Abstract
Summary
We isolate and characterize osteoblasts from humans without in vitro culture. These techniques should be broadly applicable to studying the pathogenesis of osteoporosis and other bone disorders.
Introduction
There is currently no data regarding the expression of specific genes or pathways in human osteoblasts that have not been subjected to extensive in vitro culture. Thus, we developed methods to rapidly isolate progressively enriched osteoblast populations from humans and characterized these cells.
Methods
Needle bone biopsies of the posterior iliac crest were subjected to sequential collagenase digests. The cells from the second digest were stained with an alkaline phosphatase (AP) antibody, and the AP+ cells were isolated using magnetic cell sorting.
Results
Relative to AP− cells, the AP+ cells contained virtually all of the mineralizing cells and were enriched for key osteoblast marker genes. The AP+ cells were further purified by depletion of cells expressing CD45, CD34, or CD31 (AP+/CD45/34/31− cells), which represented a highly enriched human osteoblast population devoid of hematopoietic/endothelial cells. These cells expressed osteoblast marker genes but very low to undetectable levels of SOST. We next used high-throughput RNA sequencing to compare the transcriptome of the AP+/CD45/34/31− cells to human fibroblasts and identified genes and pathways expressed only in human osteoblasts in vivo, but not in fibroblasts, including 448 genes unique to human osteoblasts.
Conclusions
We provide a detailed characterization of highly enriched human osteoblast populations without in vitro culture. These techniques should be broadly applicable to studying the pathogenesis of osteoporosis and other bone disorders.
Keywords: Bone biopsy, Osteoblasts, Osteoporosis
Introduction
Significant progress has been made in recent years in the clinical investigation of osteoporosis and other metabolic bone diseases using advanced imaging technologies [1, 2] and peripheral blood biochemical markers relevant to bone metabolism [3]. There remains, however, an important gap in knowledge in terms of understanding changes in individual genes and in gene pathway expression in highly purified or enriched bone cell populations from human samples without extensive in vitro manipulation or culture. To address this gap, several different approaches have been used, including assessment of mRNA expression in human bone biopsies [4] as well as analysis of bone marrow stromal cells following in vitro culture [5]. While providing potentially useful information, both approaches have significant limitations, especially if the target cell population of interest is the mature human osteoblast. Thus, whole bone biopsies contain a heterogeneous population of cells, and while bone marrow stromal cultures are more homogeneous, they represent an early progenitor (rather than mature osteoblast) population. Further, even short-term in vitro culture in the presence of fetal bovine serum and other growth factors is likely to alter the phenotype and gene expression profile of these cells.
In previous work, we have described the isolation and characterization of human bone marrow osteoprogenitor cells using both positive selection with alkaline phosphatase (AP) [6] or Stro-1 [7] as well as negative selection by depleting hematopoietic or endothelial cells [8]. Our goal in the present work, however, was to isolate highly enriched populations of mature osteoblasts from small needle bone biopsies and to characterize these cells at a gene expression level within ~2–4 h of obtaining the biopsy. Rather than using conventional trephine biopsies with a diameter of 7.5 mm [9], we used a modification of a clinical protocol routinely used by hematologists for bone marrow aspirates and biopsies to obtain needle biopsies of ~1–2 mm in diameter. Further, since AP is perhaps the most reliable cell surface marker for osteoblasts [10], we then rapidly isolated AP-expressing cells from these biopsies. These isolation methods were coupled to analytic tools appropriate for small numbers of cells and low RNA yields to generate microgram amounts of cDNA from the various cell populations that was amenable to further evaluation using quantitative polymerase chain reaction (QPCR) and high-throughput RNA sequencing (RNAseq) analysis. In the present report, we describe the direct isolation and characterization of human osteoblasts without in vitro culture. Moreover, since osteoblasts and fibroblasts are closely related mesenchymal cells, by comparing the transcriptomes of human osteoblasts and fibroblasts using RNAseq, we identified genes and pathways unique to human osteoblasts in vivo.
Methods
Study subjects and biopsy protocol
Study subjects were admitted to the Outpatient Mayo Clinical Research Unit following an overnight fast. All subjects provided full informed consent, and the study was approved by the Mayo Clinic Institutional Review Board. Potential subjects were rigorously screened for coexisting disease and excluded if they had diseases known to affect bone metabolism. No subjects were taking any therapies likely to affect bone metabolism such as anticonvulsants, bisphosphonates, calcitonin, glucocorticoids, or sodium fluoride.
Following local anesthesia with 1 % lidocaine and monitored IV sedation using 1–3 mg of intravenous midazolam and 50–100 µg of fentanyl, two needle biopsies of bone from the posterior iliac crest were obtained using an 8-G needle. In one subject, bone marrow aspirates were also obtained for comparison with the osteoblastic cells isolated from the bone samples. This procedure is a modification of clinical bone marrow aspirates and biopsies obtained for hematological purposes. For the validation study described, samples were obtained from six normal male subjects, age range 23 to 75 years.
µCT analysis of bone biopsy
One of the bone biopsy samples was frozen immediately in liquid nitrogen. µCT imaging was performed using a cryostatic µCTinstrument [11] which allows for the tissue samples to be scanned while frozen. The entire length of the bone biopsies was scanned by placing them on a computer-controlled rotation stage. Samples were scanned with a cubic voxel size of 20 µm and analyzed using the ANALYZE© software package (ANALYZE 9.0).
Isolation of bone cell fractions
Attached soft tissues were removed from the bone biopsies, and the samples were chopped into small fragments with a scalpel. These fragmented samples were incubated with a highly purified, endotoxin-free collagenase (0.6 WU/ml, Liberase DL solution, Roche Applied Science) for 30 min at 37 °C as the first digestion. After the first digestion, the solution was centrifuged and suspended cells were collected as the first fraction. The digested bone fragment was put into the a new Liberase solution for 60 min at 37 °C as the second digestion, and the suspended cells were again collected following centrifugation as the second digest. We found that the use of the highly purified, endotoxin-free collagenase was essential, as the generally used collagenase (Worthington Biochemical) does appear to have detrimental effects on the cells [12]. As shown in Supplementary Fig. 1 (Online Resource 1), when compared to digestion with Liberase, second digest cells obtained by standard collagenase digestions had substantially lower RNA integrity values. Cells (1 × 105) from the first and second digests were put into QIAzol (Qiagen) for future RNA extraction. The remaining cells from the second digest were incubated with a human AP Biotinylated Antibody (R&D systems) for 30 min and then incubated with Anti-Biotin MicroBeads (Miltenyi Biotec) for 15 min and positively selected by autoMACS (Miltenyi Biotec). After removing 1 × 105 cells for RNA extraction, AP+ cells were incubated with CD31PE, CD34PE, and CD45PE antibodies (Miltenyi Biotec) for 30 min and sorted by fluorescence-activated cell sorting (FACS). The fluorescence threshold was set based on excluding the highly fluorescent CD45/CD34/CD31 population, and the same fluorescence threshold for CD45/CD34/CD31 was used in all of the analyses. Cells were also stained with propidium iodide (PI) to remove dead cells. AP+/CD45/34/31−/PI− and AP+/CD45/34/31+/PI− cells were collected for further RNA extractions. Bone marrow cells were processed for isolation of AP+ and AP+/CD45/34/31−/PI− cells in a similar manner.
In vitro mineralization assay
Cells were cultured for 3 days at a density of 1 × 105/0.32 cm2 (96-well plates) in 100 µl of media containing phenol red-free αMEM, 15 % FBS, and 1 % penicillin–streptomycin mixture (Growth media), all obtained from Invitrogen. Subsequently, 100 µl of 2× osteogenic differentiation media containing growth media, 100 µM ascorbic acid, 2 mM dexamethasone, and 20 mM β-glycerolphosphate were added to each well. At days 3, 6, and 9 following the addition of differentiation media, 100 µl of media was removed from each well and replaced with 100 µl of 2× osteogenicmedia. Complete media changes were performed on days 12, 15, and 18. Cells were fixed in 10 % formalin and stained for calcium deposits using 2 % Alizarin Red on day 21.
Human fibroblast culture
Clonetics™ Human Dermal Fibroblasts (Lonza) were cultured by following the company’s instructions and RNA was extracted using QIAzol. Three independent lines of fibroblasts, which were obtained from three normal male subjects, age range 51 to 65 years, were used for the comparison with the AP+/CD45/34/31− cells.
QPCR gene expression analysis
Total RNA from the various sorted cell populations and bone biopsies was isolated using microfuge columns (MicroColumns, Qiagen). DNase treatment to digest all genomic DNA that could lead to false-positive gene expression results was performed following RNA isolation using Turbo DNA-free DNase (Ambion). RNA quality and purity were confirmed with a Nanodrop spectrophotometer (Thermo Scientific). Since the overall number of the various cell populations was limited (generally <100,000 cells) for the performance of in-depth gene expression analyses, we used the WT-Ovation™ Pico RNA whole transcriptome amplification system (NuGen Technologies, Inc.) to synthesize microgram quantities of amplified cDNA starting with a total RNA input of ~25 ng. In this linear amplification system, the relative representation of each transcript species in the original sample is maintained during and after amplification [13, 14].
For the QPCR analyses, we designed primers using the Primer Express program (Applied Biosystems). Primer sequences for any of the genes analyzed in this report are available on request. The PCR reactions were run in the ABI Prism 7900HT Real-Time System (Applied Biosystems) using SYBR Green (Qiagen) as the detection method. Normalization for variations in input RNA was performed using a panel of 10 housekeeping genes (18S, G6PDH, GAPDH, GUSB, L13A, RPII, TBP, TUBA1B, Β2M, ACTB) with the geNorm algorithm (http://medgen.ugent.be/~jvdesomp/genorm/) [15, 16] used to select the 3–4 most stable housekeeping genes from the 10 on the plate. The PCR Miner algorithm [17] was used to correct for variations in amplification efficiencies.
RNAseq analysis
RNA libraries were prepared according to the manufacturer’s instructions for the NuGen Ovation RNASeq FFPE and Ultralow Library Systems (San Carlos, CA). Briefly, first strand cDNA was generated from ~30 ng of total RNA using DNA/RNA chimeric primers and reverse transcriptase, creating a cDNA/RNA hybrid. The second strand cDNA was then synthesized containing a DNA/RNA duplex. The resulting double-stranded cDNA molecule was amplified by Single Primer Isothermal Amplification (SPIA®) using a chimeric SPIA primer, DNA polymerase, and RNase H (NuGen). Following amplification, the products were modified by random priming and extension to create double-stranded products that were suitable for generating libraries for sequencing. Unique indexes were incorporated for each sample. The double-stranded products then underwent blunt-end repair. Adapter molecules were ligated to the 5′ and 3′ ends of each fragment to facilitate PCR amplification of the fragments to produce the final library. The concentration and size distribution of the resulting libraries was determined on an Agilent Bioanalyzer DNA 1000 chip (Santa Clara, CA). The concentration values were confirmed by Qubit fluorometry (Life Technologies, Grand Island, NY). Unique indexes were incorporated at the adaptor ligation phase for multiplex sample loading on the flow cells. Libraries were loaded onto paired end flow cells at concentrations of 8–10 pM to generate cluster densities of 700,000/mm2 following Illumina’s standard protocol using the Illumina cBot and cBot Paired end cluster kit version 3. The flow cells were sequenced as 51 × 2 paired end reads on an Illumina HiSeq 2000 using TruSeq SBS sequencing kit version 3 and SCS version 1.4.8 data collection software. Base calling was performed using Illumina’s RTA version 1.12.4.2. We sequenced three samples per lane and achieved a sequencing depth of ~100 million reads per sample.
Statistical and bioinformatic analyses
The QPCR data is shown using mean ± SEM and statistical comparisons were performed using two-sided t tests. The RNAseq data was analyzed using several steps: alignment, quality control, obtaining genomic features per sample, and summarizing the data across samples for subsequent comparisons between samples (see below). Paired-end reads were aligned by TopHat 2.0.6 [18] against the hg19 genome build using the bowtie1 aligner option [19]. Initial assessment of quality control was made using RSeQC software [20] to estimate the distance between paired end reads, evaluate the sequencing depth for alternate splicing analysis, determine the rate of duplicate reads, evaluate coverage of reads across genes, etc. Gene counts were generated using HTseq software (http://www-huber.embl.de/users/anders/HTSeq/doc/overview.html), and gene annotation files were obtained from Illumina (http://cufflinks.cbcb.umd.edu/igenomes.html).
To delineate pathways unique to human osteoblasts, we identified genes not expressed in fibroblasts but present in the AP+/CD45/34/31− cells. For this type of analysis, Ramskold et al. [21] have suggested using a median reads per kilobase per million mapped reads threshold of 0.3 for calling a gene as “expressed”; in our analysis of this dataset, we found very close concordance between this criteria and simply using an absolute median gene count threshold of 10, which is what we used in the current analysis. As a more stringent criterion, we also tested for differential expression differences between groups using the edgeR package [22]. Technical variability introduced by gene GC content, gene size, and total calls per sample was accounted for using an offset obtained from the cqn package [23]. Genes with a median gene count of less than 10 in both groups were excluded. For this analysis, only genes with a false discovery rate less than 0.05 were carried forward for further investigation. For the genes uniquely expressed in the AP+/CD45/34/31− cells, pathway analysis was performed using the Ingenuity Pathway Analysis software (Ingenuity Systems).
Results
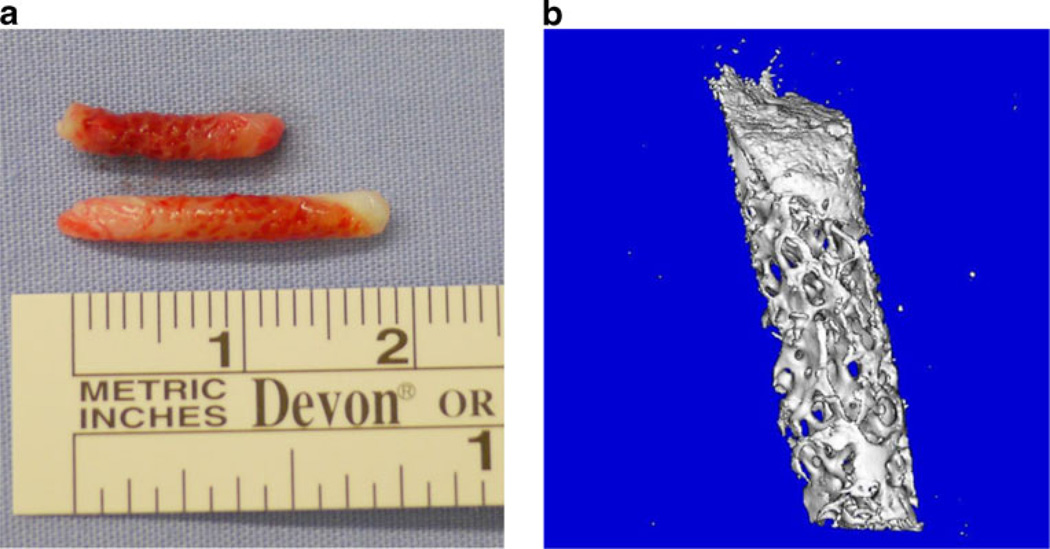
Figure 1a shows an example of the two needle bone biopsies obtained from the study subjects; each measured ~2 mm in diameter by 1–2 cm in length. Figure 1b is a µCT image of a representative biopsy, depicting both cortical and trabecular bone in these samples.
Figure 1.
a Example of needle biopsies obtained from the iliac crest and b µCT image of one of the human bone biopsies
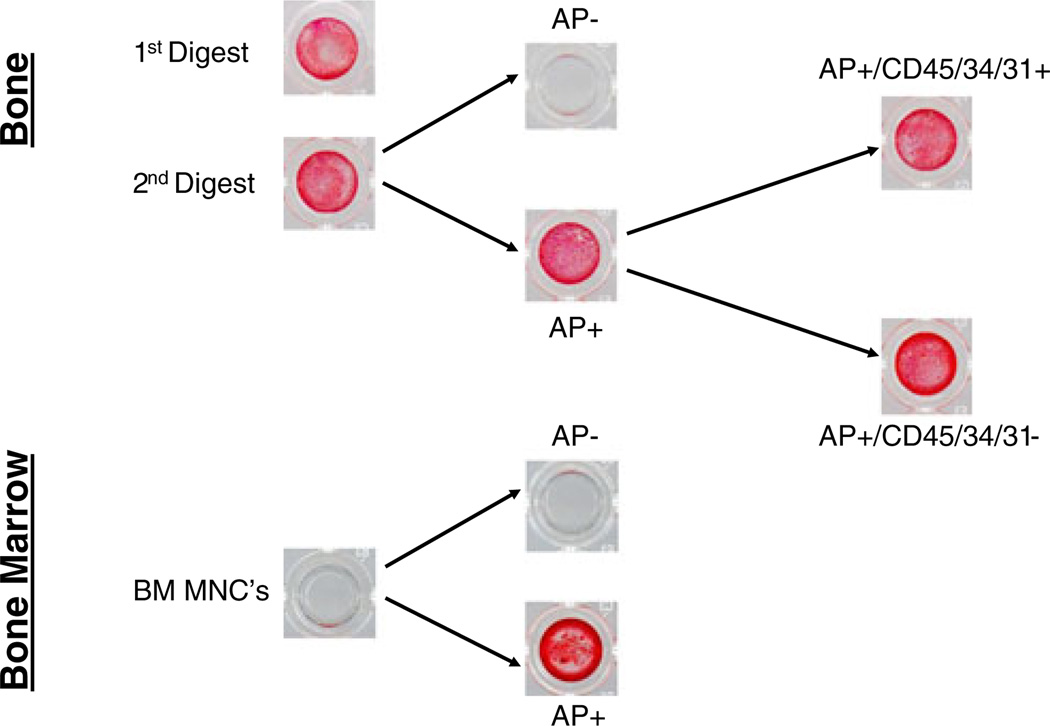
As shown in Fig. 2, cells from both the first and second digests were capable of in vitro mineralization. However, the second digest clearly contained more osteoblastic cells, as it was highly enriched for key osteoblast markers (Fig. 3a). Thus, the second digest cells were then stained with an established AP antibody (B4–78) [24], and the AP+ cells were isolated using MACS. As shown in Fig. 2, the AP+ cells contained virtually all of the mineralizing cells in the second digest, as the AP− cells from this fraction failed to mineralize. Of note, we also found that the AP antibody similarly isolated mineralizing cells from bone marrow (Fig. 2, lower panel).
Figure 2.
Alizarin Red staining of sequential fractions of cells from human bone biopsies and bone marrow
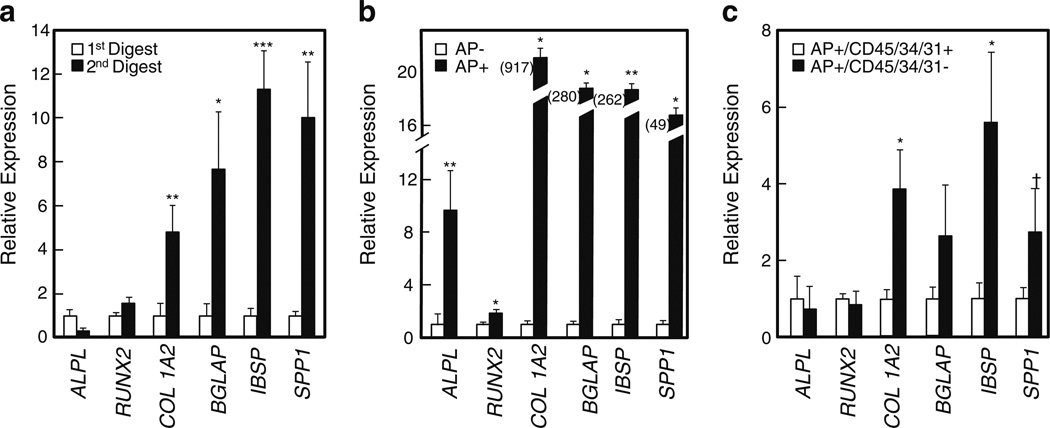
Figure 3.
Relative expression of osteoblastic genes in a first versus second digest cells; b AP− versus AP+ cells; and c AP+/CD45/34/31+ versus AP+/CD45/34/31− cells. *P <0.05; **P <0.01; ***P <0.001; †P =0.08. ALPL alkaline phosphatase, BGLAP osteocalcin, IBSP bone sialoprotein, SPP1 osteopontin
Relative to the AP− cells, the AP+ cells were highly enriched for expression of osteoblast marker genes using QPCR (Fig. 3b). Thus, the bone AP+ cells, which can be isolated and put into RNA extraction buffer within 2 h of the biopsies being taken from the subject, are a highly osteoblast-enriched fraction that can be used for further analyses.
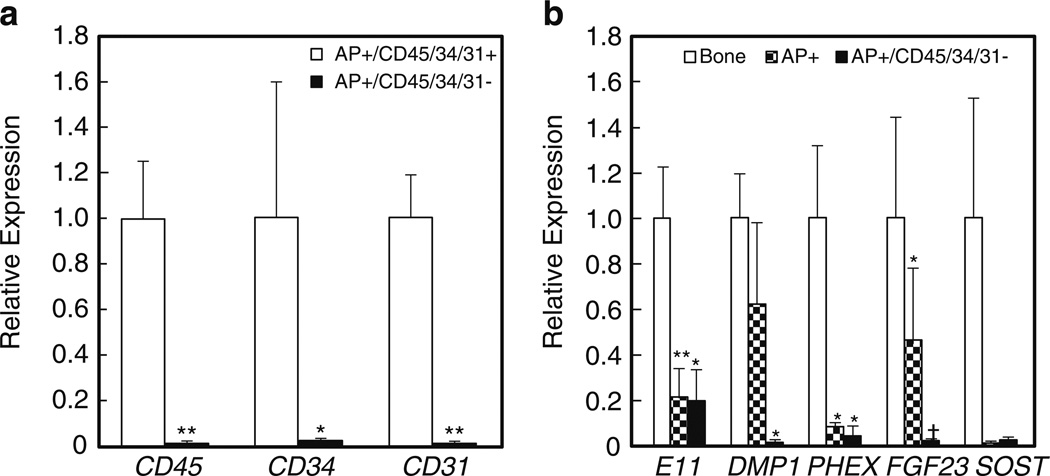
Supplementary Fig. 2 (Online Resource 1) shows a FACS analysis of the AP+ cells isolated by MACS and then stained with a cocktail of antibodies reactive against all hematopoietic/endothelial cells (CD45, CD34, CD31). Approximately 60 % of the AP+ cells were negative/dim for all of these markers (AP+/CD45/34/31−), whereas ~40 % expressed at least one marker (AP+/CD45/34/31+; since we stained with all three antibodies together, we did not distinguish which specific marker was expressed in different subsets of these cells). Thus, the next level of purification involved FACS selection for AP+/CD45/34/31− cells, and as shown in Fig. 3c, AP+/CD45/34/31− cells did express higher levels of key osteoblast markers than AP+/CD45/34/31+ cells. Moreover, relative to the AP+/CD45/34/31+ cells, the AP+/CD45/34/31− cells were almost completely depleted of the hematopoietic/endothelial markers (Fig. 4a). Interestingly, both AP+/CD45/34/31− and AP+/CD45/34/31+ cells were capable of in vitro mineralization (Fig. 2), suggesting that while the AP+/CD45/34/31− cells likely contained the true mesenchymal fraction of osteoblastic cells, AP+ cells in bone that co-express hematopoietic/endothelial markers are also capable of in vitro mineralization. The identity of these AP+/CD45/34/31+ cells remains to be further defined, but of interest, these cells were 15-fold fold enriched (P <0.001), relative to AP+/CD45/34/31− cells, for expression of mRNA levels of the macrophage/osteomac marker, CD68 [25, 26].
Figure 4.
a Relative expression of hematopoietic/endothelial marker genes in AP+/CD45/34/31+ versus AP+/CD45/34/31− cells. *P <0.05; **P <0.01. b Relative expression of osteocyte genes in the stripped bone fragments, AP+ cells, and AP+/CD45/34/31− cells. *P <0.05; **P <0.01; †P = 0.06 versus the stripped bone samples
We also examined whether the FACS sorting itself may have an independent effect on gene expression. For this, we examined the expression of 32 genes related to bone metabolism in AP+ cells before and following FACS (Supplementary Fig. 3, Online Resource 1). As is evident, we found that there were minimal effects of FACS in our hands (using a 100-µm nozzle and low flow rate) on the cycle threshold (Ct) values of these genes, particularly for Ct values <30. Thus, the FACS sorting performed with our protocol appears to have minimal effects on gene expression.
The AP+/CD45/34/31− cells isolated from the collagenase digests of bone were clearly different from the analogous cells obtained from bone marrow. Thus, relative to the bone AP+/CD45/34/31− cells, the bone marrow AP+/CD45/34/31− cells expressed markedly low (or barely detectable by QPCR) mRNA levels of COL1A2, osteocalcin, bone sialoprotein, or osteopontin, although they did express similar levels of alkaline phosphatase and RUNX2 (data not shown).
Recent data from the Bonewald laboratory has demonstrated that seven to nine sequential digests are needed to release true osteocytes from mouse bones [27]. This is consistent with our data in Fig. 4b, which shows that relative to the “stripped” bone sample (i.e., the residual bone fragments following the two sequential digests), which expressed easily detectable levels of key osteocytic genes, AP+ and AP+/CD45/34/31− cells expressed extremely low to undetectable levels of SOST. AP+/CD45/34/31− cells also expressed very low levels of all of the key osteocyte markers except for E11 (an early osteocyte marker [27]), whereas AP+ cells expressed variable levels of E11, DMP1, and FGF23.
In terms of yields of the AP+ and AP+/CD45/34/31− cells, from a mean (SEM) weight of the two combined needle biopsies of 327 (39)mg, we obtained 1.5 (0.8) million AP+ cells and 206,000 (197,000) AP+/CD45/34/31− cells.
We next used RNAseq to identify genes and pathways unique to the AP+/CD45/34/31− cells (i.e., human osteoblasts) compared to fibroblasts. As shown in Supplementary Table 1 (Online Resource 1), using the criteria of expression in the AP+/CD45/34/31− cells but not in the fibroblasts (P < 0.05) and false discovery rate (q)<0.05, 448 of the 13,306 genes expressed in the AP+/CD45/34/31− cells (3.4 %) were unique to these cells and not expressed in fibroblasts, reflecting the close similarity between osteoblasts and fibroblasts. If these criteria were made less stringent (expression in the AP+/CD45/34/31− cells but not in the fibroblasts, no P or q value threshold), this number rose to 811 (6.1 %), which likely reflects the upper limit of genes unique to the AP+/CD45/34/31− cells. Table 1 provides a list of selected genes of interest meeting the more stringent criteria, which included several of the osteoblast-related genes (osterix, osteocalcin, bone sialoprotein, BMP5 and 8, DLX5 and 6,DMP1, MEPE, PTHR1 and 2, and RANKL). Note that certain genes that might be expected to be expressed in osteoblasts (e.g., vitamin D, estrogen, androgen, or thyroid hormone receptors) are not on the list in Table 1 or Supplementary Table 1 (Online Resource 1) since they were either also expressed in fibroblasts or did not meet our criteria for being significantly different between the fibroblasts and the AP+/CD45/34/31− cells (data not shown). Interestingly, these unique genes also included several adipocyte-related genes (adiponectin, C/EBPΑ, and LPL), along with other genes known to play important roles in bone metabolism (e.g., IGF-1 and 2, and CXCR4).
Table 1.
Selected genes of interest expressed uniquely in human AP+/CD45/34/31− cells and not in fibroblasts. The entire list of 448 genes meeting these criteria is available in Supplementary File 1.Where appropriate, common names of the genes are provided in parentheses
| ADIPOQ (adiponectin) | IBSP (bone sialoprotein) |
| BGLAP (osteocalcin) | IGF1 |
| BMP5 | IGF2 |
| BMP8B | LPL |
| CEBPA | MEPE |
| CXCR4 | PTH1R |
| DLX5 | PTH2R |
| DLX6 | SP7 (osterix) |
| DMP1 | TNFSF11 (rankl) |
Table 2 shows a selected list of the gene pathways unique to osteoblasts reflected by these 448 genes, and Supplementary Table 2 (Online Resource 1) shows a more complete list of all of the statistically significant (P <0.05) pathways. These included (Table 2) LXR/RXR activation, growth hormone signaling, G protein-coupled receptor signaling, CXCR4 signaling, osteoblast-related, type II diabetes mellitus signaling, and NFAT and NFκB signaling.
Table 2.
Selected significant pathways related to bone metabolism by Ingenuity Pathway Analysis composed of genes expressed in AP+/CD45/34/31− cells, but not in fibroblasts. For full Pathway list, see Supplementary File 2. Ratio refers to the ratio of genes expressed in the AP+/CD45/34/31− cells divided by the total number of genes in that Ingenuity pathway. Where appropriate, common names of the genes are provided in parentheses
| Pathway | P value | Ratio | Genes unique to AP+/CD45/34/31 − cells in the pathway |
|---|---|---|---|
| LXR/RXR activation | 0.0005 | 0.11 | APOE, APOB, TF, NGFR, LPL,AMBP, SERPINA1, S100A8, LBP, PON3 |
| Growth hormone signaling | 0.001 | 0.10 | IGF2, PRKCQ, IGF1, PIK3CG, CEBPA, PIK3R5, PRKCB |
| VDR/RXR activation | 0.002 | 0.09 | BGLAP (osteocalcin), TNFSF11 (RANKL), PRKCQ, CAMP, TRPV5, CEBPA, PRKCB |
| G protein-coupled receptor signaling | 0.003 | 0.05 | APLNR, RGS18, PIK3R5, FPR1, P2RY13, TULP2, ADRB1 (β-adrenergic receptor 1), GNA15, VIPR1, CXCR2, PIK3CG, PTH1R, P2RY12, PRKCB |
| CXCR4 signaling | 0.003 | 0.06 | ELMO3, PRKCQ, PAK6, GNA15, CXCR4, PIK3CG, PIK3R5, MYL4, RHOH, PRKCB |
| Role of osteoblasts, osteoclasts, and chondrocytes in rheumatoid arthritis | 0.004 | 0.05 | BGLAP (osteocalcin), TNFSF11 (RANKL), IGF1, NGFR, PIK3CG, DLX5, PIK3R5, SFRP5, MMP13, SP7 (osterix), BMP5, BMP8B |
| Type II diabetes mellitus signaling | 0.005 | 0.06 | PRKCQ, SLC27A2, PKLR, NGFR, PIK3CG, ADIPOQ (adiponectin), PIK3R5, PRKCB |
| Role of NFAT in regulation of the immune response | 0.016 | 0.05 | BTK, FCGR3B, PRKCQ, GNA15, PIK3CG, SYK, PIK3R5, FCGR3A, LCP2 |
| NF-κB signaling | 0.035 | 0.05 | TLR2, TNFSF11 (RANKL), PRKCQ, NGFR, PIK3CG, NTRK1, PIK3R5, PRKCB |
NFAT nuclear factor of activated Tcells, VDR/RXR vitamin D receptor-retinoid X receptor, NF-κB nuclear factor kappa light chain enhancer of activated B cells
Discussion
In the present study, we isolate and characterize progressively purified populations (AP+ and AP+/CD45/34/31− cells) of human osteoblasts that can be studied further without the need for in vitro culture. The AP+ cells mineralize in vitro and, relative to AP− cells, are highly enriched for key osteoblast-related genes. This enriched human osteoblast fraction can be isolated and placed into RNA extraction buffer within ~2 h of the small needle biopsies. To further deplete these AP+ cells of cells co-expressing hematopoietic/endothelial markers requires an additional FACS step, which leads to the isolation of AP+/CD45/34/31− cells, but this prolongs the processing time to ~4–4.5 h. The residual bone fragments have easily measurable mRNA levels for all of the key osteocyte markers and thus represent an osteocyte-enriched fraction. The methods described here should be broadly applicable to the study of the pathophysiology of osteoporosis and other metabolic bone diseases. In addition, based on the gene expression analysis of these cell populations, serum-based assays for secreted proteins could be developed as novel biomarkers for various bone diseases.
The bone AP+/CD45/34/31− cells isolated using this method are clearly mature osteoblastic cells, as these cells differ markedly in their osteoblast gene expression profile compared to bone marrow AP+/CD45/34/31− cells isolated using similar methods. If desired, parallel processing of bone marrow aspirates could yield both early (from marrow aspirates) and mature (from the bone samples) osteoblastic cells from the same patient.
Previous studies of mouse bones [28] have found that the initial collagenase digests (20–40 min) largely release hematopoietic cells that respond to calcitonin but fail to respond to PTH, whereas the cells released following 40–80 min of digestion are PTH responsive, consistent with an osteoblast phenotype. Our first digest of 30 min and pooled second digest of 60 min fall essentially into this time frame, and while both our first and second digest cells are capable of in vitro mineralization, the second digest cells were markedly enriched for osteoblastic gene expression as compared to the first digest cells. Conversely, as mentioned earlier, work from the Bonewald laboratory [27] has recently demonstrated that true osteocytic cells are not released from mouse bones until approximately the seventh serial digestion and continue to be released through the ninth digestion. Consistent with these findings, we observed that while AP+ cells and, to a lesser extent, AP+/CD45/34/31− cells expressed low levels of osteocyte markers, these populations expressed undetectable (or barely detectable) levels of SOST mRNA compared to the stripped bone fragments. These findings, combined with our osteoblast gene expression and in vitro mineralization data, indicate that the AP+ and AP+/CD45/34/31− cells likely represent mature osteoblastic cells, although some of these cells may be transitioning to osteocytes.
We also found that the type of collagenase used in isolating cells from bone is quite important, since use of less purified collagenase (Worthington) led to much lower RNA integrity values in the released cells compared to using a highly purified, endotoxin-free collagenase (Roche).
One of the notable findings from the present study was that even though the AP+/CD45/34/31− cells were enriched for osteoblastic gene expression compared to AP+/CD45/34/31+ cells, both cell populations (as long as they expressed surface AP) were capable of in vitro mineralization. These findings are consistent with mounting evidence demonstrating that various hematopoietic and endothelial populations are capable of mineralization and, under certain circumstances, bone formation. For example, Suda et al. [29] have described a circulating CD45+ bone marrow-derived type I collagen + cell population that also expresses AP and is capable of mineralization as well as heterotopic ossification. In addition, Fadini and colleagues [30] characterized myeloid cells expressing osteocalcin and AP that may contribute to vascular calcification in patients with diabetes. Finally, we have previously identified endothelial progenitor cells co-staining for bone-related proteins and capable of in vitro mineralization [31–33]. Of particular interest is the recent description by Chang et al. [25] of osteal macrophages whose presence in proximity to osteoblastic cells seems to be critical for optimal mineralization and bone formation by the osteoblasts. In humans, these osteal macrophages have been identified by staining with CD68 [25] and these cells may also express AP [26]. As such, the marked enrichment for CD68 mRNA levels in the AP+/CD45/34/31+ compared to the AP+/CD45/34/31− cells raises the possibility that these two AP+ populations may reside in close proximity to each other on bone surfaces, with the myeloid AP+ cells providing key supportive cytokines/growth factors for optimal bone formation by the mesenchymal AP+ cells. Clearly, further studies are needed to test this hypothesis.
Recently, Ayturk et al. [12] proposed a novel approach to electronically deplete genes expressed in whole mouse bone samples by those highly expressed in blood, bone marrow, or muscle. This in silico approach could allow for examination of osteoblast-related genes in mouse bones without the need for any in vitro processing. While this is an intriguing and viable approach, the isolation of a highly enriched osteoblast population (AP+/CD45/34/31− cells) allows for the possibility of performing the reverse selection—specifically, selecting the genes in the whole bone samples that reflect those expressed in the osteoblasts derived from that sample. Thus, the physical isolation of human osteoblasts (AP+/CD45/34/31− cells) could be combined with a parallel analysis of unfractionated bone biopsies without any processing, and then, an examination of the osteoblast-specific genes from the AP+/CD45/34/31− cells in the bone biopsies could be performed. This would allow for both a physical and an in silico isolation of osteoblasts, with comparison of changes in the genes in common between the AP+/CD45/34/31− cells and the whole bone biopsies with age or drug treatment providing complementary data.
Since osteoblasts and fibroblasts are closely related mesenchymal cells, we also compared, using RNAseq, the transcriptomes of these cells and found that 3.4–6.1 % (depending on the stringency of the criteria applied, see “Methods” section) of the expressed transcripts in the AP+/CD45/34/31− cells were unique to these cells and not expressed in fibroblasts. In addition to the expected genes (e.g., osterix and osteocalcin), Supplementary Tables 1 and 2 (Online Resource 1) provide a complete list of the transcripts that were unique to the AP+/CD45/34/31− cells along with specific pathways reflected by these genes. Beyond the expected pathways (e.g., osteoblast-related, VDR activation, Table 2), perhaps of particular interest were two of the pathways related to energy metabolism (LXR/RXR activation and type II diabetes mellitus signaling). Thus, there is growing evidence for a link between bone and energy metabolism [34], and recent evidence has implicated both LXR [35] and insulin [36, 37] signaling in osteoblasts in the regulation of bone formation. Compared to the closely related mesenchymal cells, fibroblasts, these pathways appear to be relatively more important in osteoblasts. These data, along with the additional genes and pathways identified in Supplementary Tables 1 and 2 (Online Resource 1), may therefore be useful for the further exploration of osteoblast-specific genes/pathways or in the identification of novel drug targets to modulate osteoblast function in humans. Finally, the techniques described and validated here to isolate and characterize human osteoblasts without in vitro culture should also be broadly applicable to studying the pathogenesis of osteoporosis and other metabolic bone diseases.
Supplementary Material
Acknowledgments
We would like to thank the staff of the Mayo Clinical Research Unit for their assistance with the studies. This work was supported by NIH Grants AG004875 and UL1 TR000135 (Mayo Clinical and Translational Science Award).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00198-013-2529-9) contains supplementary material, which is available to authorized users.
Conflicts of interest None.
Contributor Information
K. Fujita, College of Medicine, Mayo Clinic, Rochester, MN, USA
M. M. Roforth, College of Medicine, Mayo Clinic, Rochester, MN, USA
E. J. Atkinson, College of Medicine, Mayo Clinic, Rochester, MN, USA
J. M. Peterson, College of Medicine, Mayo Clinic, Rochester, MN, USA
M. T. Drake, College of Medicine, Mayo Clinic, Rochester, MN, USA
L. K. McCready, College of Medicine, Mayo Clinic, Rochester, MN, USA
J. N. Farr, College of Medicine, Mayo Clinic, Rochester, MN, USA
D. G. Monroe, College of Medicine, Mayo Clinic, Rochester, MN, USA
S. Khosla, Email: khosla.sundeep@mayo.edu, College of Medicine, Mayo Clinic, Rochester, MN, USA; Endocrine Research Unit and Kogod Center on Aging, Mayo Clinic, Guggenheim 7–11, 200 First Street SW, Rochester, MN 55905, USA.
References
- 1.Genant HK, Engelke K, Prevrhal S. Advanced CT bone imaging in osteoporosis. Rheumatology (Oxford) 2008;47:iv9–iv16. doi: 10.1093/rheumatology/ken180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Majumdar S. Magnetic resonance imaging for osteoporosis. Skeletal Radiol. 2008;37:95–97. doi: 10.1007/s00256-007-0412-5. [DOI] [PubMed] [Google Scholar]
- 3.Leeming DJ, Alexandersen P, Karsdal MA, Qvist P, Schaller S, Tanko LB. An update on biomarkers of bone turnover and their utility in biomedical research and clinical practice. Eur J Clin Pharmacol. 2006;62:781–792. doi: 10.1007/s00228-006-0174-3. [DOI] [PubMed] [Google Scholar]
- 4.Patsch JM, Kohler T, Berzlanovich A, Muschitz C, Bieglmayr C, Roscher P, Resch H, Pietschmann P. Trabecular bone micro-structure and local gene expression in iliac crest biopsies of men with idiopathic osteoporosis. J Bone Miner Res. 2011;26:1584–1592. doi: 10.1002/jbmr.344. [DOI] [PubMed] [Google Scholar]
- 5.Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS, Glowacki J. Age-related intrinsic changes in human bone marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7:335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drake MT, Srinivasan B, Modder UI, Ng AC, Undale AH, Roforth MM, Peterson JM, McCready LK, Riggs BL, Khosla S. Effects of intermittent parathyroid hormone treatment on osteoprogenitor cells in postmenopausal women. Bone. 2011;49:349–355. doi: 10.1016/j.bone.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modder UI, Roforth MM, Hoey K, McCready LK, Peterson JM, Monroe DG, Oursler MJ, Khosla S. Effects of estrogen on osteoprogenitor cells and cytokines/bone regulatory factors in post-menopausal women. Bone. 2011;49:202–207. doi: 10.1016/j.bone.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Modder UI, Roforth MM, Nicks KM, Peterson JM, McCready LK, Monroe DG, Khosla S. Characterization of mesenchymal progenitor cells isolated from human bone marrow by negative selection. Bone. 2012;50:807–810. doi: 10.1016/j.bone.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodgson SF, Johnson KA, Muhs JM, Lufkin EG, McCarthy JT. Outpatient percutaneous biopsy of the iliac crest: methods, morbidity, and patient acceptance. Mayo Clin Proc. 1986;61:28–33. doi: 10.1016/s0025-6196(12)61395-0. [DOI] [PubMed] [Google Scholar]
- 10.Aubin JE. Advances in the osteoblast lineage. Biochem Cell Biol. 1998;76:899–910. [PubMed] [Google Scholar]
- 11.Kantor B, Jorgensen SM, Lund PE, Chmelik MS, Reyes DA, RItman EL. Cryostatic micro-computed tomography imaging of arterial wall perfusion. Scanning. 2002;24:186–190. doi: 10.1002/sca.4950240405. [DOI] [PubMed] [Google Scholar]
- 12.Ayturk UM, Jacobsen CM, Christodoulou D, Gorham J, Seidman JG, Seidman CE, Robling AG, Warman ML. An RNA-seq protocol to identify mRNA expression changes in mouse diaphyseal bone: applications in mice with bone property altering Lrp5 mutations. J Bone Miner Res. 2013 doi: 10.1002/jbmr.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dafforn A, Chen P, Deng G, et al. Linear mRNA amplification from as little as 5 ng total RNA for global gene expression. Biotechniques. 2004;37:854–857. doi: 10.2144/04375PF01. [DOI] [PubMed] [Google Scholar]
- 14.Nygaard V, Hovig E. Options available for profiling small samples: a review of sample amplification technology when combined with microarray profiling. Nucleic Acids Res. 2006;34:996–1014. doi: 10.1093/nar/gkj499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radonic A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A. Guideline to reference gene selection for quantitative realtime PCR. Biochem Biophys Res Commun. 2004;313:856–862. doi: 10.1016/j.bbrc.2003.11.177. [DOI] [PubMed] [Google Scholar]
- 16.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging ofmultiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. research0034.0031-0030-0034.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao S, Fernald RD. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J Comput Biol. 2005;12:1047–1064. doi: 10.1089/cmb.2005.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Wang S, Li W. RSeQC: quality control of RNA-seq experiments. Bioinformatics. 2012;28:2184–2185. doi: 10.1093/bioinformatics/bts356. [DOI] [PubMed] [Google Scholar]
- 21.Ramskold D, Wang ET, Burge CB, Sandberg R. An abundance of ubiquitously expressed genes revealed by tissue transcriptome sequence data. PLoS Comput Biol. 2009;5:e1000598. doi: 10.1371/journal.pcbi.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen KD, Irizarry RA. Removing technical variability in RNA-seq data using conditional quantile normalization. Biostatistics. 2012;13:204–216. doi: 10.1093/biostatistics/kxr054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawson GM, Katzmann JA, Kimlinger TK, O’Brien JF. Isolation and preliminary characterization of a monoclonal antibody which interacts preferentially with the liver isoenzymes of human alkaline phosphatase. Clinical chemistry. 1985;31:381–385. [PubMed] [Google Scholar]
- 25.Chang MK, Raggatt L-J, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, Maylin ER, Ripoll VM, Hume DA, Pettit AR. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 2008;181:1232–1244. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- 26.Pettit AR, Chang MK, Hume DA, Raggatt L-J. Osteal macrophages: a new twist on coupling during bone dynamics. Bone. 2008;43:976–982. doi: 10.1016/j.bone.2008.08.128. [DOI] [PubMed] [Google Scholar]
- 27.Stern AR, Stern MM, Van Dyke ME, Jahn K, Prideaux M, Bonewald LF. Isolation and culture of primary osteocytes from the long bones of skeletally mature and aged mice. Biotechniques. 2012;52:361–373. doi: 10.2144/0000113876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luben RA, Wong GL, Cohn DV. Biochemical characterization with parathormone and calcitonin of isolated bone cells: provisional identification of osteoclasts and osteoblasts. Endocrinology. 1976;99:526–534. doi: 10.1210/endo-99-2-526. [DOI] [PubMed] [Google Scholar]
- 29.Suda RK, Billings PC, Egan KP, Kim JH, McCarrick-Walmsley R, Glaser DL, Porter DL, Shore EM, Pignolo RJ. Circulating osteogenic precursor cells in heterotopic bone formation. Stem Cells. 2009;27:2209–2219. doi: 10.1002/stem.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fadini GP, Albiero M, Menegazzo L, et al. Widespread increase in myeloid calcifying cells contributes to ectopic vascular calcification in type 2 diabetes. Circ Res. 2011;108:1112–1121. doi: 10.1161/CIRCRESAHA.110.234088. [DOI] [PubMed] [Google Scholar]
- 31.Gossl M, Modder UIL, Atkinson EJ, Lerman A, Khosla S. Osteocalcin expression by circulating endothelial progenitor cells in patients with coronary artherosclerosis. J Am Coll Cardiol. 2008;52:1314–1325. doi: 10.1016/j.jacc.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gossl M, Modder UI, Gulati R, Rihal CS, Prasad A, Loeffler D, Lerman LO, Khosla S, Lerman A. Coronary endothelial dysfunction in humans is associated with coronary retention of osteogenic endothelial progenitor cells. Eur Heart J. 2010;31:2909–2914. doi: 10.1093/eurheartj/ehq373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peris P, Atkinson EJ, Gossl M, Kane TL, McCready LK, Lerman A, Khosla S, McGregor UI. Effects of bisphosphonate treatment on circulating osteogenic endothelial progenitor cells in postmenopausal women. Mayo Clin Proc. 2013;88:46–55. doi: 10.1016/j.mayocp.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karsenty G, Oury F. The central regulation of bone mass, the first link between bone remodeling and energy metabolism. J Clin Endocrinol Metab. 2010;95:4795–4801. doi: 10.1210/jc.2010-1030. [DOI] [PubMed] [Google Scholar]
- 35.Robertson KM, Norgard M, Windahl SH, Hultenby K, Ohlsson C, Andersson G, Gustafsson JA. Cholesterol-sensing receptors, liver X receptor alpha and beta, have novel and distinct roles in osteoclast differentiation and activation. J Bone Miner Res. 2006;21:1276–1287. doi: 10.1359/jbmr.060503. [DOI] [PubMed] [Google Scholar]
- 36.Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fulzele K, Riddle RC, DiGirolamo DJ, et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142:309–319. doi: 10.1016/j.cell.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.