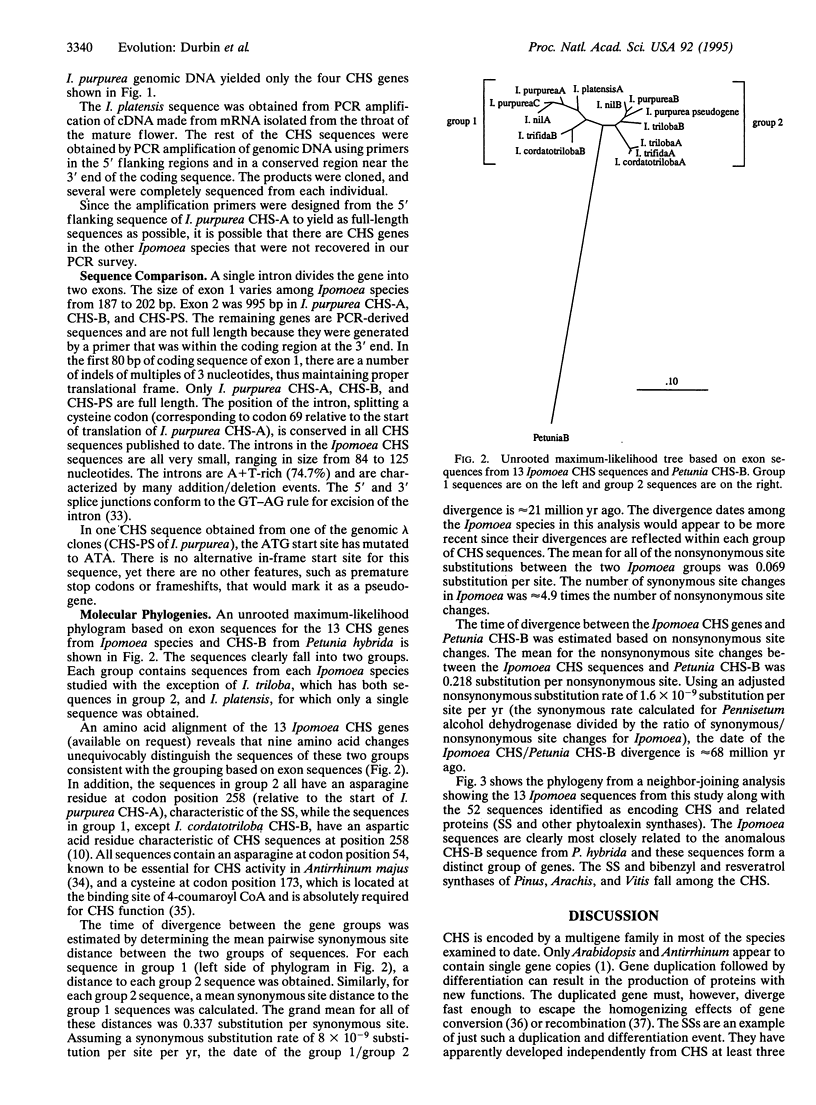
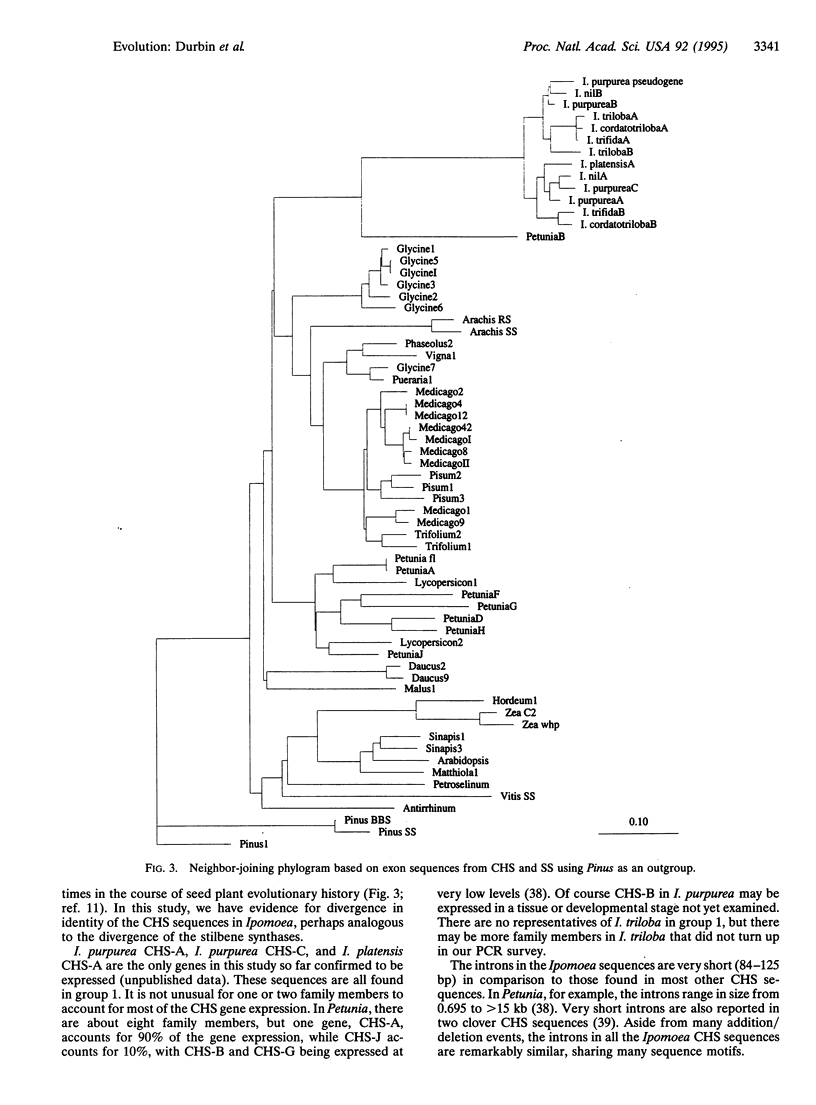
The evolution of the chalcone synthase [CHS; malonyl-CoA:4-coumaroyl-CoA malonyltransferase (cyclizing), EC 2.3.1.74] multigene family in the genus Ipomoea is explored. Thirteen CHS genes from seven Ipomoea species (family Convolvulaceae) were sequenced--three from genomic clones and the remainder from PCR amplification with primers designed from the 5' flanking region and the end of the 3' coding region of Ipomoea purpurea Roth. Analysis of the data indicates a duplication of CHS that predates the divergence of the Ipomoea species in this study. The Ipomoea CHS genes are among the most rapidly evolving of the CHS genes sequenced to date. The CHS genes in this study are most closely related to the Petunia CHS-B gene, which is also rapidly evolving and highly divergent from the rest of the Petunia CHS sequences.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arioli T., Howles P. A., Weinman J. J., Rolfe B. G. In Trifolium subterraneum, chalcone synthase is encoded by a multigene family. Gene. 1994 Jan 28;138(1-2):79–86. doi: 10.1016/0378-1119(94)90785-4. [DOI] [PubMed] [Google Scholar]
- Brown J. W. A catalogue of splice junction and putative branch point sequences from plant introns. Nucleic Acids Res. 1986 Dec 22;14(24):9549–9559. doi: 10.1093/nar/14.24.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Dey P. M., Lamb C. J. Phytoalexins: enzymology and molecular biology. Adv Enzymol Relat Areas Mol Biol. 1983;55:1–136. doi: 10.1002/9780470123010.ch1. [DOI] [PubMed] [Google Scholar]
- Drouin G., Dover G. A. Independent gene evolution in the potato actin gene family demonstrated by phylogenetic procedures for resolving gene conversions and the phylogeny of angiosperm actin genes. J Mol Evol. 1990 Aug;31(2):132–150. doi: 10.1007/BF02109482. [DOI] [PubMed] [Google Scholar]
- Epperson B. K. Spatial autocorrelation of genotypes under directional selection. Genetics. 1990 Mar;124(3):757–771. doi: 10.1093/genetics/124.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 1981;17(6):368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- Gaut B. S., Clegg M. T. Molecular evolution of alcohol dehydrogenase 1 in members of the grass family. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2060–2064. doi: 10.1073/pnas.88.6.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M., Rubery P. H. Naturally occurring auxin transport regulators. Science. 1988 Jul 15;241(4863):346–349. doi: 10.1126/science.241.4863.346. [DOI] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980 Dec;16(2):111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Koes R. E., Spelt C. E., van den Elzen P. J., Mol J. N. Cloning and molecular characterization of the chalcone synthase multigene family of Petunia hybrida. Gene. 1989 Sep 30;81(2):245–257. doi: 10.1016/0378-1119(89)90185-6. [DOI] [PubMed] [Google Scholar]
- Lamb C. J., Lawton M. A., Dron M., Dixon R. A. Signals and transduction mechanisms for activation of plant defenses against microbial attack. Cell. 1989 Jan 27;56(2):215–224. doi: 10.1016/0092-8674(89)90894-5. [DOI] [PubMed] [Google Scholar]
- Lanz T., Tropf S., Marner F. J., Schröder J., Schröder G. The role of cysteines in polyketide synthases. Site-directed mutagenesis of resveratrol and chalcone synthases, two key enzymes in different plant-specific pathways. J Biol Chem. 1991 May 25;266(15):9971–9976. [PubMed] [Google Scholar]
- Long S. R. Rhizobium-legume nodulation: life together in the underground. Cell. 1989 Jan 27;56(2):203–214. doi: 10.1016/0092-8674(89)90893-3. [DOI] [PubMed] [Google Scholar]
- Luo D., Coen E. S., Doyle S., Carpenter R. Pigmentation mutants produced by transposon mutagenesis in Antirrhinum majus. Plant J. 1991 Jul;1(1):59–69. [PubMed] [Google Scholar]
- Meyerhans A., Vartanian J. P., Wain-Hobson S. DNA recombination during PCR. Nucleic Acids Res. 1990 Apr 11;18(7):1687–1691. doi: 10.1093/nar/18.7.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M., Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol. 1986 Sep;3(5):418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- O'Neill S. D., Tong Y., Spörlein B., Forkmann G., Yoder J. I. Molecular genetic analysis of chalcone synthase in Lycopersicon esculentum and an anthocyanin-deficient mutant. Mol Gen Genet. 1990 Nov;224(2):279–288. doi: 10.1007/BF00271562. [DOI] [PubMed] [Google Scholar]
- Rausher M. D., Fry J. D. Effects of a locus affecting floral pigmentation in Ipomoea purpurea on female fitness components. Genetics. 1993 Aug;134(4):1237–1247. doi: 10.1093/genetics/134.4.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzer E., Jahnen W., Hahlbrock K. In situ localization of light-induced chalcone synthase mRNA, chalcone synthase, and flavonoid end products in epidermal cells of parsley leaves. Proc Natl Acad Sci U S A. 1988 May;85(9):2989–2993. doi: 10.1073/pnas.85.9.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropf S., Lanz T., Rensing S. A., Schröder J., Schröder G. Evidence that stilbene synthases have developed from chalcone synthases several times in the course of evolution. J Mol Evol. 1994 Jun;38(6):610–618. doi: 10.1007/BF00175881. [DOI] [PubMed] [Google Scholar]
- Walsh J. B. Sequence-dependent gene conversion: can duplicated genes diverge fast enough to escape conversion? Genetics. 1987 Nov;117(3):543–557. doi: 10.1093/genetics/117.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]


