Abstract
Background
Although obesity is commonly linked with metabolic disease risk, some obese adults do not develop metabolic abnormalities, such as insulin resistance.
Objectives
The primary aim of this study was to determine whether alterations in fatty acid mobilization and uptake underlie differences in insulin sensitivity (Si) among a seemingly homogeneous cohort of obese women.
Methods
Insulin sensitivity (FSIVGTT), basal fatty acid rate of disappearance from plasma (Rd), resting whole-body fat oxidation, intramyocellular triacylglycerol (IMTG) concentration, and markers of skeletal muscle inflammation were measured in 21 obese women. Participants were divided into tertiles based on their Si. The subset of participants with the lowest Si (LOW-Si; Si ≤2.1 (mU/L)−1·min−1; n=7) was compared with the subset of participants with the highest Si, who exhibited relatively normal insulin sensitivity (NORM-Si; Si ≥3.4 (mU/L)−1·min−1; n=8).
Results
Despite nearly identical physical characteristics in LOW-Si vs. NORM-Si (BMI: 34±2 vs. 34±1 kg/m2; %body fat: 48±1% vs. 47±1%; waist circumference: 104±2 vs. 104±2 cm; VO2max: 2.2±0.2 vs. 2.3±0.1 L/min), fatty acid Rd was nearly 30% lower in NORM (P=0.02). Importantly, the greater rate of fatty acid uptake in LOW-Si vs. NORMSi did not translate to higher rate of fat oxidation (3.5±0.2 vs. 3.7±0.2 μmol/kg/min) or to a measureable difference in IMTG content, (68.3±12.7 vs. 63.7±6.7 μmol/g dry weight). In conjunction with the lower fatty acid Rd in NORM-Si vs. LOW-Si, activation of inflammatory pathways known to impair insulin action in skeletal muscle was also lower (i.e. lower phosphorylated JNK, higher IκBα abundance). In contrast, LOW-Si and NORM-Si exhibited no differences in plasma markers of inflammation (i.e. TNFα, IL-6, MCP-1).
Conclusion
These findings suggest that obese women who maintain a relatively low rate of endogenous fatty acid uptake may be somewhat “protected” against the development of insulin resistance potentially by less activation of inflammatory pathways within skeletal muscle.
Keywords: insulin resistance, obesity, pre-diabetes, inflammation
INTRODUCTION
Obesity is often associated with insulin resistance, which is a major contributor to the development of several chronic diseases, including type 2 diabetes1. Despite the common link between obesity and disease risk, there are still many obese adults who appear to be somewhat “protected” against the development of many chronic health issues. In fact, it has been reported that over 30% of obese adults in the United States were classified as “metabolically healthy”2. However, it is still unclear why some obese adults are more prone to develop metabolic complications, like insulin resistance, while others are not, even when they are very similar in terms of the magnitude and distribution of their adiposity.
Excessive systemic fatty acid availability and uptake has been identified as a key factor underlying insulin resistance in obesity. Indeed, abdominally obese individuals exhibit elevated systemic fatty acid availability compared with their lean counterparts3,4, and lipid infusions in lean, healthy humans (used to elevate systemic fatty acid availability) readily impair insulin sensitivity5,6. Conversely, acute reduction in systemic fatty acid availability (via lipolytic inhibition) can improve insulin sensitivity in obesity7–9. Evidence suggests that the deleterious effects of elevated fatty acid availability and uptake are mediated in part via skeletal muscle accumulation of lipid intermediates, including diacylglycerol, ceramides, and fatty acyl-CoA10,11. These intermediates can increase activation of pro-inflammatory pathways (e.g.; c-jun N-terminal kinase (JNK), and inhibitor of κB/nuclear factor-κB (IκB/NF-κB)) known to disrupt insulin signaling12–14. Thus, elevated systemic fatty acid availability and subsequent skeletal muscle inflammatory pathway activation are believed to underlie the development of insulin resistance obesity.
Although lipolytic rate and fatty acid availability are typically elevated in obesity 3, there is still considerable variability in fatty acid mobilization among the obese adults15,16. It remains unclear whether the magnitude of endogenous fatty acid flux contributes to differences in the severity of insulin resistance among obese individuals. The primary aim of this study was to determine whether the magnitude of fatty acid uptake and markers of inflammatory pathway activation in skeletal muscle were related with the degree of insulin resistance in a cohort of obese sedentary women. We hypothesized that obese women with the lowest rates of fatty acid flux would exhibit relatively low pro-inflammatory pathway activation in skeletal muscle, and would be the least insulin resistant among the cohort.
METHODS
Participants
Twenty-one obese women (body mass index (BMI): 30–40 kg·m−2; waist circumference >100 cm) participated in this study. All women were premenopausal and considered to be in good health after a medical examination. The medical evaluation included a history questionnaire, physical examination, 12-lead electrocardiogram, and standard blood and urine tests. None of the participants were taking regular medications except for some who were taking contraceptive medication. All participants were non-smokers, weight stable (±2 kg) for 6 months, and had not participated in regular exercise for at least 6 months before participating in the study. Participants with coronary heart disease, type 2 diabetes, hypertension, or clinically significant hypertriacylglycerolemia (plasma triacylglycerol > 150mg·dl−1) were excluded. Some of these subjects participated in a previous study from our laboratory17. All participants were fully informed of the possible risks associated with the study and signed an informed consent document, which was approved by the University of Michigan Institutional Review Board, in accordance with the Declaration of Helsinki.
Preliminary testing
Body composition was determined using dual x-ray absorptiometry (Lunar DPX DEXA Scanner, Madison, WI, USA). Aerobic fitness was analyzed by measuring peak oxygen uptake (VO2 peak) during cycle ergometer exercise. The protocol consisted of a 4 minute warm-up, followed by a progressive increase in work rate every minute until volitional fatigue.
Experimental procedures
Participants were admitted to the Michigan Clinical Research Unit (MCRU) at the University of Michigan hospital at 1800h and stayed overnight. A standardized meal was provided at 2000h and the participants remained fasted until completion of the trial the next day.
At 0700h the next morning a skeletal muscle biopsy was obtained from the vastus lateralis. The muscle sample was separated from any adipose and connective tissue, rinsed with saline, blotted dry, frozen in liquid nitrogen, and store at −80°C until analysis. Intravenous catheters were placed in a hand vein for blood sampling and in a forearm vein of the opposite arm for infusion of the stable isotope tracer, [13C] -palmitate (Cambridge Isotope Laboratories, Andover, MA, USA). A blood sample was obtained before the start of the tracer infusion to assess background [13C]-palmitate enrichment and to measure baseline concentrations of plasma pro-inflammatory markers (i.e., TNF-a, IL-6, and MCP-1). At 0800h, a constant infusion of [13C] palmitate (0.04 μmol kg−1 min−1) bound to human albumin (Baxter, Deerfield, IL, USA) was initiated. Four arterialized blood samples were collected in 5 min intervals from a heated hand vein at minutes 45, 50, 55, and 60 for determination of the rate of fatty acid disappearance from plasma (Rd), to assess fatty acid uptake. Resting whole-body fat oxidation was calculated from the rates of oxygen consumption (VO2) and carbon dioxide production (VCO2) using a metabolic cart (Delta Trac, Sensor Medics Yorba Linda, CA, USA). After all of the fatty acid metabolism measurements, a frequently sampled intravenous glucose tolerance test (FSIVGTT) was conducted to assess insulin sensitivity (Si) using the minimal model technique, as previously described18. The trial was performed during the first 2 weeks of the participants’ menstrual cycle.
Analytical procedures
Plasma fatty acid kinetics
The tracer-to-tracee ratio (TTR) for plasma palmitate was determined by gas chromatography-mass spectrometry (GC-MS) with an MSD 5973 system (Agilent Technologies; Wilmington, DE, USA) with capillary column as previously described19,20. Plasma palmitate concentration was measured by the internal standard method using GC/flame ionization detection (GC/FID)21.
Plasma glucose, fatty acid and insulin concentrations
Plasma glucose (Thermo Scientific, Waltham MA, USA) and fatty acid (Wako Chemicals USA, Richmond, VA, USA) concentrations were measured using a commercially available colorimetric assay kits. Plasma insulin concentration was measured by radioimmunoassay (Linco Research Inc., St Louis, MO, USA).
Intramyocellular triacylglycerol concentration
Skeletal muscle triacylglycerol concentration was determined with saponification techniques as previously described20,22 Briefly, triacylglycerols were extracted from the dried muscle sample using a 2:1 chloroform:methanol solution and saponified in 4% ethanolic KOH. Free glycerol concentration was then determined fluorometrically.
Tissue lysate preparation
Frozen muscle samples were weighed and transferred into pre-chilled microfuge tubes containing 1 ml ice cold lysis buffer and a steel ball bearing. Tissue samples were homogenized in the microfuge tubes for 5 minutes using a Qiagen TissueLyser II (Qiagen, Hilden, Germany). The lysis buffer contained T-PER Tissue Protein Extraction Reagent (#78510, Fisher Scientific, USA), 1mM EDTA, 1mM EGTA, 2.5 mM sodium pyrophosphate, 1mM sodium orthovanadate, 1mM β-glycerophosphate, 1 μg/ml leupeptin, and 1mM phenylmethylsulfonyl fluoride. In order to remove insoluble material, the homogenates were transferred to new microfuge tubes and rotated for one hour at 4°C and then centrifuged at 15,000 g for 15 min at 4°C. Protein concentration was measured using the bicinchoninic acid method (Thermo Scientific, Rockford, IL, USA).
Multiplex analysis
Plasma cytokines and skeletal muscle proteins associated with inflammation were quantified using a commercially available Multiplex bead assay per manufacturer recommendations (Luminex L200, Luminex, Austin, TX, USA). Plasma was assayed for IL-6, TNF-α, and MCP-1 as (#HCVD3-67CK, Milliplex MAP Kit, Millipore, Billerica, MA, USA). Prepared tissue lysates were analyzed for total protein abundance of JNK and IkBα and separately analyzed for the total phosphorylation of JNKThr185/Tyr185 (#48-602, Milliplex MAP Kit, Millipore, Billerica, MA, USA).
Calculations
Fat oxidation
Whole body fat/triacylglycerol oxidation (g/min) was calculated from VO2 and VCO2 measurements using the equations of Frayn23. Whole body fatty acid oxidation was calculated by dividing triacylglycerol oxidation by an estimated molecular weight of triacylglycerol (860 g/mol) and multiplying by 3.
Fatty acid uptake
Palmitate rate of disappearance (Rd) from plasma was calculated using the Steele equation for steady-state conditions24. Fatty acid Rd was calculated by dividing palmitate Rd by the ratio of plasma palmitate to total plasma fatty acid concentration.
Insulin sensitivity index (Si)
The insulin sensitivity index (Si) was calculated from least squares fitting of the insulin and glucose concentration curves from the IVGTT using the Minimal Model Millenium (version 6.02; MiniMod Inc.) computer analysis software. Although not the gold standard, Si has been found to match well with insulin sensitivity measured by the hyperinsulinemic-euglycaemic clamp technique25,26.
Fatty acid area under the curve during the IVGTT
Area under the curve (AUC) for plasma fatty acid concentration during time 0–180 min of the IVGTT was calculated using the trapezoidal rule27.
Statistics
Unpaired Student’s t-tests were used to test for significant between group (NORM-Si vs. LOW-Si) differences in outcome variables. Simple linear regression was used to examine the relationship between Si, fatty acid uptake, IκBα, and JNK in all participants (n=21). Statistical significance was defined as P < 0.05. All results are presented as mean ± standard error (SE). All analysis was performed on SigmaPlot version 11.0 computer software.
RESULTS
Insulin sensitivity index and cohort stratification
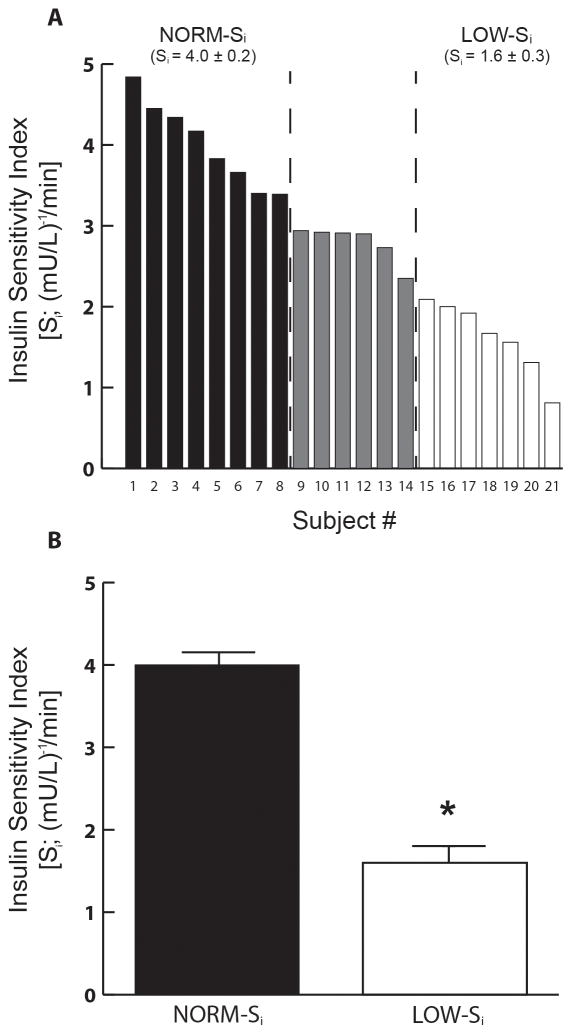
As designed, the participant pool was largely homogeneous in terms of BMI, adiposity, waist circumference, and cardiorespiratory fitness (Table 1); however, Si varied widely among the 21 participants (Figure 1), ranging from 4.8 to 0.8 (mU/L)−1·min−1. As noted in Figure 1A, participants with Si in the lowest one-third of the overall participant pool (≤2.1 (mU/L)−1·min−1) were grouped into the “low” insulin sensitive cohort (LOW-Si; n=7), and those in the highest one-third (≥3.4 (mU/L)−1·min−1) were grouped into the “normal” insulin sensitivity cohort (NORM-Si; n=8). The term “normal” was used to define the Si of the most insulin sensitive participants because these values were very similar to those previously reported by our laboratory and others for lean, healthy adults20,25,28,29. As designed, the difference in Si between NORM- Si and LOW- Si was highly significant (Figure 1B; P<0.000001); but importantly, these groups were very well matched for BMI, adiposity, waist circumference, and cardiorespiratory fitness (Table 1). In addition, fasting plasma glucose and insulin concentrations were similar in NORM-Si and LOW-Si (Table 1; p=0.47, p=0.28, respectively). In order to compare groups with distinct differences in insulin sensitivity, primary comparisons did not include participants with Si values between 2.1 and 3.4 (mU/L)−1·min−1 (grey bars in Figure 1). The participants with intermediate Si were included in correlation analyses, which incorporated the entire participant pool. The racial profile within our groups were as follows: NORM-Si - 2 African American and 6 Caucasian women; LOW-Si –1 African American, 1 Asian, 1 Hispanic/Latino and 4 Caucasian women; Intermediate-Si – 2 African American and 4 Caucasian women.
Table 1.
Subject Characteristics
| All Subjects | NORM-Si | LOW-Si | |
|---|---|---|---|
| Age (y) | 30 ± 1 | 32 ± 2 | 29 ± 3 |
| Body Mass (kg) | 93 ± 2 | 92 ± 2 | 91 ± 5 |
| BMI (kg/m2) | 34 ± 1 | 34 ± 1 | 34 ± 2 |
| Waist circumfrence (cm) | 104 ± 1 | 104 ± 2 | 104 ± 2 |
| Fat Mass (kg) | 44 ± 2 | 44 ± 1 | 44 ± 4 |
| Fat free mass (kg) | 49 ± 1 | 49 ± 1 | 48 ± 2 |
| Body Fat (%) | 48 ± 1 | 47 ± 1 | 48 ± 1 |
| VO2peak (ml/kg/min) | 24 ± 1 | 25 ± 1 | 23 ± 1 |
| Fasting glucose (mM) | 4.8 ± 0.2 | 5.0 ± 0.2 | 4.7 ± 0.4 |
| Fasting insulin (μU/mL) | 14.2 ± 0.9 | 15.2 ± 1.2 | 13.3 ± 1.2 |
| HOMA-IR | 3.0 ± 0.2 | 3.4 ± 0.3 | 2.7 ± 0.4 |
Figure 1. Insulin sensitivity.
(A) Insulin sensitivity index (Si) measured the morning after an overnight fast in all subjects (n=21). Subjects were ordered from highest to lowest Si, and stratified into tertiles to identify a low insulin sensitivity cohort (LOW- Si; Si ≤2.1 (mU/L)−1·min−1; n=7) and a normal insulin sensitivity cohort (NORM-Si; Si ≥3.4 (mU/L)−1·min−1) (B) mean Si in NORM-Si and LOW-Si cohorts. *P < 0.000001 vs. NORM- Si
Fat metabolism
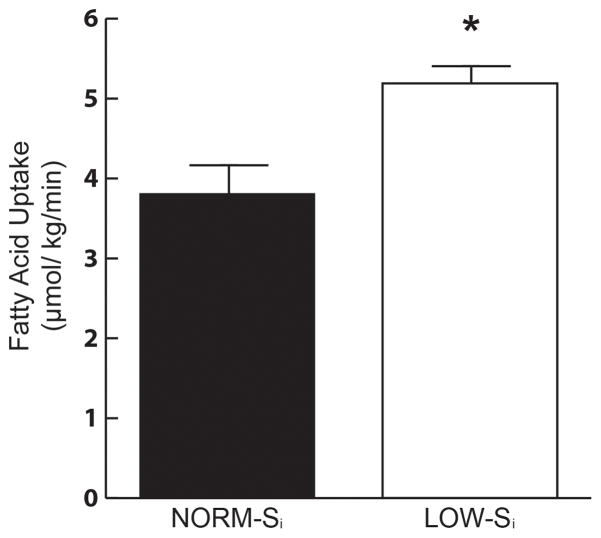
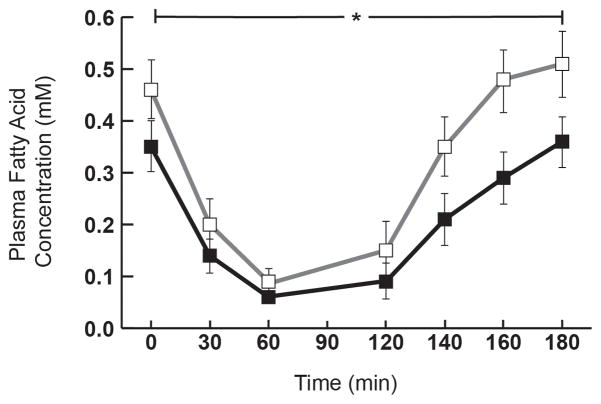
In conjunction with higher insulin sensitivity, fatty acid Rd was nearly 30% lower in NORM-Si vs. LOW-Si (Figure 2; P<0.02). Because we measured fatty acid kinetics under steady-state conditions, fatty acid Rd was equivalent to the rate of fatty acid appearance into the systemic circulation. Therefore, not only is fatty acid uptake lower in NORM-Si vs. LOW-Si, but fatty acid mobilization from adipose tissue was lower as well. Despite the lower rates of fatty acid mobilization and uptake in NORM-Si vs. LOWSi, resting whole-body fatty acid oxidation was not different between groups (3.7 ± 0.2 vs. 3.5 ± 0.2 μmol/kg/min, respectively). Interestingly, fatty acid uptake was reasonably well matched to the rate of fatty acid oxidation in NORM-Si (3.8 ± 0.5 vs. 3.7±0.2 umol/kg/min, respectively), but in LOW-Si the rate of fatty acid uptake exceeded fatty acid oxidation by nearly 50% (5.2 ± 0.4 vs. 3.5 ± 0.2 umol/kg/min, respectively). However, this disparity between fatty acid uptake and fat oxidation in LOW-Si did not translate to a measureable difference in IMTG content, which was similar between groups (63.7 ± 6.7 vs. 68.3 ± 12.7 μmol/g dry weight for NORM- Si and LOW- Si, respectively). In addition to the lower rate of fatty acid uptake in the overnight fasted state in NORM-Si compared with LOW-Si, plasma fatty acid concentration during the IVGTT was also significantly lower in NORM-Si vs. LOW-Si in response to insulin during the IVGTT (AUC: 29±10 vs. 47±15 mM•min, respectively, P=0.02; Figure 3)
Figure 2. Fatty acid uptake.
Fatty acid rate of disappearance from plasma (Rd) in NORM-Si and LOW-Si cohorts, measured the morning after an overnight fast. *P < 0.05 vs. NORM- Si
Figure 3. Insulin-induced suppression of plasma fatty acid concentration.
Plasma fatty acid concentration during the 3h intravenous glucose tolerance test (IVGTT) in LOW-Si (white square □) and NORM-Si (black square ■). Values are mean ± SE. * Significant difference in Area under the curve (AUC) between the groups, P = 0.02.
Markers of inflammation
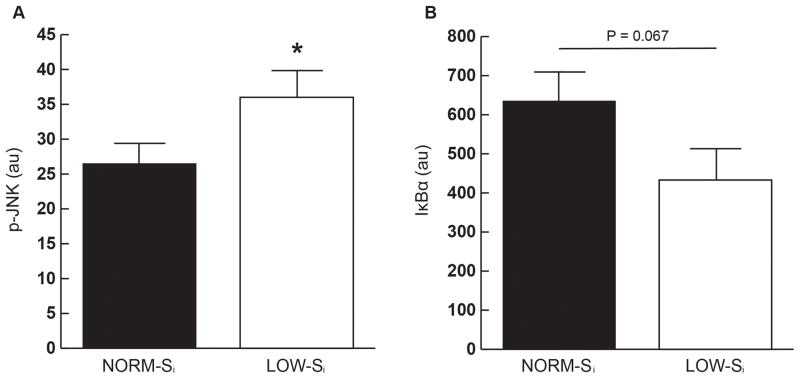
Within skeletal muscle, JNK phosphorylation (p-JNK) was significantly lower in NORM- Si compared with LOW-Si (Figure 4A), suggestive of attenuated inflammatory pathway activation in muscle from NORM-Si participants. This may have been due in part to a lower total JNK protein abundance in NORM-Si vs. LOW-Si (101±10 vs. 149±20 arbitrary units (AU), respectively; p ≤0.05). Skeletal muscle protein abundance of IκB-α tended to be greater in NORM-Si compared with LOW-Si (Figure 4B), but this difference between groups did not quite reach statistical significance (p=0.067). Because IκB-α suppresses activation of the IKK-NFκB inflammatory pathway30, the trend for greater abundance of IκB-α in muscle from NORM-Si vs. LOW-Si is also indicative of reduced inflammation in NORM-Si. In contrast to the differences in markers of inflammation in skeletal muscle, there were no significant differences in fasting plasma concentrations of IL-6 (15.0 ± 8.4 vs. 23.8 ± 10.0 pg/ml), TNF-α (4.3 ±1.0 vs. 7.9 ± 2.2 pg/ml), or MCP- 1 (128.5 ± 12.6 vs. 114.5 ± 32.7 pg/ml) between NORM-Si and LOW-Si, respectively.
Figure 4. Markers of inflammation.
(A) Total protein abundance of phosphorylated c- Jun N-terminal kinase (p-JNK) (B) Total protein abundance of Inhibitor of NF-κB α (IκB-α). *P < 0.05 for NORM-Si vs. LOW-Si
Relationships between insulin sensitivity, fatty acid uptake, and inflammatory markers
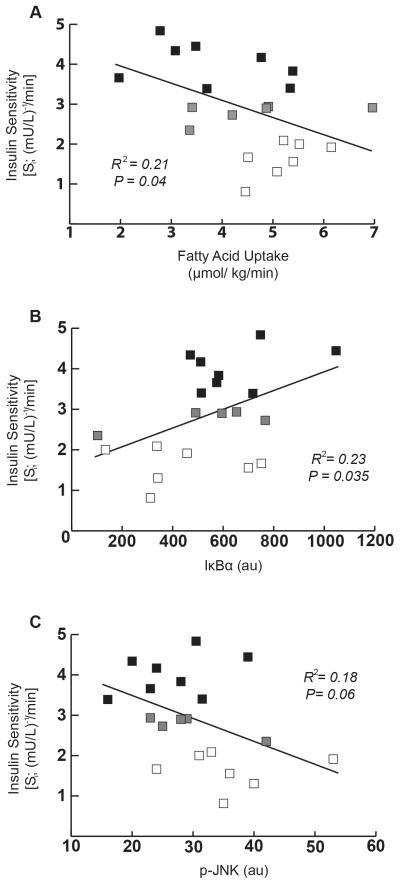
Among the entire participant population (n=21), fatty acid uptake was inversely correlated with Si, (Figure 5A; R2=0.21; p = 0.04), further supporting the notion that those with relatively low fatty acid uptake were the most insulin sensitive. Similarly, skeletal muscle IκB-α content was positively correlated with Si (Figure 5B; R2=0.22; p=0.035) and skeletal muscle p-JNK tended to be inversely correlated with Si (Figure 5C; R² = 0.18; p= 0.06), both suggesting that lower inflammatory pathway activation in skeletal muscle is related to insulin sensitivity in obesity. We found no correlations between IL-6, MCP-1, or TNF-α in relation to fatty acid uptake (IL-6: R2=0.052, p=0.25; MCP-1: R2=0.0004, p=.94; TNF-α: R2=0.002, p=0.90) or Si (IL-6: R2=0.017, p=0.65; MCP-1: R2=0.0003, p=.70; TNF-α: R2=0.072, p=0.31).
Figure 5. Correlational analyses for insulin sensitivity with fatty acid uptake and inflammatory markers in skeletal muscle.
(A) Correlation between insulin sensitivity index (Si) and fatty acid uptake. (B) Correlation between Si and IκB-α abundance. (C) Correlation between Si and p-JNK abundance. All correlational analyses were performed using the entire subject cohort (n=21) [LOW-Si (n=7; white square □), NORM- Si (n=8; black square ■) and participants that did not fall into either category (n=6; grey square
 ).
).
DISCUSSION
While many obese adults have serious cardio-metabolic complications and/or are at high risk for developing chronic diseases, such as type 2 diabetes, there are some obese adults who appear to be in relatively good health2,31. We found that just over one-third of our obese cohort exhibited “normal” insulin sensitivity, which matched very well with epidemiological evidence using data collected from the National Health and Nutrition Examination Survey (NHANES)2. However, it is not clear why some obese adults are prone to develop insulin resistance (as well as other cardio-metabolic complications), while others appear to remain somewhat “protected”. In our participants, body mass, BMI, waist circumference, fat mass, fat free mass, cardiorespiratory fitness, resting fat oxidation, and skeletal muscle triacylglycerol content could not explain the disparity in insulin resistance. Instead, our main findings suggest that a relatively low basal rate of fatty acid uptake may be a key factor helping to preserve insulin sensitivity in some obese adults.
An overabundance of fatty acids has long been known to induce insulin resistance5,6. Excessive fatty acid uptake into insulin responsive tissues like skeletal muscle is thought to induce insulin resistance in part via the intracellular accumulation of lipid intermediates, such as diacylglycerol, ceramides, and fatty acyl-CoA10,11. Theoretically, high rates of fatty acid oxidation may help compensate for elevated fatty acid uptake, thereby attenuating the accumulation of lipid intermediates, which may help protect against the development of insulin resistance32. However, the impact that variation in resting fatty acid oxidation may have on the development of insulin resistance is questioned33,34. In our study, rates of fatty acid oxidation were identical in NORM-Si and LOW-Si, suggesting differences in oxidative disposal of fatty acids was not likely contributing to the differences in insulin sensitivity observed between these groups. In contrast, a ~40% greater rate of fatty acid uptake in the LOW-Si vs. NORM-Si contributed to a large mismatch between the rates of fatty acid uptake and oxidation in this group. Although we did not measure the muscle accumulation of diacylglycerol, ceramide, or fatty acyl-CoA in this study due to limitations in the amount of tissue available, it is plausible that a disparity between fatty acid uptake and oxidation of this magnitude may have led to an accumulation of these lipid intermediates. In turn, the accumulation of these lipids within the muscle cell can increase the activation of inflammatory pathways, which may contribute to the impairment in insulin action35,36.
It has become evident that increased inflammatory pathway activation in skeletal muscle is an important link between altered fatty acid metabolism and impaired insulin signaling11,14,37,38. The high rates of fatty acid mobilization and uptake commonly found in obesity3 is often accompanied by an elevated activation of JNK12,39 and/or IKK37,39 in skeletal muscle. Importantly, pharmacologically induced suppression of fatty acid availability in humans was found to suppress the activation of inflammatory pathways in skeletal muscle, and improve insulin action7–9. Furthermore, previous work from our laboratory demonstrated that the improvement in insulin sensitivity found after weight loss was largely attributed to the weight loss-induced reduction in fatty acid availability, with an accompanying decline in the activation of the inflammatory JNK and IKK-NFκB pathways17. Our present finding that the relatively low rate of fatty acid uptake in NORM-Si was accompanied by lower p-JNK and a higher IκBα abundance compared with LOW-Si, highlights the importance of the magnitude of fatty acid uptake as a key mediator of inflammation and insulin sensitivity within skeletal muscle.
When examining health disparities among obese adults, there is considerable emphasis on the role that differences in anatomical distribution of body fat (i.e., subcutaneous vs. visceral) may play on determining metabolic health in obesity. Abdominal obesity is most commonly linked with insulin resistance40,41, as well as other cardiovascular disease risk factors42,43. More specifically, accumulation of fat in the visceral region is typically identified as being an especially potent contributor to the development of metabolic disorders44–46. Our NORM-Si and LOW-Si groups were tightly matched for waist circumference, which provides a reasonably accurate surrogate measure for visceral adiposity47. Nevertheless, because we did not definitively assess visceral fat mass, we cannot rule out the possibility that potential differences in visceral adiposity may help explain some of the observed difference in insulin sensitivity among our participants. Many reports suggest that visceral adiposity contributes to insulin resistance as a consequence of the relatively high lipolytic rate measured in visceral compared with subcutaneous adipocytes48,49. However, in vivo measurements indicate that about 85% of systemic fatty acids are derived from subcutaneous adipose tissue, whereas only about 15% come from visceral adipose tissue50. Therefore, differences in the regulation of fatty acid release from subcutaneous (rather than visceral) adipose tissue are likely responsible for the observed disparity in systemic fatty acid mobilization between NORM-Si and LOW-Si.
The rate of fatty acid mobilization from subcutaneous adipose tissue has been reported to vary widely among obese adults15,16. The rate of fatty acid release from adipose tissue is determined in large part by the rate of lipolysis, which liberates fatty acids from triacylglycerol. Therefore, a relatively low lipolytic rate within adipose tissue would suppress the mobilization of fatty acids into the systemic circulation, which may lower the risk for developing insulin resistance. Along these lines, it has recently been reported that specific inhibition of hormone sensitive lipase in mice decreased fatty acid mobilization and lead to an increase in insulin sensitivity16. These same authors also found lipolytic rate to be positively correlated with insulin resistance in humans, independent of BMI16. Importantly, the rate of triacylglycerol synthesis (i.e., fatty acid esterification) can also modulate the rate fatty acid release, and it has been reported that obese individuals with a relatively high capacity for triacylglycerol synthesis in subcutaneous adipose tissue were largely protected from insulin resistance15. Future studies designed to examine the abundance and activity of key factors that regulate fatty acid release and triacylglycerol synthesis in adipose tissue will help identify potential mechanisms mediating the differences in fatty acid mobilization and uptake in obesity.
Insulin potently suppresses lipolysis51, therefore individual variability in the magnitude of the insulin-mediated suppression of fatty acid mobilization may have also contributed to the variability in insulin resistance among our participants. Our finding that plasma fatty acid concentration during the IVGTT was lower in NORM-Si vs LOW-Si suggests that LOW-Si may have also been more resistant to the anti-lipolytic effects of insulin than NORM-Si. This is in accord with recent findings demonstrating that the magnitude of the insulin-induced suppression in fatty acid mobilization was significantly correlated with insulin-mediated glucose uptake52. Interestingly, despite also demonstrating a significant relationship between post-absorptive fatty acid availability and insulin resistance (similar to our present findings), the authors challenged the notion that fatty acid mobilization in the post-absorptive state was an important contributor to the development of insulin resistance52. We agree that if fatty acid availability remains relatively high despite insulin exposure (e.g., after a meal, during an IVGTT, or hyperinsulinemic clamp) – this certainly could contribute to the development of insulin resistance. However, even though the anti-lipolytic response to insulin appeared to be blunted in LOW-Si vs. NORM Si, plasma fatty acid concentration during the IVGTT was still suppressed to very low levels in LOW-Si (i.e., 60–80% below overnight fasted concentrations (Figure 3)). If the accumulation of lipid intermediates within the muscle cell does indeed play an important role in the development of insulin resistance10,11, these intracellular lipids likely accumulate in large part in the post-absorptive state, when fatty acid availability is highest, and muscle fatty acid oxidation does not increase sufficiently to accommodate the high rate of fatty acid uptake. Therefore, we contend that the exposure to high availability of fatty acids that is often found in the post-absorptive state of many obese adults can have a potent impact on insulin resistance.
Interestingly, we did not find any differences in plasma cytokines known to be involved with systemic inflammation between the NORM-Si and LOW-Si groups. The chronic low-grade inflammation observed in obesity is partly attributed to elevated systemically circulating inflammatory cytokines such as TNF-α and Interleukin 6 (IL-6)53,54. We can possibly attribute these results to our relatively small sample size and the inherent variability typically observed in these circulating cytokines. Alternatively, it may be indicative that the inflammatory response is primarily mediated within the peripheral tissues as opposed to the systemically circulating cytokines. However, it is possible that other cytokines and/or adipokines not measured in this study (i.e. adiponectin, IL-1β, etc.) may be different.
In conclusion, our findings indicate that variability in systemic fatty acid uptake is a key mediator of the observed differences in insulin resistance among sedentary obese adults. More specifically, obese participants who had a relatively low rate of fatty acid uptake appear to be somewhat “protected” against the development of insulin resistance. This lower rate of fatty acid uptake was accompanied by a lower activation of markers for inflammatory pathways within the skeletal muscle, and consequently, higher insulin sensitivity when compared with our participants with relatively high rates of fatty acid uptake. Importantly, the observed variability in insulin resistance among our obese participants could not be attributed to differences in basal fat oxidation, common markers of systemic inflammation (e.g., IL-6, TNF-α, MCP-1), skeletal muscle triacylglycerol accumulation, or cardiorespiratory fitness. It is likely that differences in the regulation of fatty acid storage and release from adipose tissue mediates a large portion of this observed variability in fatty acid availability and uptake. Therefore, it is now very important to identify the factors underlying the relatively low fatty acid availability and uptake among obese individuals who are not insulin resistant (and/or factors underlying the high fatty acid availability among those who are) in order to develop preventative, and/or therapeutic approaches in the treatment of insulin resistance and related diseases.
Acknowledgments
This work was primarily supported by grants from the American Diabetes Association (1-03JF10) and the National institutes of Health (R01DK077966). Additional support was provided by the Michigan Clinical Research Center (2UL1TR000433), and the University of Michigan Nutrition and Obesity Research Center (P30DK089503). We are very thankful to Dr. Alexander Hinko for his assistance with the fatty acid tracer analysis, the nursing staff of the Michigan Clinical Research Center for their clinical support throughout the study, to the Chemistry Core of the Michigan Diabetes Research Center (P30DK020572) for measuring plasma insulin concentration, and finally, we are particularly grateful to the study subjects for their participation in this project.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes care. 2010;33:2692–2696. doi: 10.2337/dc10-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Archives of internal medicine. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 3.Horowitz JF, Coppack SW, Paramore D, Cryer PE, Zhao G, Klein S. Effect of short-term fasting on lipid kinetics in lean and obese women. The American journal of physiology. 1999;276:E278–284. doi: 10.1152/ajpendo.1999.276.2.E278. [DOI] [PubMed] [Google Scholar]
- 4.Roust LR, Jensen MD. Postprandial free fatty acid kinetics are abnormal in upper body obesity. Diabetes. 1993;42:1567–1573. doi: 10.2337/diab.42.11.1567. [DOI] [PubMed] [Google Scholar]
- 5.Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, et al. Mechanism of free fatty acid-induced insulin resistance in humans. The Journal of clinical investigation. 1996;97:2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelley DE, Mokan M, Simoneau JA, Mandarino LJ. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. The Journal of clinical investigation. 1993;92:91–98. doi: 10.1172/JCI116603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bajaj M, Suraamornkul S, Romanelli A, Cline GW, Mandarino LJ, Shulman GI, et al. Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty Acyl-CoAs and insulin action in type 2 diabetic patients. Diabetes. 2005;54:3148–3153. doi: 10.2337/diabetes.54.11.3148. [DOI] [PubMed] [Google Scholar]
- 8.Santomauro AT, Boden G, Silva ME, Rocha DM, Santos RF, Ursich MJ, et al. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes. 1999;48:1836–1841. doi: 10.2337/diabetes.48.9.1836. [DOI] [PubMed] [Google Scholar]
- 9.Worm D, Henriksen JE, Vaag A, Thye-Ronn P, Melander A, Beck-Nielsen H. Pronounced blood glucose-lowering effect of the antilipolytic drug acipimox in noninsulin-dependent diabetes mellitus patients during a 3-day intensified treatment period. The Journal of clinical endocrinology and metabolism. 1994;78:717–721. doi: 10.1210/jcem.78.3.8126147. [DOI] [PubMed] [Google Scholar]
- 10.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 11.Boden G. Obesity and free fatty acids. Endocrinology and metabolism clinics of North America. 2008;37:635–646. viii–ix. doi: 10.1016/j.ecl.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 13.Sriwijitkamol A, Christ-Roberts C, Berria R, Eagan P, Pratipanawatr T, DeFronzo RA, et al. Reduced skeletal muscle inhibitor of kappaB beta content is associated with insulin resistance in subjects with type 2 diabetes: reversal by exercise training. Diabetes. 2006;55:760–767. doi: 10.2337/diabetes.55.03.06.db05-0677. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen MTA, Satoh H, Favelyukis S, Babendure JL, Imamura T, Sbodio JI, et al. JNK and tumor necrosis factor-alpha mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. Journal of Biological Chemistry. 2005;280:35361–35371. doi: 10.1074/jbc.M504611200. [DOI] [PubMed] [Google Scholar]
- 15.Tuvdendorj D, Chandalia M, Batbayar T, Saraf M, Beysen C, Murphy EJ, et al. Altered subcutaneous abdominal adipose tissue lipid synthesis in obese, insulin-resistant humans. American journal of physiology Endocrinology and metabolism. 2013;305:E999–E1006. doi: 10.1152/ajpendo.00194.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girousse A, Tavernier G, Valle C, Moro C, Mejhert N, Dinel AL, et al. Partial inhibition of adipose tissue lipolysis improves glucose metabolism and insulin sensitivity without alteration of fat mass. PLoS biology. 2013;11:e1001485. doi: 10.1371/journal.pbio.1001485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schenk S, Harber MP, Shrivastava CR, Burant CF, Horowitz JF. Improved insulin sensitivity after weight loss and exercise training is mediated by a reduction in plasma fatty acid mobilization, not enhanced oxidative capacity. The Journal of physiology. 2009;587:4949–4961. doi: 10.1113/jphysiol.2009.175489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schenk S, Cook JN, Kaufman AE, Horowitz JF. Postexercise insulin sensitivity is not impaired after an overnight lipid infusion. American journal of physiology Endocrinology and metabolism. 2005;288:E519–525. doi: 10.1152/ajpendo.00401.2004. [DOI] [PubMed] [Google Scholar]
- 19.Patterson BW, Zhao G, Klein S. Improved accuracy and precision of gas chromatography/mass spectrometry measurements for metabolic tracers. Metabolism: clinical and experimental. 1998;47:706–712. doi: 10.1016/s0026-0495(98)90035-x. [DOI] [PubMed] [Google Scholar]
- 20.Newsom SA, Schenk S, Thomas KM, Harber MP, Knuth ND, Goldenberg N, et al. Energy deficit after exercise augments lipid mobilization but does not contribute to the exercise-induced increase in insulin sensitivity. J Appl Physiol. 2010;108:554–560. doi: 10.1152/japplphysiol.01106.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis. Wiley-Liss; 1992. [Google Scholar]
- 22.Frayn KN, Maycock PF. Skeletal muscle triacylglycerol in the rat: methods for sampling and measurement, and studies of biological variability. Journal of lipid research. 1980;21:139–144. [PubMed] [Google Scholar]
- 23.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. Journal of applied physiology. 1983;55:628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 24.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Annals of the New York Academy of Sciences. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 25.Bergman RN, Prager R, Volund A, Olefsky JM. Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. The Journal of clinical investigation. 1987;79:790–800. doi: 10.1172/JCI112886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saad MF, Anderson RL, Laws A, Watanabe RM, Kades WW, Chen YD, et al. A comparison between the minimal model and the glucose clamp in the assessment of insulin sensitivity across the spectrum of glucose tolerance. Insulin Resistance Atherosclerosis Study. Diabetes. 1994;43:1114–1121. doi: 10.2337/diab.43.9.1114. [DOI] [PubMed] [Google Scholar]
- 27.Tai MM. A mathematical model for the determination of total area under glucose tolerance and other metabolic curves. Diabetes care. 1994;17:152–154. doi: 10.2337/diacare.17.2.152. [DOI] [PubMed] [Google Scholar]
- 28.Caumo A, Bergman RN, Cobelli C. Insulin sensitivity from meal tolerance tests in normal subjects: a minimal model index. The Journal of clinical endocrinology and metabolism. 2000;85:4396–4402. doi: 10.1210/jcem.85.11.6982. [DOI] [PubMed] [Google Scholar]
- 29.Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. The Journal of clinical investigation. 2007;117:1690–1698. doi: 10.1172/JCI30566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karin M. The beginning of the end: IkappaB kinase (IKK) and NF-kappaB activation. The Journal of biological chemistry. 1999;274:27339–27342. doi: 10.1074/jbc.274.39.27339. [DOI] [PubMed] [Google Scholar]
- 31.Pajunen P, Kotronen A, Korpi-Hyovalti E, Keinanen-Kiukaanniemi S, Oksa H, Niskanen L, et al. Metabolically healthy and unhealthy obesity phenotypes in the general population: the FIN-D2D Survey. BMC public health. 2011;11:754. doi: 10.1186/1471-2458-11-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55 (Suppl 2):S9–S15. doi: 10.2337/db06-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holloszy JO. “Deficiency” of mitochondria in muscle does not cause insulin resistance. Diabetes. 2013;62:1036–1040. doi: 10.2337/db12-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han DH, Hancock CR, Jung SR, Higashida K, Kim SH, Holloszy JO. Deficiency of the mitochondrial electron transport chain in muscle does not cause insulin resistance. PloS one. 2011;6:e19739. doi: 10.1371/journal.pone.0019739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. The Journal of clinical investigation. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375:2267–2277. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nature medicine. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 38.Gual P, Le Marchand-Brustel Y, Tanti JF. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie. 2005;87:99–109. doi: 10.1016/j.biochi.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 39.Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM. Increased p85/55/50 expression and decreased phosphotidylinositol 3-kinase activity in insulin-resistant human skeletal muscle. Diabetes. 2005;54:2351–2359. doi: 10.2337/diabetes.54.8.2351. [DOI] [PubMed] [Google Scholar]
- 40.Kissebah AH, Vydelingum N, Murray R, Evans DJ, Hartz AJ, Kalkhoff RK, et al. Relation of body fat distribution to metabolic complications of obesity. The Journal of clinical endocrinology and metabolism. 1982;54:254–260. doi: 10.1210/jcem-54-2-254. [DOI] [PubMed] [Google Scholar]
- 41.Bjorntorp P. Abdominal obesity and the development of noninsulin-dependent diabetes mellitus. Diabetes/metabolism reviews. 1988;4:615–622. doi: 10.1002/dmr.5610040607. [DOI] [PubMed] [Google Scholar]
- 42.Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Archives of internal medicine. 2002;162:2074–2079. doi: 10.1001/archinte.162.18.2074. [DOI] [PubMed] [Google Scholar]
- 43.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 44.Goodpaster BH, Krishnaswami S, Harris TB, Katsiaras A, Kritchevsky SB, Simonsick EM, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Archives of internal medicine. 2005;165:777–783. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]
- 45.Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA : the journal of the American Medical Association. 2012;308:1150–1159. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 47.Janssen I, Heymsfield SB, Allison DB, Kotler DP, Ross R. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. The American journal of clinical nutrition. 2002;75:683–688. doi: 10.1093/ajcn/75.4.683. [DOI] [PubMed] [Google Scholar]
- 48.Bolinder J, Kager L, Ostman J, Arner P. Differences at the receptor and postreceptor levels between human omental and subcutaneous adipose tissue in the action of insulin on lipolysis. Diabetes. 1983;32:117–123. doi: 10.2337/diab.32.2.117. [DOI] [PubMed] [Google Scholar]
- 49.Ostman J, Arner P, Engfeldt P, Kager L. Regional differences in the control of lipolysis in human adipose tissue. Metabolism: clinical and experimental. 1979;28:1198–1205. doi: 10.1016/0026-0495(79)90131-8. [DOI] [PubMed] [Google Scholar]
- 50.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. The Journal of clinical investigation. 2004;113:1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nurjhan N, Campbell PJ, Kennedy FP, Miles JM, Gerich JE. Insulin dose-response characteristics for suppression of glycerol release and conversion to glucose in humans. Diabetes. 1986;35:1326–1331. doi: 10.2337/diab.35.12.1326. [DOI] [PubMed] [Google Scholar]
- 52.Magkos F, Fabbrini E, Conte C, Patterson BW, Klein S. Relationship between adipose tissue lipolytic activity and skeletal muscle insulin resistance in nondiabetic women. The Journal of clinical endocrinology and metabolism. 2012;97:E1219–1223. doi: 10.1210/jc.2012-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. American journal of physiology Endocrinology and metabolism. 2001;280:E745–751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 54.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nature reviews Immunology. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]