Abstract
The characteristics of reentrant circuits during short duration ventricular fibrillation (SDVF; 20 s in duration) and the role of Ca++ and rapid-activating delayed rectifier potassium currents during long duration ventricular fibrillation (LDVF; up to 10 min in duration) were investigated using verapamil and sotalol. Activation mapping of the LV epicardium with a 21 × 24 electrode plaque was performed in 12 open-chest pigs. Pigs were given either verapamil (0.136 mg/kg) or sotalol (1.5 mg/kg) and verapamil. Reentry patterns were quantified for SDVF, and, for LDVF, activation patterns were compared with our previously reported control LDVF data. Verapamil significantly increased conduction velocity around the reentrant core by 10% and reduced the reentrant cycle length by 15%, with a net reduction in reentry incidence of 70%. Sotolol had an opposite effect of decreasing the conduction velocity around the core by 6% but increasing the reentrant cycle length by 13%, with a net reduction of reentry incidence of 50%. After 200 s of VF, verapamil significantly slowed wavefront conduction velocity and activation rate compared with control data. Verapamil decreased the incidence of reentry in SDVF by accelerating conduction velocity to increase the likelihood of conduction block, possibly through increased sympathetic tone. The drug slowed activation rate and conduction velocity after 200 s of VF, suggesting that L-type Ca++ channels remain active and may be important in the maintenance of LDVF. Sotalol in addition to verapamil caused no additional antiarrhythmic effect.
Keywords: reentry, ventricular fibrillation, verapamil
whether ventricular fibrillation (VF) consists of multiple wandering wavelets (33) or a single stable mother rotor that generates many daughter wavelets (54), reentry is generally agreed to be the mechanism that maintains short duration VF (SDVF). The ability of a drug to prevent, reduce, or eliminate reentrant circuits is often used to estimate its antifibrillation efficacy (52). There are three conditions that cause an impulse to continue to travel around a pathway to cause re-entry. First, the pathway around the circuit must be sufficiently long, so that by the time the impulse returns to its original position, the tissue will no longer be refractory, thus allowing the impulse to continue around the circuit again. This effect is influenced by heart size. However, during VF, the pathway must not be too long or it will be invaded by other wavefronts. Second, the conduction velocity must be sufficiently slow, and, third, the refractory period of the muscle must be sufficiently short, so that a large enough interval of time will elapse before the impulse returns to the original activated position to allow this tissue to be out of the refractory state and permit the impulse to continue around the circuit. A drug that alters either condition 2 or 3 could decrease the likelihood that the impulse would continue around the circuit and may have an antifibrillatory effect. However, a drug that alters any of these three conditions could also increase the likelihood that the impulse could continue around the circuit and then would be expected to have a proarrhythmia effect.
Likewise, a drug that decreases the wave breaks and myocardial heterogeneity, which is a favorable condition for reentry formation and maintenance, may also be expected to prevent and/or interrupt fibrillation. It has been reported that a decrease in the slope of the action potential duration (APD) restitution curve decreases the incidence of wave breaks (27).
Although not listed as a principal drug to treat VT or VF during clinical practice, verapamil has been effective in stopping electrical storm for some patients when other drugs failed to stop these arrhythmias (10, 29, 48). We hypothesized that 1) verapamil achieves an antifibrillatory effect by altering one or more of those three conditions required to maintain reentry in such a way that it decreases the incidence of reentry during SDVF and that this decreased incidence of reentry caused by verapamil would be prevented by the β-blocker sotalol (1). Verapamil decreases the incidence of reentry during SDVF also by flattening the slope of the restitution curve.
Both Na+ and Ca++ currents are active during SDVF (55). During VF lasting more than 1 min (long duration VF, or LDVF), global ischemia would be expected to decrease the resting membrane potential (14). If sufficiently large, this decrease in resting potential could inactivate Na+ channels so that Ca++ channels become more important in LDVF maintenance. We used verapamil to block the Ca++ channels to reveal whether the Ca++ channels were activated during LDVF, and, if so, the time course of this deactivation by verapamil compared with LDVF without verapamil.
METHODS
Animals were managed in accordance with the American Heart Association guidelines on research animal use (2), and the protocol was approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
Animal preparation.
Fourteen pigs (37 ± 8 kg, means ± SD) were injected intramuscularly with Telazol (4.4 mg/kg), xylazine (2.2 mg/kg), and atropine (0.04 mg/kg) for anesthesia induction. Anesthesia was maintained with isoflurane in 100% oxygen by inhalation. Core body temperature, arterial blood pressure, arterial blood gases, and serum electrolytes were monitored and maintained within normal ranges throughout the study. ECG Lead II was continuously monitored. The heart was exposed through a median sternotomy and supported in a pericardial sling. Although intramural mapping can provide more accurate data during VF, it requires more aggressive and time consuming techniques with multiple plunge needle electrode insertions (30). The reentry incidence calculated from intramural (30) and epicardial (4) mapping techniques are comparable and parallel during the early stage of VF. Thus we applied epidcardial mapping techniques in this study. A plaque containing 504 electrodes (24 × 21) with 2-mm spacing between each electrode was sutured to the posterior lateral LV, with one edge of the plaque adjacent to the posterior descending artery. A stainless steel wire was attached to the left leg as the return electrode for the unipolar mapping electrodes. The mapping electrodes were made from Teflon-coated silver wires with a diameter of 0.013 inches. The diameter of the tip of each mapping electrode was ∼0.2 mm. A catheter (SPRINT 6942-65 cm; Medtronic, Minneapolis, MN) containing a 5-cm-long RV coil (479 mm2 surface area) and a 8-cm-long SVC coil (766 mm2 surface area) defibrillation electrode was inserted into the right jugular vein. One electrode was in the right ventricular apex, and the other was near the junction of the superior vena cava and the right atrium. To pace the heart, a pair of barbed silver wire electrodes, insulated except at the tips, was inserted into the LV epicardium via 21G needles. The electrodes were 0.2–0.5 cm away from the edge of the mapping plaque. After LDVF was recorded for 10 min, the heart was continuously monitored until all electrical activity ceased and the animal was declared dead. The animals were divided into two groups. Group 1 (n = 8) was given verapamil, and Group 2 (n = 6) was given sotalol and verapamil combined.
Pacing protocol.
The restitution relationship was determined during pacing. Pacing stimuli were delivered at twice diastolic threshold to the pacing wires described above. Trains of 30 S1 stimuli were repeated at the intervals described below. Depending on the intrinsic heart rate, the pacing cycle length (PCL) was initially 450 or 400 ms and was decreased to 300 ms by 50 ms steps. The PCL was decreased by 10-ms steps after 300 ms to an interval that induced VF or lost 1:1 capture, which was defined as the target interval. To capture during shorter PCLs, the S1-S1 interval was initially 300 ms and then decreased in 10-ms steps to the target interval, and thereafter was kept at the target interval for 30 beats.
VF induction and rescue defibrillation.
We have previously shown that there are no significant differences in VF parameters among multiple short VF episodes (22). Four VF episodes (2 before and 2 after verapamil administration) were induced by a 9 V battery applied briefly to the right ventricle. The first three VF episodes were recorded for 20 s as a SDVF episode before it was halted by a 400–600 V biphasic shock (6/4 ms) delivered from a defibrillator (Ventritex HVSO2; St. Jude Medical) via the catheter electrodes. The last VF episode was allowed to continue for at least 10 min (LDVF), and the animal was not resuscitated. We compared the LDVF data with our previously published LDVF data (control group) in which no drug was given in six animals (4). The first 20 s VF data during this LDVF episode served as the fourth SDVF episode.
Verapamil administration (Group 1).
After the baseline pacing protocol and the induction of two episodes of 20 s of SDVF, verapamil at 0.136 mg/kg body wt was injected intravenously over 5 min. The pacing protocol, another two VF episodes (a SDVF and a LDVF), was repeated beginning 5 min postinjection.
Sotalol and verapamil administration (Group 2).
After two baseline episodes of VF, sotalol was infused intravenously at a dose of 1.5 mg/kg to achieve class III anti-arrhythmic action. Two more episodes of VF induction were repeated. After the sotalol measurements, a dose of verapamil at 0.136 mg/kg was injected over 5 min and all the same measurements, except for restitution, were repeated at 15 min post-injection.
Activation recovery interval measurements.
The activation recovery interval (ARI) was determined as an estimate of the APD (18) and, hence, the refractory period. A 15-point quadratic differentiating filter was applied to determine the first derivatives of the extracellular potentials and to minimize interference from noise. Activation time in the unipolar electrograms was defined as the steepest downslope of the QRS complex and recovery time as the fastest upslope of the T wave (6, 18, 37). The ARI was defined as the interval between the activation time and the recovery time. Less than 5% of unipolar recordings were discarded from analysis because of electrograms in which the fastest upslope of the T wave was ambiguous.
Restitution properties.
An exponential function, ARI = a + b × e−DI/c, was used to fit a restitution curve for each mapping electrode (3, 38, 51). DI (diastolic interval) is the difference between the pacing cycle length (PCL) and the last ARI, and a, b, and c are model parameters that are fit by a least-square procedure, which halted when the difference in calculated ARI between the last two iterations converged to <10−8. The restitution curves were calculated based on the measurement of ARI from the unipolar electrogram. The T waves on some electrograms were indistinct, which made the ARI measurement difficult on those recordings; hence only those restitution curves for which the square of the correlation coefficient (R2) was >0.8 were included in the analysis. Restitution slopes were calculated as the first derivatives of the exponential function at each DI, and the maximum slope was identified.
Quantitative analysis of VF activation.
We applied quantitative wavefront isolation methods to compute five reentry parameters for each 5-s interval and five other quantitative VF parameters for each 1-s interval of VF during each of the four SDVF episodes and one LDVF episode. Quantitative analysis of VF activation patterns was performed on a Linux system computer using algorithms discussed in detail elsewhere (43, 44, 45). We defined the effects of verapamil on five reentry parameters as 1) incidence of reentry, the fraction of components (46) that were reentrant; 2) reentrant core size (the region enclosed by the reentrant path, which is excitable but not excited by the wave) (43); 3) reentrant core perimeter; 4) the cycle length of reentry, the time it takes the reentrant wavefront to travel around the core (43); and 5) reentrant speed, the reentrant core perimeter divided by the reentrant cycle length.
The five quantitative VF parameters were as follows: 1) number of wavefronts; 2) wavefront fractionation incidence; 3) wavefront multiplicity; 4) activation rate; and 5) wavefront conduction velocity.
A previous study demonstrated that the effective refractory period determined with the extrastimulus method at a basic cycle length of 300 ms correlated well with mean VF cycle length measured at the same sites (35, 36). Our (21) and other (36) studies did not show a diastolic interval between successive action potentials during early VF. Thus the mean VF activation cycle length from 504 electrodes was used as an index of effective refractory period during early VF. VF activation cycle length was defined as the reciprocal of the activation rate and was determined for each animal.
Statistical analysis.
Results are expressed as means ± SD. The mean of the nonreentry SDVF variables for the two SDVF episodes before verapamil administration for all 1-s segments were compared with the mean of these same two SDVF episodes after verapamil administration using ANOVA with repeated measures. The mean of the reentry SDVF variable for the two SDVF episodes before verapamil for all 5-s segments were compared with the mean of these same two SDVF episodes after verapamil (ANOVA). Dispersion of refractoriness was quantified using the standard deviation (SD) of the ARIs and the coefficient of variation (the SD divided by the mean ARI, in percentage) across all of the plaque electrodes. The number of wavefronts, propagation velocity, and activation rate of the LDVF in 1-s segments between control data from a previous study (4) and the verapamil group were compared with Student's t-test before and after 200 s, where parameters of the two groups crossed each other. A value of P < 0.05 was considered statistically significant.
RESULTS
Two animals developed hypotension during the rapid pacing phase of the restitution measurements after verapamil was given in Group 1. Thus six animals completed the entire protocol of Group 1. Verapamil significantly lowered the systolic blood pressure from 110 ± 17 mmHg to 80 ± 11 mmHg in these six animals (P < 0.05). Verapamil significantly increased the heart rate from 77 ± 10 beats/min to 96 ± 12 beats/min in these six animals (P < 0.05).
Effects of verapamil on electrical restitution properties.
Of the 504 electrodes in the plaque, 368 ± 82 had restitution curves that fit an exponential function with an R2 greater than 0.8 and were therefore used for analysis. After administration of verapamil, the restitution curves were flattened with the mean maximal slope (0.84 ± 0.32) significantly reduced 24% compared with baseline (1.10 ± 0.15; Fig. 1).
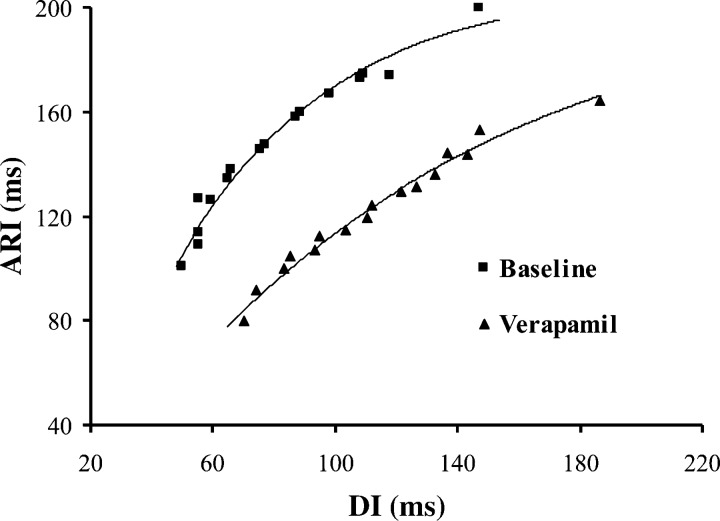
Fig. 1.
The restitution curves from 1 of the 6 animals at the same electrode at baseline (■) and after verapamil (▲). The maximum slope of the restitution curve is 1.10 at baseline and 0.84 after verapamil administration. ARI, activation recovery intervals; DI, diastolic interval.
Dispersion of refractoriness.
To estimate the dispersion of refractoriness, ARIs at each recording electrode were compared at four PCLs: 200 ms, 250 ms, 300 ms, and 350 ms (Fig. 2). When compared with baseline, verapamil significantly decreased the ARI at each PCL (Fig. 2A). Although the drug significantly decreased the spatial SD of the ARIs from 12.0 ms to 7.7 ms (Fig. 2B) and the coefficient of variation from 5.8% to 4.3% at a PCL of 350 ms (Fig. 2C), it significantly increased the SD from 5.1 ms to 6.2 ms (Fig. 2B) and the coefficient of variation from 3.8% to 5.2% (Fig. 2C) when the PCL was reduced to 200 ms.
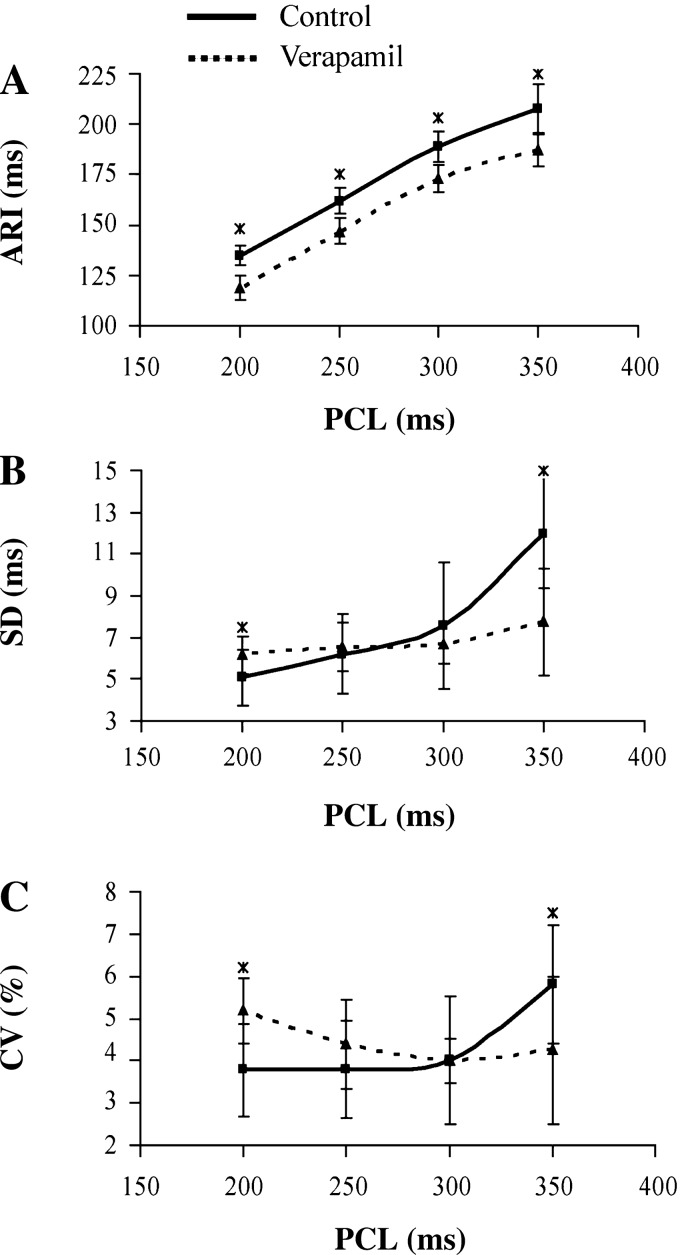
Fig. 2.
ARIs and dispersion of refractoriness at the pacing cycle lengths (PCLs) of 350, 300, 250, and 200 ms at baseline (■) and after verapamil (▲). At each PCL, verapamil significantly reduced the ARI compared with baseline (A). Verapamil significantly decreased the SD (B) and the coefficient of variation (CV; C) of the ARIs at a PCL of 350 ms, but significantly increased them at a PCL of 200 ms. *P < 0.05.
Effect of verapamil on reentry.
Quantitative data from six animals of all 5-s intervals for reentry parameters during the first 20 s of SDVF are pooled in Table 1. Verapamil markedly reduced the incidence of reentry by 70% and significantly reduced the reentry cycle length by 15% compared with baseline during SDVF (Table 1). However, it did not significantly change the core size and perimeter of the reentrant circuits. In the examples shown in Figs. 3 and 4, there was a complete reentrant circuit at baseline (Fig. 3A) and an incomplete circuit after verapamil (Fig. 4A). The core area of the reentrant circuit was 40 mm2 in Fig. 3A and 44 mm2 in Fig. 4A. The cycle length for the wavefront to complete the circuit was 105 ms in Fig. 3A. The time for the wavefront to propagate back to near the previously excited area was 70 ms in Fig. 4A. The previously excited area may still have been in its refractory period so that the wavefront could not pass through this area and was blocked. The amplitude of the unipolar recordings within the cores (I in Figs. 3B and 4B) was markedly decreased when reentry was present, less decreased near the perimeter of the core (II in Figs. 3B and 4B), and normal at a distance from the core (III in Figs. 3B and 4B).
Table 1.
Effects of verapamil on characteristics of reentry during 20-s VF episodes
| Baseline | Verapamil | Percent Change | P Value | |
|---|---|---|---|---|
| Reentry incidence, % | 3.3 ± 2.1 | 1.0 ± 0.9 | 70 ± 11↓ | 0.01 |
| Reentrant core size, mm2 | 83 ± 49 | 77 ± 50 | 7 ± 8↓ | 0.22 |
| Reentrant perimeter, mm | 47 ± 15 | 43 ± 17 | 9 ± 17↓ | 0.25 |
| Reentrant cycle length, ms | 82 ± 10 | 70 ± 9.6 | 15 ± 4↓ | <0.001 |
| Reentrant speed, m/s | 0.51 ± 0.06 | 0.56 ± 0.04 | 10 ± 2↑ | <0.001 |
Values are means ± SD. VF, ventricular fibrillation.
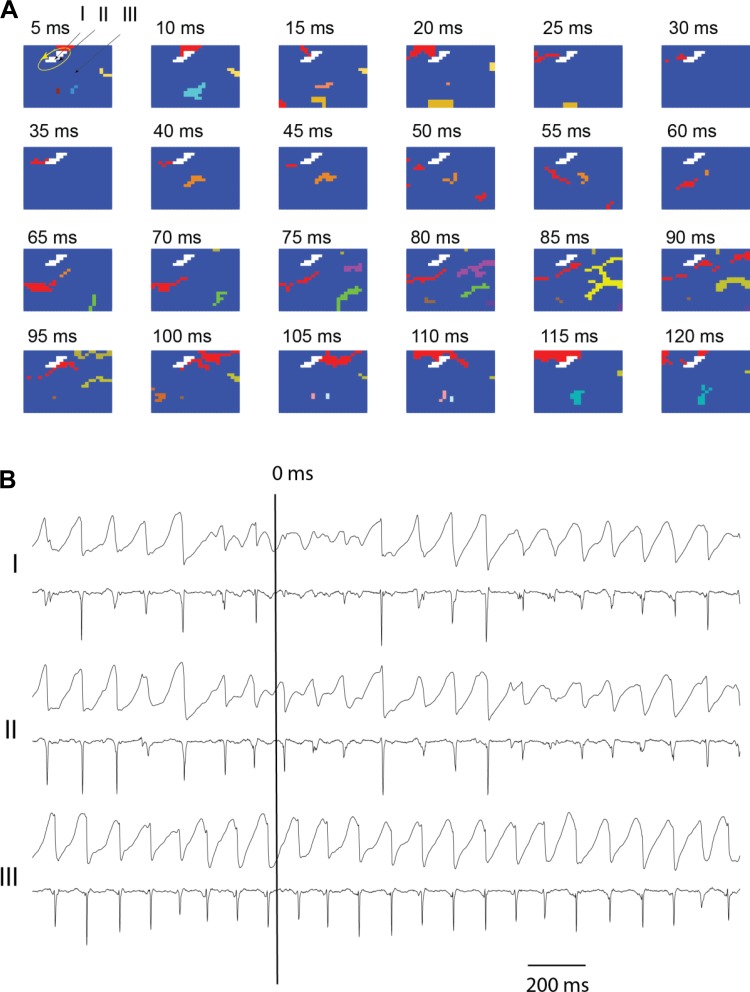
Fig. 3.
Snapshots of activation of short duration ventricular fibrillation (SDVF) in 1 animal at baseline (A) 5 s after the initiation of SDVF. A: activation sequences in which each colored pixel represents an electrode site at which the rate of voltage change (dV/dt) was less than or equal to the activation threshold of −0.5 V/s sometime during the 5-ms interval represented by each frame. The numbers above each frame represent the time of onset of the 5-ms interval with time 0 ms indicated by a long vertical line in B. Each color indicates an individual wavefront, except for red, which indicates all wavefronts that form a reentrant circuit, and white, which indicates the core of the reentrant circuit. Recordings from sites I, II, and III are shown in B, with I within the reentry core, II at the periphery of the core, and III at a site distant from the core. In all 3 cases, the top tracing is the unipolar recording and the bottom tracing is its temporal derivative. See the article for additional explanation.
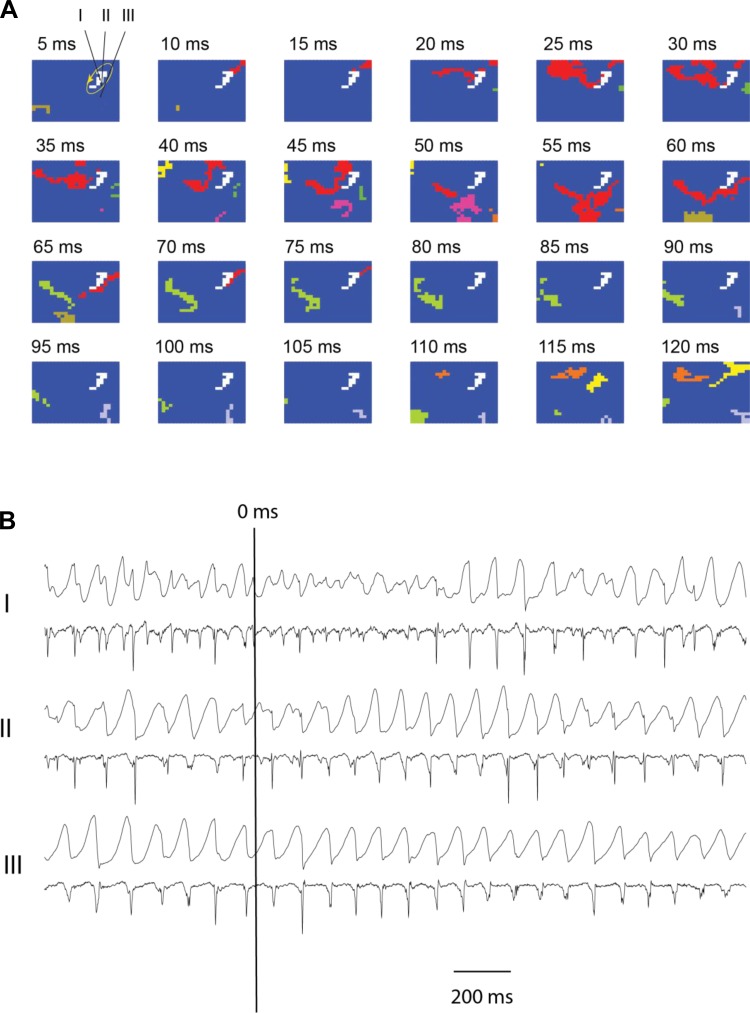
Fig. 4.
Snapshots of activation of SDVF in 1 animal after verapamil (A and B) 5 s after the initiation of SDVF. A: activation sequences. B: 3 unipolar recordings as described in Fig. 3.
Quantitative analysis of SDVF activation patterns.
Quantitative data from six animals of all 1-s intervals for other VF parameters during the first 20 s of SDVF are pooled in Table 2.
Table 2.
Effects of verapamil on activation patterns during 20-s VF episodes
| Baseline | Verapamil | Percent Change | P Value | |
|---|---|---|---|---|
| Number of wavefronts | 72 ± 24 | 75 ± 31 | 4 ± 0.05↑ | 0.07 |
| Fractionation incidence | 0.14 ± 0.05 | 0.12 ± 0.05 | 14 ± 7↓ | 0.009 |
| Multiplicity | 11.3 ± 5.4 | 15.2 ± 6.4 | 35 ± 14↑ | <0.001 |
| Activation rate, b/s | 10 ± 0.9 | 10.4 ± 1.3 | 4 ± 2.6↑ | 0.043 |
| Conduction velocity, m/s | 0.44 ± 0.09 | 0.49 ± 0.09 | 11 ± 1.2↑ | <0.001 |
Values are means ± SD.
Although verapamil did not significantly increase the number of wavefronts during the first 20 s of SDVF, it significantly reduced the incidence of wavefront fractionation by 14% (Table 2).
When compared with baseline, verapamil significantly increased multiplicity by 35%. Verapamil significantly increased the SDVF activation rate by 4% compared with baseline (Table 2). For example, in Fig. 4 the mean SDVF cycle length before verapamil was 109 ms (Fig. 4B) and 100 ms after verapamil (Fig. 4D). When compared with baseline, verapamil significantly increased conduction velocity by 11%.
Evolution of activation patterns by verapamil in LDVF.
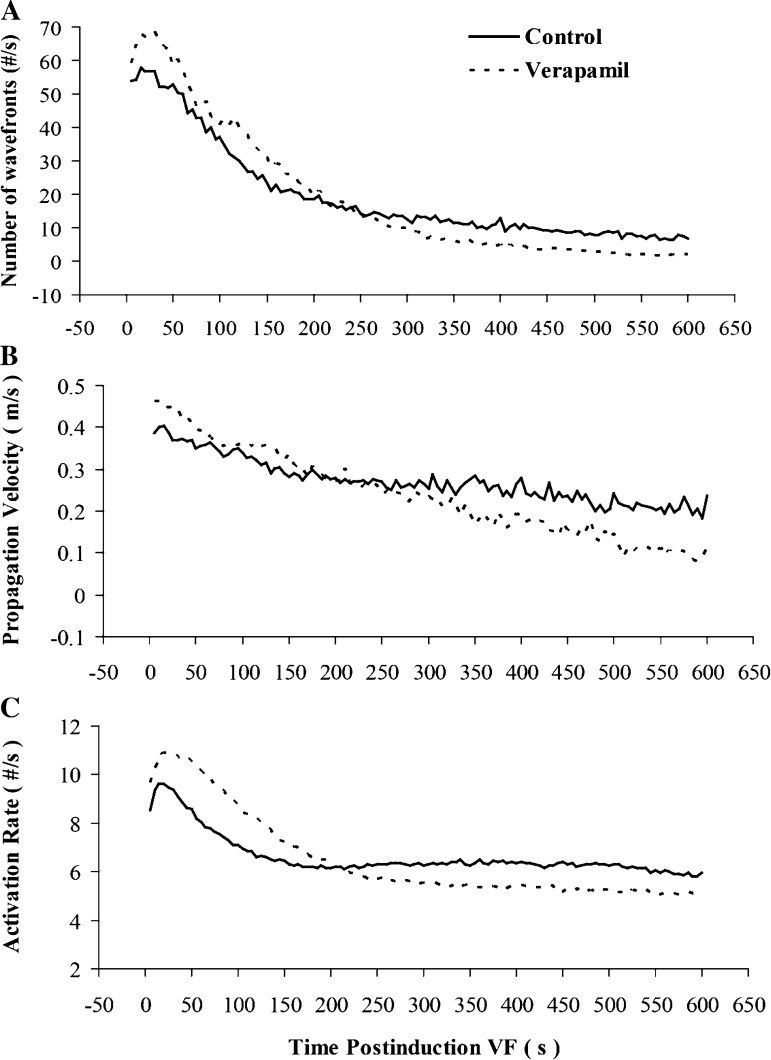
When compared with the control data, the number of wavefronts, the epicardial conduction velocity, and the activation rate were all significantly increased for the first 200 s of VF in the verapamil group (Fig. 5). From 200 s to the end of analysis at 10 min of LDVF, all three of these variables were significantly decreased after verapamil compared with the control animals.
Fig. 5.
A: evolution of the number of wavefronts during 600 s of ventricular fibrillation (VF). When compared with those of the control group, wavefronts were significantly increased from the onset of VF to 200 s and were reduced after 200 s of VF in the verapamil group. B: evolution of wavefront propagation velocity. C: activation rate during 600 s of VF. When compared with those of the control, wavefronts propagated significantly more slowly, and the activation rate was significantly slower in pigs after 200 s treated with verapamil.
Effect of sotalol and verapamil on reentry.
In Group 2, sotalol significantly reduced the incidence of reentry by 50%. Although verapamil reduced the incidence of reentry in Group 1 by 70%, adding verapamil to sotalol did not further alter reentry incidence compared with sotalol alone (Table 3). Adding verapamil to sotalol did not significantly affect reentrant core size, perimeter, cycle length, or speed (Table 3).
Table 3.
Effects of sotalol and verapamil on characteristics of reentry during 20-s VF episodes
| Sotalol |
Sotalol + Verapamil |
Sotalol vs. Sotalol + Verapamil |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Percent Change | P Value | Percent Change | P Value | Percent Change | P Value | |||
| Reentry incidence, % | 3.8 ± 1.9 | 1.9 ± 1.5 | 50↓ | <0.01 | 1.8 ± 1.1 | 53↓ | <0.01 | 5↓ | NS |
| Core size, mm2 | 81 ± 40 | 84 ± 34 | 4↑ | NS | 82 ± 38 | 1↑ | NS | 2↓ | NS |
| Reentrant perimeter, mm | 48 + 13 | 49 + 15 | 2↑ | NS | 47 ± 12 | 2↓ | NS | 4↓ | NS |
| Reentrant cycle length, ms | 83 ± 16 | 93 ± 12 | 13↑ | <0.01 | 86 ± 7 | 4↓ | NS | 8↓ | NS |
| Reentrant speed, m/s | 0.49 ± 0.08 | 0.46 ± 0.05 | 6↓ | <0.05 | 0.48 ± 0.06 | 2↓ | NS | 4↑ | NS |
Values are means ± SD. NS, not significant.
DISCUSSION
Our data indicate the following: 1) verapamil markedly decreased the incidence of reentry during SDVF; 2) verapamil increased conduction velocity without changing reentrant core size and perimeter during SDVF; and 3) verapamil significantly reduced the number of wavefronts, slowed the activation rate, and decreased the conduction velocity after 200 s of LDVF, suggesting that although Na+ channels are active during the early stages of VF, their activity decreases after 200 s of VF, so that Ca++ channels become important for the maintenance of LDVF; and 4) the effects of solalol and verapamil combined are not additive.
The effects of verapamil on reentry during SDVF.
As a Class IV antiarrhythmic drug, verapamil is mainly used clinically to treat supraventricular arrhythmias. The principal electrophysiologic effect of verapamil is inhibition of the slow inward calcium current. Verapamil stops supraventricular tachycardia by prolonging the activation propagation time and tissue refractoriness in the sinus and AV nodes, where the slow inward calcium current dominates conduction activity (7, 31). However, the ability of verapamil to convert VF to VT is not based on the same mechanism because verapamil does not change conduction velocity and refractoriness during slow and rapid heart rates in isolated atria and ventricles (11).
In pigs, we found that verapamil decreased the ARI during pacing consistent with a decrease in the refractory period. We also found that verapamil increased the SDVF activation rate by 4%, suggesting a decreased refractory period also during SDVF in the in vivo swine heart. The shortening of refractoriness would allow earlier restimulation of cardiac tissue, which would be expected to increase the incidence of reentry and, hence, increase the likelihood that VF would be sustained.
However, verapamil also increased wavefront conduction velocity by 11%, to a greater extent than the 4% shortening of refractoriness during SDVF. So the net action of the effect of verapamil was to increase the wavelength, making reentry less likely.
Verapamil may have affected conduction velocity in two ways. First, verapamil prevented slowing of conduction velocity caused by hypoxia (13). During VF, the myocardial cells rapidly become hypoxic due to ischemia. The APD shortens (6) progressively and conduction velocity decreases appreciably (53). However, verapamil has been shown to have a protective effect against conduction delays in hypoxic conditions (13). Second, sympathetic tone may have been affected by verapamil. The hemodynamic changes in pigs due to verapamil are more pronounced than in humans and some other species (28). After verapamil infusion, the systolic pressure drops significantly in pigs, which causes sympathetic activation including norepinephrine release from sympathetic nerve endings to the heart (24). The shortened ARIs at all pacing rates after verapamil and the increased heart rate might have been caused by increased sympathetic activation (32). If increased norepinephrine (25) with verapamil was still present during the first 20 s of SDVF before the effect of the hypotension caused by VF had fully occurred, then it may have also been responsible for the increased conduction velocity and the decreased incidence of reentry during SDVF in pigs. The finding that verapamil did not further decrease the incidence of reentry when the β-blocker sotalol had previously been given supports this possibility.
Effect of verapamil on restitution properties.
Although the kinetics of electrical restitution are not the only determining factor of VF initiation and maintenance, especially measured during rapid pacing (5, 12, 21), many studies have shown that verapamil prevents VF initiation and converts VF to VT by flattening the restitution curve (15, 41, 47). It is believed that a restitution curve with slope >1 amplifies APD alternans, which may lead to wavebreak, whereas a slope <1 dampens alternans and decreases the incidence of reentry. In addition, verapamil decreases ARI during both pacing and VF, because increased ARI (APD) can prevent wave breaks and might therefore convert VF to VT by eliminating fibrillatory wave breaks (47). The reduction of the fractionation incidence and the increase of multiplicity by verapamil in our study supports this possibility.
Rate dependence of verapamil on dispersion of refractoriness.
Rate dependent effects of verapamil have been documented previously in experimental and clinical studies on atrioventricular nodal conduction (9, 49) and atrial refractoriness (17), but not on the dispersion of ventricular refractoriness and VF activation patterns. Verapamil blocks both inward calcium currents and the rapid component of the delayed rectifier potassium current (Ikr) (8). Although an Ikr blocker could reduce the dispersion of refractoriness (39), verapamil also has reverse use-dependent actions, which reduce the efficacy of the Ikr block at a rapid activation rate (34, 50). Moreover, blockade of calcium currents by verapamil could be enhanced at rapid heart rates (8) and increase the dispersion of refractoriness (40). This mechanism could explain why verapamil reduced the dispersion of refractoriness at a PCL of 350 ms yet increased the dispersion of refractoriness at a PCL of 200 ms (Figs. 2 and 3).
Alteration of LDVF activation patterns by verapamil.
The membrane potential immediately before activation (the takeoff potential) is an important determinant of Na+ channel opening (23). The takeoff potential does not return to −85 mV during VF because of the rapid activation rate during early stage VF and because of severe ischemia during late stage VF (27). During early VF, the Na+ channels were still active (55) because the takeoff potential was still lower than the threshold needed to activate Na+ channels. As VF continued, global ischemia became increasingly severe and the takeoff potential became elevated to a level close to or above the Na+ channels activation threshold and Na+ channels were inactivated. Thus LDVF activation should be maintained by slow action potentials dominated by Ca++ currents (1). However, it is not clear when the excitable cardiac cells convert from fast Na+ channel to slow Ca++ channel as VF progresses.
The myocardium activated at a much slower rate and wavefronts propagated more slowly after 200 s of VF induction in the verapamil group compared with the control group (Fig. 5). The results suggest that calcium currents play a larger role in LDVF than in SDVF.
Clinical relevance.
Although we did not provide direct evidence that verapamil could prevent VF initiation or terminate VF in this study, we found that verapamil decreased the number of wavefronts, propagation velocity, and activation rate during LDVF. Those changes alter VF activation toward more organized patterns, which might allow a lower defibrillation threshold to terminate VF or might reduce the incidence of refibrillation after the shock. More studies are needed to confirm these hypotheses. Because of its negative hemodynamic and inotropic effects, verapamil is not a preferred drug to treat ventricular arrhythmias, especially in heart failure patients. However, in hemodynamically normal patients with recurrent VF, when other antiarrhythmic drugs fail to stop malignant ventricular arrhythmias, verapamil might be worth consideration and used with caution to treat the arrhythmia or manipulate the reentrant circuits so they could be more easy terminated by other antiarrhythmic drugs. However, this study also indicates that there may be no additional benefit to using verapamil in combination with the IKr blocker sotalol.
Study limitations.
One limitation of our study is that we only observed the effects of low-dose verapamil on VF. The effect of verapamil on the level of L-type Ca++ block achieved in vivo is dose responsive (16). Because the hemodynamic response of pigs is very sensitive to verapamil, we could not investigate the effects of verapamil at higher doses. The antifibrillatory effect of verapamil might be more significant at a higher dose of verapamil. Another limitation is that refractoriness was estimated from the activation rate during SDVF and the conduction velocity was only estimated during SDVF and LDVF using our previously reported methods (19). A constant pacing and S1-S2 protocol might provide additional information for evaluation of the efficacy of verapamil. As we have reported, short diastolic intervals are present during early VF (21). However, in that study we measured the diastolic interval based on the APD at 60% repolarization. The real diastolic interval may be smaller or even close to nonexistent if we measured diastolic intervals from APD at 90% repolarization.
GRANTS
This work was supported in part by National Natural Science Foundation of China Grant 81000081, Shanghai Science and Technology Committee Grant 13140903702, an American Heart Association Scientist Development Grant (to J. Huang), and National Heart, Lung, and Blood Institute Research Grants HL-28429, HL-66256, and HL-85370.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Q.J., D.J.D., J.M.R., R.E.I., and J.H. conception and design of research; Q.J., D.J.D., L.L., and J.H. performed experiments; Q.J., D.J.D., L.L., J.M.R., and J.H. analyzed data; Q.J., L.L., J.M.R., R.E.I., and J.H. interpreted results of experiments; Q.J., L.L., and J.H. prepared Figs.; Q.J. drafted manuscript; Q.J., D.J.D., J.M.R., R.E.I., and J.H. edited and revised manuscript; Q.J., D.J.D., L.L., J.M.R., R.E.I., and J.H. approved final version of manuscript.
REFERENCES
- 1.Akiyama T. Intracellular recording of in situ ventricular cells during ventricular fibrillation. Am J Physiol Heart Circ Physiol 240: H465–H471, 1981 [DOI] [PubMed] [Google Scholar]
- 2.Anon. Position of the American Heart Association on Research Animal Use. Circulation 71: 849A–850A, 1985 [PubMed] [Google Scholar]
- 3.Banville I, Gray RA. Effect of action potential duration and conduction velocity restitution and their spatial dispersion on alternans and the stability of arrhythmias. J Cardiovasc Electrophysiol 13: 1141–1149, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Cheng KA, Dosdall DJ, Li L, Rogers JM, Ideker RE, Huang J. Evolution of activation patterns during long-duration ventricular fibrillation in pigs. Am J Physiol Heart Circ Physiol 302: H992–H1002, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conrath CE, Opthof T. Ventricular repolarization: an overview of (patho)physiology, sympathetic effects and genetic aspects. Prog Biophys Mol Biol 92: 269–307, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Coronel R, de Bakker JM, Wilms-Schopman FJ, Opthof T, Linnenbank AC, Belterman CN, Janse MJ. Monophasic action potentials and activation recovery intervals as measures of ventricular action potential duration: experimental evidence to resolve some controversies. Heart Rhythm 3: 1043–1050, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Duridanova DB, Gagov HS, Boev KK. Two types of Ca2+-channels in cells from the circular layer of guinea-pig ileum. Gen Physiol Biophys 12: 325–338, 1993 [PubMed] [Google Scholar]
- 8.Ehara T, Daufmann R. The voltage- and time-dependent effects of (-)-verapamil on the slow inward current in isolated cat ventricular myocardium. J Pharmacol Exp Ther 207: 49–55, 1978 [PubMed] [Google Scholar]
- 9.Ellenbogen KA, German LD, O′Callaghan WG, Colavita PG, Marchese AC, Gilbert MR, Strauss HC. Frequency-dependent effects of verapamil on atrioventricular nodal conduction in man. Circulation 72: 344–352, 1985 [DOI] [PubMed] [Google Scholar]
- 10.Erkapic D, Neumann T, Schmitt J, Sperzel J, Berkowitsch A, Kuniss M, Hamm CW, Pitschner HF. Electrical storm in a patient with arrhythmogenic right ventricular cardiomyopathy and SCN5A mutation. EP Europace 8: 884–887, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Fedorov VV, Glukhov AV, Sharifov OF, Beloshapko GG, Iushmanova AV, Rozenshtraukh LV. [Effects of verapamil on atrial fibrillation spontaneous initiation in the intact canine heart.] Kardiologiia 43: 55–70, 2003 [PubMed] [Google Scholar]
- 12.Franz MR. The electrical restitution curve revisited: steep or flat slope–which is better? J Cardiovasc Electrophysiol 14: S140–S147, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Furuta T, Kodama I, Shimizu T, Toyama J, Yamada K. Effects of hypoxia on conduction velocity of ventricular muscle. Jpn Heart J 24: 417–425, 1983 [DOI] [PubMed] [Google Scholar]
- 14.Ganitkevich V, Reil S, Schwethelm B, Schroeter T, Benndorf K. Dynamic responses of single cardiomyocytes to graded ischemia studied by oxygen clamp in on-chip picochambers. Circ Res 99: 165–171, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Garfinkel A, Kim YH, Voroshilovsky O, Qu Z, Kil JR, Lee MH, Karagueuzian HS, Weiss JN, Chen PS. Preventing ventricular fibrillation by flattening cardiac restitution. Proc Natl Acad Sci USA 97: 6061–6066, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hara K, Saito Y, Kirihara Y, Sakura S, Kosaka Y. Antinociceptive effects of intrathecal L-type calcium channel blockers on visceral and somatic stimuli in the rat. Anesth Analg 87: 382–387, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Hassan SA, Oral H, Scharf C, Chugh A, Pelosi F, Knight BP, Strickberger SA, Morady F. Rate-dependent effect of verapamil on atrial refractoriness. J Am Coll Cardiol 41: 446–451, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Haws CW, Lux RL. Correlation between in vivo transmembrane action potential durations and activation-recovery intervals from electrograms. Effects of interventions that alter repolarization time. Circulation 81: 281–288, 1990 [DOI] [PubMed] [Google Scholar]
- 19.Huang J, Dosdall DJ, Cheng KA, Li L, Rogers JM, Ideker RE. The importance of Purkinje activation in long duration ventricular fibrillation. J Am Heart Assoc 3: e000495, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang J, Zhou X, Smith WM, Ideker RE. Restitution properties during ventricular fibrillation in the in situ swine heart. Circulation 110: 3161–3167, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Jin Q, Chen X, Smith WM, Ideker RE, Huang J. Effects of procainamide and sotalol on restitution properties, dispersion of refractoriness, and ventricular fibrillation activation patterns in pigs. J Cardiovasc Electrophysiol 19: 1090–1097, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz A. The cardiac action potential. In: Physiology of the Heart, edited by Katz A. Philadelphia: Lippincott Williams & Wilkins, 2000, p. 478 [Google Scholar]
- 24.Katz A. Neurohumoral responses and the hemodynamic defense reaction. In: Physiology of the Heart, edited by Katz A. Philadelphia: Lippincott Williams & Wilkins, 2000, p. 287 [Google Scholar]
- 25.Killingsworth CR, Wei CC, Dell′Italia LJ, Ardell JL, Kingsley MA, Smith WM, Ideker RE, Walcott GP. Short-acting beta-adrenergic antagonist esmolol given at reperfusion improves survival after prolonged ventricular fibrillation. Circulation 109: 2469–2474, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Koller ML, Riccio ML, Gilmour RF, Jr. Dynamic restitution of action potential duration during electrical alternans and ventricular fibrillation. Am J Physiol Heart Circ Physiol 275: H1635–H1642, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Landmark K, Amlie JP. A study of the verapamil-induced changes in conductivity and refractoriness and monophasic action potentials of the dog heart in situ. Eur J Cardiol 4: 419–427, 1976 [PubMed] [Google Scholar]
- 29.Leenhardt A, Glaser E, Burguera M, Nurnberg M, Maison-Blanche P, Coumel P. Short-coupled variant of torsade de pointes. A new electrocardiographic entity in the spectrum of idiopathic ventricular tachyarrhythmias. Circulation 89: 206–215, 1994 [DOI] [PubMed] [Google Scholar]
- 30.Li L, Jin Q, Dosdall DJ, Huang J, Pogwizd SM, Ideker RE. Activation becomes highly organized during long-duration ventricular fibrillation in canine hearts. Am J Physiol Heart Circ Physiol 298: H2046–H2053, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonald TF. The slow inward calcium current in the heart. Annu Rev Physiol 44: 425–434, 1982 [DOI] [PubMed] [Google Scholar]
- 32.Millar CK, Kralios FA, Lux RL. Correlation between refractory periods and activation-recovery intervals from electrograms: effects of rate and adrenergic interventions. Circulation 72: 1372–1379, 1985 [DOI] [PubMed] [Google Scholar]
- 33.Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. Am Heart J 67: 200–220, 1964 [DOI] [PubMed] [Google Scholar]
- 34.Nattel S. The molecular and ionic specificity of antiarrhythmic drug actions. J Cardiovasc Electrophysiol 10: 272–282, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Opthof T, Dekker LR, Coronel R, Vermeulen JT, van Capelle FJ, Janse MJ. Interaction of sympathetic and parasympathetic nervous system on ventricular refractoriness assessed by local fibrillation intervals in the canine heart. Cardiovascular Research 27: 753–759, 1993 [DOI] [PubMed] [Google Scholar]
- 36.Opthof T, Ramdat Misier AR, Coronel R, Vermeulen JT, Verberne HJ, Frank RGJ, Moulijn AC, van Capelle FJL, Janse MJ. Dispersion of refractoriness in canine ventricular myocardium: effects of sympathetic stimulation. Circ Res 68: 1204–1215, 1991 [DOI] [PubMed] [Google Scholar]
- 37.Potse M, Vinet A, Opthof T, Coronel R. Validation of a simple model for the morphology of the T wave in unipolar electrograms. Am J Physiol Heart Circ Physiol 297: H792–H801, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Qin H, Huang J, Rogers JM, Walcott GP, Rollins DL, Smith WM, Ideker RE. Mechanisms for the maintenance of ventricular fibrillation: the nonuniform dispersion of refractoriness, restitution properties, or anatomic heterogeneities? J Cardiovasc Electrophysiol 16: 888–897, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Rahme MM, Ungab GA, Wadhwa M, Al-Kandari F, Yao B, Gupta A, Lee K, Kim HY, Feld GK. Electrophysiologic and antiarrhythmic effects of the new class III antiarrhythmic drug KCB-328 in experimental canine atrial flutter. J Cardiovasc Pharmacol Ther 6: 297–306, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Ramanna H, Elvan A, Wittkampf FH, de Bakker JM, Hauer RN, Robles de Medina EO. Increased dispersion and shortened refractoriness caused by verapamil in chronic atrial fibrillation. J Am Coll Cardiol 37: 1403–1407, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Riccio ML, Koller ML, Gilmour RF, Jr. Electrical restitution and spatiotemporal organization during ventricular fibrillation. Circ Res 84: 955–963, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Rogers JM, Huang J, Smith WM, Ideker RE. Incidence, evolution, and spatial distribution of functional reentry during ventricular fibrillation in pigs. Circ Res 84: 945–954, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Rogers JM, Usui M, KenKnight BH, Ideker RE, Smith WM. A quantitative framework for analyzing epicardial activation patterns during ventricular fibrillation. Ann Biomed Eng 25: 749–760, 1997 [DOI] [PubMed] [Google Scholar]
- 45.Rogers JM, Usui M, KenKnight BH, Ideker RE, Smith WM. Recurrent wavefront morphologies: a method for quantifying the complexity of epicardial activation patterns. Ann Biomed Eng 25: 761–768, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Rogers JM, Walcott GP, Gladden JD, Melnick SB, Ideker RE, Kay MW. Epicardial wavefronts arise from widely distributed transient sources during ventricular fibrillation in the isolated swine heart. New J Phys 10: 015004, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swissa M, Qu Z, Ohara T, Lee MH, Lin SF, Garfinkel A, Karagueuzian HS, Weiss JN, Chen PS. Action potential duration restitution and ventricular fibrillation due to rapid focal excitation. Am J Physiol Heart Circ Physiol 282: H1915–H1923, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Takeuchi T, Sato N, Kawamura Y, Takahashi F, Sato M, Kikuchi K, Akasaka N, Go K, Fujimoto K, Hasebe N. A case of a short-coupled variant of torsades de pointes with electrical storm. Pacing Clin Electrophysiol 26: 632–636, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Talajic M, Nattel S. Frequency-dependent effects of calcium antagonists on atrioventricular conduction and refractoriness: demonstration and characterization in anesthetized dogs. Circulation 74: 1156–1167, 1986 [DOI] [PubMed] [Google Scholar]
- 50.Tsujimae K, Suzuki S, Murakami S, Kurachi Y. Frequency-dependent effects of various IKr blockers on cardiac action potential duration in a human atrial model. Am J Physiol Heart Circ Physiol 293: H660–H669, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Watanabe MA, Koller ML. Mathematical analysis of dynamics of cardiac memory and accommodation: theory and experiment. Am J Physiol Heart Circ Physiol 282: H1534–H1547, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Weiss JN, Garfinkel A, Karagueuzian HS, Qu Z, Chen PS. Chaos and the transition to ventricular fibrillation: a new approach to antiarrhythmic drug evaluation. Circulation 99: 2819–2826, 1999 [DOI] [PubMed] [Google Scholar]
- 53.Wu TJ, Lin SF, Weiss JN, Ting CT, Chen PS. Two types of ventricular fibrillation in isolated rabbit hearts: importance of excitability and action potential duration restitution. Circulation 106: 1859–1866, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Zhou S, Cao JM, Tebb ZD, Ohara T, Huang HL, Omichi C, Lee MH, Kenknight BH, Chen LS, Fishbein MC, Karagueuzian HS, Chen PS. Modulation of QT interval by cardiac sympathetic nerve sprouting and the mechanisms of ventricular arrhythmia in a canine model of sudden cardiac death. J Cardiovasc Electrophysiol 12: 1068–1073, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Zhou X, Guse P, Wolf PD, Rollins DL, Smith WM, Ideker RE. Existence of both fast and slow channel activity during the early stages of ventricular fibrillation. Circ Res 70: 773–786, 1992 [DOI] [PubMed] [Google Scholar]