Abstract
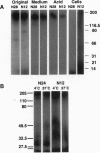
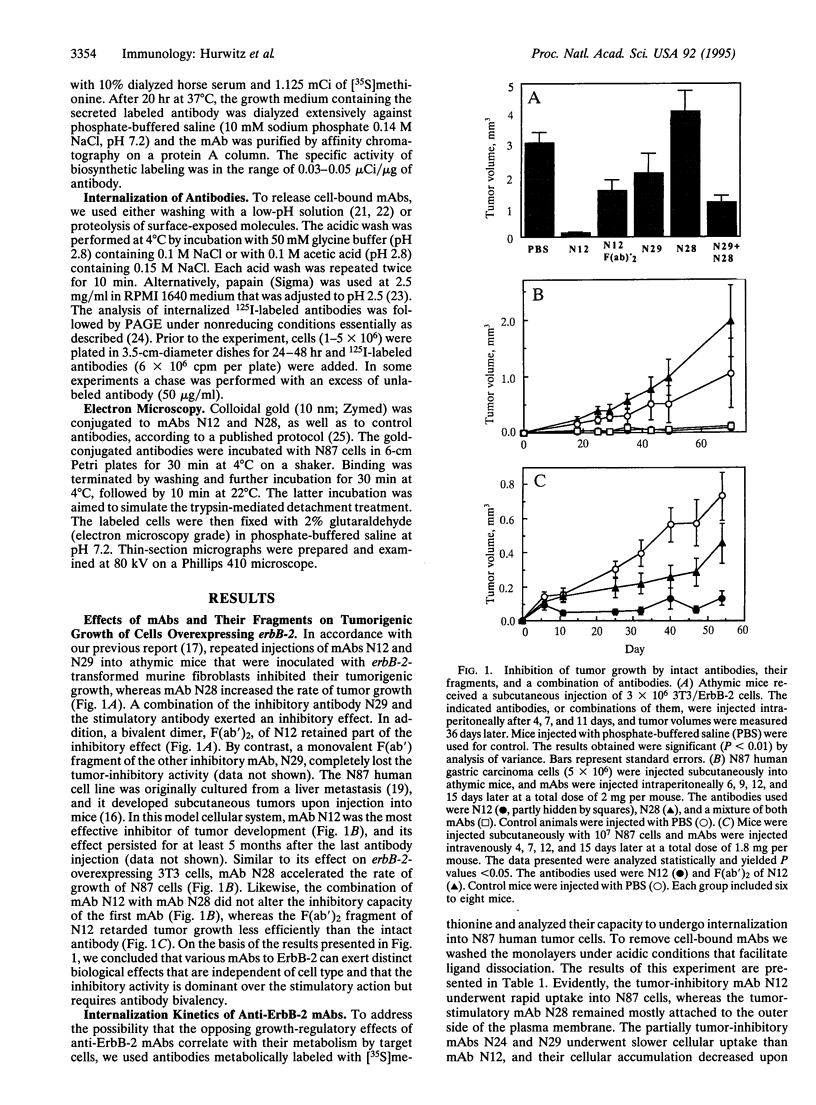
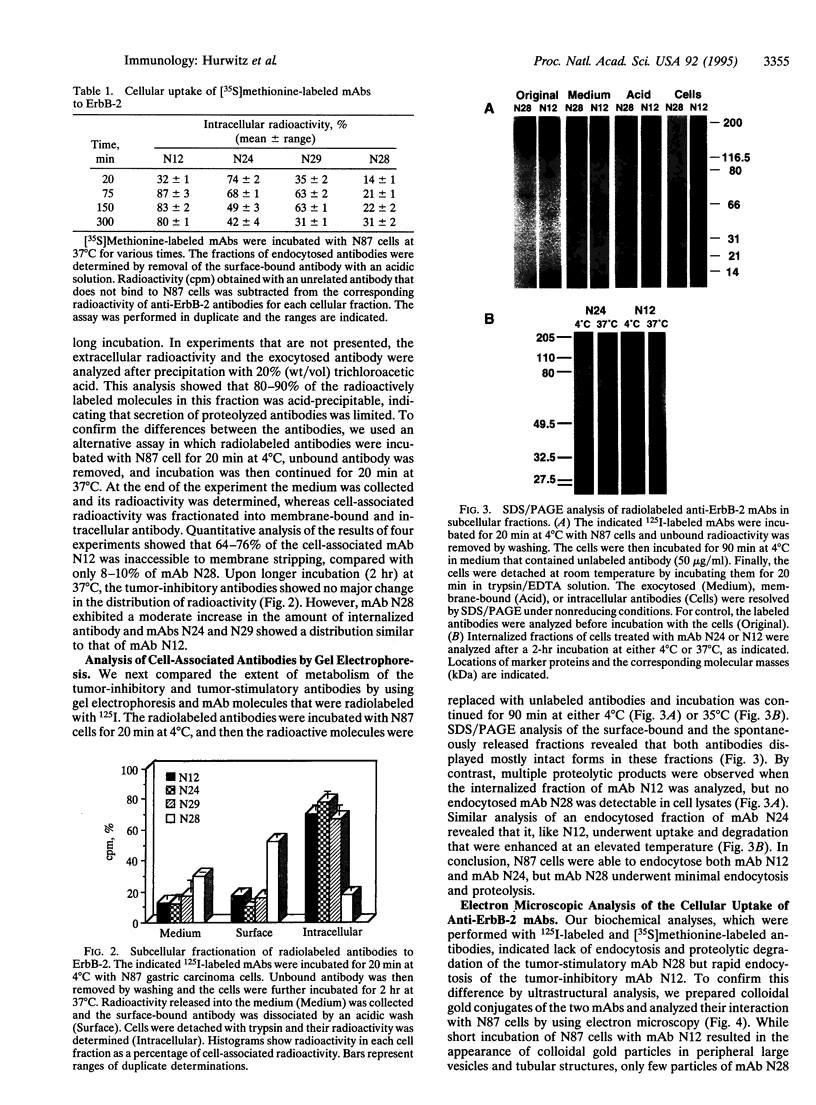
Amplification and overexpression of the erbB-2/neu protooncogene are frequently associated with aggressive clinical course of certain human adenocarcinomas, and therefore the encoded surface glycoprotein is considered a candidate target for immunotherapy. We previously generated a series of anti-ErbB-2 monoclonal antibodies (mAbs) that either accelerate or inhibit the tumorigenic growth of erbB-2-transformed murine fibroblasts. The present study extended this observation to a human tumor cell line grown as xenografts in athymic mice and addressed the biochemical differences between the two classes of mAbs. We show that the inhibitory effect is dominant in an antibody mixture, and it depends on antibody bivalency. By using radiolabeled mAbs we found that all of three tumor-inhibitory mAbs became rapidly inaccessible to acid treatment when incubated with tumor cells. However, a tumor-stimulatory mAb remained accessible to extracellular treatments, indicating that it did not undergo endocytosis. In addition, intracellular fragments of the inhibitory mAbs, but not of the stimulatory mAb, were observed. Electron microscopy of colloidal gold-antibody conjugates confirmed the absence of endocytosis of the stimulatory mAb but detected endocytic vesicles containing an inhibitory mAb. We conclude that acceleration of cell growth by ErbB-2 correlates with cell surface localization, whereas inhibition of tumor growth is associated with an intrinsic ability of anti-ErbB-2 mAbs to induce endocytosis. These conclusions are relevant to the selection of optimal mAbs for immunotherapy and may have implications for the mechanism of cellular transformation by an overexpressed erbB-2 gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronheim A., Engelberg D., Li N., al-Alawi N., Schlessinger J., Karin M. Membrane targeting of the nucleotide exchange factor Sos is sufficient for activating the Ras signaling pathway. Cell. 1994 Sep 23;78(6):949–961. doi: 10.1016/0092-8674(94)90271-2. [DOI] [PubMed] [Google Scholar]
- Bacus S. S., Stancovski I., Huberman E., Chin D., Hurwitz E., Mills G. B., Ullrich A., Sela M., Yarden Y. Tumor-inhibitory monoclonal antibodies to the HER-2/Neu receptor induce differentiation of human breast cancer cells. Cancer Res. 1992 May 1;52(9):2580–2589. [PubMed] [Google Scholar]
- Bargmann C. I., Hung M. C., Weinberg R. A. Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell. 1986 Jun 6;45(5):649–657. doi: 10.1016/0092-8674(86)90779-8. [DOI] [PubMed] [Google Scholar]
- Ben-Levy R., Paterson H. F., Marshall C. J., Yarden Y. A single autophosphorylation site confers oncogenicity to the Neu/ErbB-2 receptor and enables coupling to the MAP kinase pathway. EMBO J. 1994 Jul 15;13(14):3302–3311. doi: 10.1002/j.1460-2075.1994.tb06632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiore P. P., Helin K., Kraus M. H., Pierce J. H., Artrip J., Segatto O., Bottaro D. P. A single amino acid substitution is sufficient to modify the mitogenic properties of the epidermal growth factor receptor to resemble that of gp185erbB-2. EMBO J. 1992 Nov;11(11):3927–3933. doi: 10.1002/j.1460-2075.1992.tb05486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiore P. P., Pierce J. H., Kraus M. H., Segatto O., King C. R., Aaronson S. A. erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science. 1987 Jul 10;237(4811):178–182. doi: 10.1126/science.2885917. [DOI] [PubMed] [Google Scholar]
- Drebin J. A., Link V. C., Greene M. I. Monoclonal antibodies specific for the neu oncogene product directly mediate anti-tumor effects in vivo. Oncogene. 1988 Apr;2(4):387–394. [PubMed] [Google Scholar]
- Drebin J. A., Link V. C., Stern D. F., Weinberg R. A., Greene M. I. Down-modulation of an oncogene protein product and reversion of the transformed phenotype by monoclonal antibodies. Cell. 1985 Jul;41(3):697–706. doi: 10.1016/s0092-8674(85)80050-7. [DOI] [PubMed] [Google Scholar]
- Fendly B. M., Winget M., Hudziak R. M., Lipari M. T., Napier M. A., Ullrich A. Characterization of murine monoclonal antibodies reactive to either the human epidermal growth factor receptor or HER2/neu gene product. Cancer Res. 1990 Mar 1;50(5):1550–1558. [PubMed] [Google Scholar]
- Garrigues J., Garrigues U., Hellström I., Hellström K. E. Ley specific antibody with potent anti-tumor activity is internalized and degraded in lysosomes. Am J Pathol. 1993 Feb;142(2):607–622. [PMC free article] [PubMed] [Google Scholar]
- Goldman R., Levy R. B., Peles E., Yarden Y. Heterodimerization of the erbB-1 and erbB-2 receptors in human breast carcinoma cells: a mechanism for receptor transregulation. Biochemistry. 1990 Dec 18;29(50):11024–11028. doi: 10.1021/bi00502a002. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Helping out in Bhopal. Nature. 1984 Dec 13;312(5995):579–580. doi: 10.1038/312579a0. [DOI] [PubMed] [Google Scholar]
- Hudziak R. M., Lewis G. D., Winget M., Fendly B. M., Shepard H. M., Ullrich A. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol. 1989 Mar;9(3):1165–1172. doi: 10.1128/mcb.9.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudziak R. M., Schlessinger J., Ullrich A. Increased expression of the putative growth factor receptor p185HER2 causes transformation and tumorigenesis of NIH 3T3 cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7159–7163. doi: 10.1073/pnas.84.20.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprzyk P. G., Song S. U., Di Fiore P. P., King C. R. Therapy of an animal model of human gastric cancer using a combination of anti-erbB-2 monoclonal antibodies. Cancer Res. 1992 May 15;52(10):2771–2776. [PubMed] [Google Scholar]
- Lotti L. V., Di Lazzaro C., Zompetta C., Frati L., Torrisi M. R. Surface distribution and internalization of erbB-2 proteins. Exp Cell Res. 1992 Oct;202(2):274–280. doi: 10.1016/0014-4827(92)90075-j. [DOI] [PubMed] [Google Scholar]
- Maier L. A., Xu F. J., Hester S., Boyer C. M., McKenzie S., Bruskin A. M., Argon Y., Bast R. C., Jr Requirements for the internalization of a murine monoclonal antibody directed against the HER-2/neu gene product c-erbB-2. Cancer Res. 1991 Oct 1;51(19):5361–5369. [PubMed] [Google Scholar]
- McKenzie S. J., Marks P. J., Lam T., Morgan J., Panicali D. L., Trimpe K. L., Carney W. P. Generation and characterization of monoclonal antibodies specific for the human neu oncogene product, p185. Oncogene. 1989 May;4(5):543–548. [PubMed] [Google Scholar]
- Park J. G., Frucht H., LaRocca R. V., Bliss D. P., Jr, Kurita Y., Chen T. R., Henslee J. G., Trepel J. B., Jensen R. T., Johnson B. E. Characteristics of cell lines established from human gastric carcinoma. Cancer Res. 1990 May 1;50(9):2773–2780. [PubMed] [Google Scholar]
- Peles E., Bacus S. S., Koski R. A., Lu H. S., Wen D., Ogden S. G., Levy R. B., Yarden Y. Isolation of the neu/HER-2 stimulatory ligand: a 44 kd glycoprotein that induces differentiation of mammary tumor cells. Cell. 1992 Apr 3;69(1):205–216. doi: 10.1016/0092-8674(92)90131-u. [DOI] [PubMed] [Google Scholar]
- Peles E., Yarden Y. Neu and its ligands: from an oncogene to neural factors. Bioessays. 1993 Dec;15(12):815–824. doi: 10.1002/bies.950151207. [DOI] [PubMed] [Google Scholar]
- Press O. W., Hansen J. A., Farr A., Martin P. J. Endocytosis and degradation of murine anti-human CD3 monoclonal antibodies by normal and malignant T-lymphocytes. Cancer Res. 1988 Apr 15;48(8):2249–2257. [PubMed] [Google Scholar]
- Slamon D. J., Clark G. M., Wong S. G., Levin W. J., Ullrich A., McGuire W. L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Godolphin W., Jones L. A., Holt J. A., Wong S. G., Keith D. E., Levin W. J., Stuart S. G., Udove J., Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989 May 12;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Sorkin A., Waters C. M. Endocytosis of growth factor receptors. Bioessays. 1993 Jun;15(6):375–382. doi: 10.1002/bies.950150603. [DOI] [PubMed] [Google Scholar]
- Stancovski I., Hurwitz E., Leitner O., Ullrich A., Yarden Y., Sela M. Mechanistic aspects of the opposing effects of monoclonal antibodies to the ERBB2 receptor on tumor growth. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8691–8695. doi: 10.1073/pnas.88.19.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliabue E., Centis F., Campiglio M., Mastroianni A., Martignone S., Pellegrini R., Casalini P., Lanzi C., Ménard S., Colnaghi M. I. Selection of monoclonal antibodies which induce internalization and phosphorylation of p185HER2 and growth inhibition of cells with HER2/NEU gene amplification. Int J Cancer. 1991 Apr 1;47(6):933–937. doi: 10.1002/ijc.2910470625. [DOI] [PubMed] [Google Scholar]
- Tzahar E., Levkowitz G., Karunagaran D., Yi L., Peles E., Lavi S., Chang D., Liu N., Yayon A., Wen D. ErbB-3 and ErbB-4 function as the respective low and high affinity receptors of all Neu differentiation factor/heregulin isoforms. J Biol Chem. 1994 Oct 7;269(40):25226–25233. [PubMed] [Google Scholar]
- Wada T., Qian X. L., Greene M. I. Intermolecular association of the p185neu protein and EGF receptor modulates EGF receptor function. Cell. 1990 Jun 29;61(7):1339–1347. doi: 10.1016/0092-8674(90)90697-d. [DOI] [PubMed] [Google Scholar]
- Wells A., Welsh J. B., Lazar C. S., Wiley H. S., Gill G. N., Rosenfeld M. G. Ligand-induced transformation by a noninternalizing epidermal growth factor receptor. Science. 1990 Feb 23;247(4945):962–964. doi: 10.1126/science.2305263. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Gabbay M., Schlessinger J. Primary amines do not prevent the endocytosis of epidermal growth factor into 3T3 fibroblasts. Biochim Biophys Acta. 1981 May 5;674(2):188–203. doi: 10.1016/0304-4165(81)90377-9. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]
- Zschiesche W., Schönborn I., Minguillon C., Spitzer E. Significance of immunohistochemical c-erbB-2 product localization pattern for prognosis of primary human breast cancer. Cancer Lett. 1994 Jun 15;81(1):89–94. doi: 10.1016/0304-3835(94)90169-4. [DOI] [PubMed] [Google Scholar]
- van Leeuwen F., van de Vijver M. J., Lomans J., van Deemter L., Jenster G., Akiyama T., Yamamoto T., Nusse R. Mutation of the human neu protein facilitates down-modulation by monoclonal antibodies. Oncogene. 1990 Apr;5(4):497–503. [PubMed] [Google Scholar]