Abstract
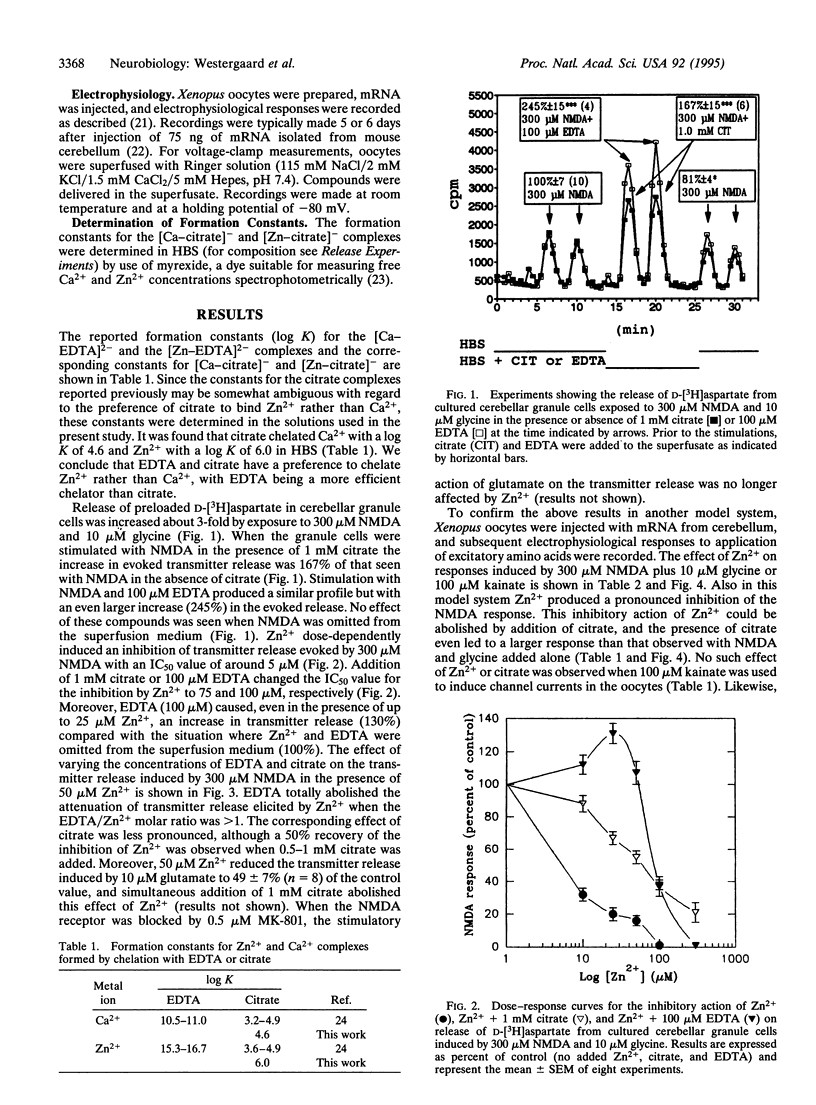
The effect of the two metal-ion chelators EDTA and citrate on the action of N-methyl-D-aspartate (NMDA) receptors was investigated by use of cultured mouse cerebellar granule neurons and Xenopus oocytes, respectively, to monitor either NMDA-evoked transmitter release or membrane currents. Transmitter release from the glutamatergic neurons was determined by superfusion of the cells after preloading with the glutamate analogue D-[3H]aspartate. The oocytes were injected with mRNA isolated from mouse cerebellum and, after incubation to allow translation to occur, currents mediated by NMDA were recorded electrophysiologically by voltage clamp at a holding potential of -80 mV. It was found that citrate as well as EDTA could attenuate the inhibitory action of Zn2+ on NMDA receptor-mediated transmitter release from the neurons and membrane currents in the oocytes. These effects were specifically related to the NMDA receptor, since the NMDA receptor antagonist MK-801 abolished the action and no effects of Zn2+ and its chelators were observed when kainate was used to selectively activate non-NMDA receptors. Since it was additionally demonstrated that citrate (and EDTA) preferentially chelated Zn2+ rather than Ca2+, the present findings strongly suggest that endogenous citrate released specifically from astrocytes into the extracellular space in the brain may function as a modulator of NMDA receptor activity. This is yet another example of astrocytic influence on neuronal activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assaf S. Y., Chung S. H. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984 Apr 19;308(5961):734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- Belhage B., Rehder V., Hansen G. H., Kater S. B., Schousboe A. 3H-D-aspartate release from cerebellar granule neurons is differentially regulated by glutamate- and K(+)-stimulation. J Neurosci Res. 1992 Nov;33(3):436–444. doi: 10.1002/jnr.490330309. [DOI] [PubMed] [Google Scholar]
- Bell J. D., Brown J. C., Sadler P. J., Macleod A. F., Sönksen P. H., Hughes R. D., Williams R. High resolution proton nuclear magnetic resonance studies of human cerebrospinal fluid. Clin Sci (Lond) 1987 May;72(5):563–570. doi: 10.1042/cs0720563. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Charton G., Rovira C., Ben-Ari Y., Leviel V. Spontaneous and evoked release of endogenous Zn2+ in the hippocampal mossy fiber zone of the rat in situ. Exp Brain Res. 1985;58(1):202–205. doi: 10.1007/BF00238969. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988 Oct;1(8):623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Drejer J., Honoré T., Schousboe A. Excitatory amino acid-induced release of 3H-GABA from cultured mouse cerebral cortex interneurons. J Neurosci. 1987 Sep;7(9):2910–2916. doi: 10.1523/JNEUROSCI.07-09-02910.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drejer J., Schousboe A. Selection of a pure cerebellar granule cell culture by kainate treatment. Neurochem Res. 1989 Aug;14(8):751–754. doi: 10.1007/BF00964953. [DOI] [PubMed] [Google Scholar]
- Eimerl S., Schramm M. Potentiation of 45Ca uptake and acute toxicity mediated by the N-methyl-D-aspartate receptor: the effect of metal binding agents and transition metal ions. J Neurochem. 1993 Aug;61(2):518–525. doi: 10.1111/j.1471-4159.1993.tb02154.x. [DOI] [PubMed] [Google Scholar]
- Harrison N. L., Gibbons S. J. Zn2+: an endogenous modulator of ligand- and voltage-gated ion channels. Neuropharmacology. 1994 Aug;33(8):935–952. doi: 10.1016/0028-3908(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Koh J. Y., Choi D. W. Zinc alters excitatory amino acid neurotoxicity on cortical neurons. J Neurosci. 1988 Jun;8(6):2164–2171. doi: 10.1523/JNEUROSCI.08-06-02164.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr The action of zinc on synaptic transmission and neuronal excitability in cultures of mouse hippocampus. J Physiol. 1989 Aug;415:351–365. doi: 10.1113/jphysiol.1989.sp017725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Westbrook G. L. Modulation of excitatory amino acid receptors by group IIB metal cations in cultured mouse hippocampal neurones. J Physiol. 1989 Aug;415:329–350. doi: 10.1113/jphysiol.1989.sp017724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Peters S., Koh J., Choi D. W. Zinc selectively blocks the action of N-methyl-D-aspartate on cortical neurons. Science. 1987 May 1;236(4801):589–593. doi: 10.1126/science.2883728. [DOI] [PubMed] [Google Scholar]
- Petroff O. A., Yu R. K., Ogino T. High-resolution proton magnetic resonance analysis of human cerebrospinal fluid. J Neurochem. 1986 Oct;47(4):1270–1276. doi: 10.1111/j.1471-4159.1986.tb00750.x. [DOI] [PubMed] [Google Scholar]
- Rassendren F. A., Lory P., Pin J. P., Nargeot J. Zinc has opposite effects on NMDA and non-NMDA receptors expressed in Xenopus oocytes. Neuron. 1990 May;4(5):733–740. doi: 10.1016/0896-6273(90)90199-p. [DOI] [PubMed] [Google Scholar]
- Scarpa A. Spectrophotometric measurement of calcium by murexide. Methods Enzymol. 1972;24:343–351. doi: 10.1016/0076-6879(72)24082-4. [DOI] [PubMed] [Google Scholar]
- Schousboe A., Westergaard N., Sonnewald U., Petersen S. B., Yu A. C., Hertz L. Regulatory role of astrocytes for neuronal biosynthesis and homeostasis of glutamate and GABA. Prog Brain Res. 1992;94:199–211. doi: 10.1016/s0079-6123(08)61751-3. [DOI] [PubMed] [Google Scholar]
- Sonnewald U., Westergaard N., Krane J., Unsgård G., Petersen S. B., Schousboe A. First direct demonstration of preferential release of citrate from astrocytes using [13C]NMR spectroscopy of cultured neurons and astrocytes. Neurosci Lett. 1991 Jul 22;128(2):235–239. doi: 10.1016/0304-3940(91)90268-x. [DOI] [PubMed] [Google Scholar]
- Westbrook G. L., Mayer M. L. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987 Aug 13;328(6131):640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- Westergaard N., Sonnewald U., Unsgård G., Peng L., Hertz L., Schousboe A. Uptake, release, and metabolism of citrate in neurons and astrocytes in primary cultures. J Neurochem. 1994 May;62(5):1727–1733. doi: 10.1046/j.1471-4159.1994.62051727.x. [DOI] [PubMed] [Google Scholar]


