Abstract
The DNA damage response (DDR) orchestrates DNA repair and halts cell cycle. If damage is not resolved, cells can enter into an irreversible state of proliferative arrest called cellular senescence. Organismal ageing in mammals is associated with accumulation of markers of cellular senescence and DDR persistence at telomeres. Since the vast majority of the cells in mammals are non-proliferating, how do they age? Are telomeres involved? Also oncogene activation causes cellular senescence due to altered DNA replication and DDR activation in particular at the telomeres. Is there a common mechanism shared among apparently distinct types of cellular senescence? And what is the role of telomeric DNA damage?
Current Opinion in Genetics & Development 2014, 26:89–95
This review comes from a themed issue on Molecular and genetic bases of disease
Edited by Cynthia T McMurray and Jan Vijg
For a complete overview see the Issue and the Editorial
Available online 11th August 2014
http://dx.doi.org/10.1016/j.gde.2014.06.009
0959-437X/© 2014 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Introduction
Genome integrity is preserved by the DNA damage response (DDR) that, in the presence of DNA damage, arrests the cell cycle progression while coordinating DNA repair events [1]. The DDR pathway is composed of a complex protein network, regulated mainly by post-translational modifications such as phosphorylation, ubiquitylation, SUMOylation, acetylation and PARylation [1]. Recently a direct role of small non-coding RNAs in DDR modulation has also been proposed [2, 3]. Among the different types of damage, DNA double-strand breaks (DSBs) are considered the most deleterious, because they can cause cell death, a permanent proliferative arrest termed cellular senescence or, in checkpoint-impaired cells, genomic instability leading to cancer development. DSBs are repaired by two major mechanisms, the homologous recombination (HR) pathway, an error-free mechanism that uses a homologous chromosome as template for repair [4], and the non-homologous end joining (NHEJ) pathway in which the two DNA ends are ligated together with no need for homologous sequences [5]. If unrepaired, DNA damage fuels persistent DDR signalling and cellular senescence establishment. Which kind of DNA damages is refractory to DNA repair and triggers a permanent cell cycle arrest was not clear until recently. An example of terminal arrest was first observed by Hayflick and Moorhead in 1961, who reported that normal human cells in culture can undergo only a limited number of population doublings and eventually stop proliferating [6]. This was later explained by the so-called end replication problem, the inability of most normal cells to completely replicate linear genomes thus causing progressive shortening of chromosome ends, the telomeres, at every cell division [7]. When telomeres become critically short, they are sensed as damaged DNA, which triggers a DDR-initiated cellular senescence [8, 9, 10]. Despite the fact that chromosomes bear ends that resemble a DNA discontinuity such as a DSB, telomeres are generally not recognized as DSBs and do not activate a DDR. This is achieved by the joint action of different telomere-binding proteins, collectively named as a shelterin complex [11, 12]. It is becoming evident that there is a key role of telomeres in DDR modulation that is not restricted to their shortening. In this review we will dissect the impact of telomeric DNA damage on different types of cellular senescence.
Replicative senescence in ageing
In the past years, a strong link between telomere-initiated cellular senescence and organismal ageing has emerged [13]. Evidence that cellular senescence is a biologically active response in tissue has been found in mouse stem and somatic cells as well as in baboon and human skin fibroblasts [14, 15, 16, 17, 18, 19]. These senescent cells are thought to contribute to tissue ageing by at least two mechanisms. First of all intrinsically, by their inability to further proliferate and thus to replenish tissues with new cells; secondly, by up-regulating genes that encode extracellular-matrix-degrading enzymes, inflammatory cytokines and growth factors [20, 21]. These secreted factors, which are responsible for the senescent-associated secretory phenotype (SASP), act also on the neighbouring cells [22, 23], and fuelling DDR by still ill-defined mechanisms [24]. The association between cellular senescence and tissue ageing seems to be causative, since lack of p16, which precludes senescence establishment, prevents the age-related decline, thereby increasing healthspan [25, 26, 27]. Similarly, clearance of p16-expressing cells leads to a delay in age-related pathologies and to attenuation of established age-related disorders [28••]. Telomeres seem to play a fundamental role in senescence-mediated organismal ageing. Indeed dysfunctional telomeres have been found in senescent cells in vivo in primates [16, 29], and loss of telomerase function in mice causes senescence and physiological impairment of many tissues [30, 31, 32, 33]. Moreover deletion of p21 in telomerase-deficient mice with dysfunctional telomeres prolongs the lifespan [34]. Telomere shortening seems to be the driving force, since elongation of telomeres by reactivation of telomerase is sufficient to eliminate the degenerative phenotypes in multiple organs observed in telomerase knock out mice [35••].
A role for telomeres in ageing of non-proliferating compartments
Telomere-initiated cellular senescence seems to be a plausible mechanism to explain the ageing-associated functional decline of proliferating tissues in vivo. However, it is reasonable to assume that some other mechanisms may be in place in non-proliferating cells in which no telomeric attrition due to the end replication problem is expected to occur, either because these cells are quiescent or differentiated. Surprisingly however, we and others have shown that telomeres might have a central role in senescence establishment independently from their shortening [36••, 37••]. In these reports, random DNA damage generated by ionizing radiation, genotoxic drugs, or H2O2, leads to DDR activation that preferentially persists at telomeres over time. Cells with persistent DDR activation show a senescent phenotype that cannot be prevented by exogenous expression of telomerase, further excluding a contribution of telomere shortening. The mechanism proposed to explain this phenomenon is the suppression of effective DNA repair at telomeres by TRF2, a telomeric DNA binding protein [36••]. Inhibition of DNA repair might reflect the evolutionary role of telomeres in preventing chromosomal fusions, illegitimate DNA repair events among chromosome ends, in order to maintain the linear structure of chromosomes. TRF2 and the associated RAP1 protein are indeed able to inhibit NHEJ in vitro [38, 39, 40] and knock out of TRF2 leads to dramatic chromosomal fusions [41, 42], most of which depend on NHEJ [43•]. Similarly, TRF2 has been shown to inhibit NHEJ also when a DSB occurs within a telomere, and not only at its end (Figure 1), revealing that telomeric proteins, rather than telomeric DNA, are responsible for telomere irreparability. Consistent with this model, DDR activation at telomeres is more frequent in mouse and baboon tissues from aged animals, when compared with their young counterparts [36••, 37••]. This observation also suggests that having long telomeres may have an important drawback, since more telomeric DNA can offer a wider target for random DNA damage that cannot be repaired. Indeed, in different mammalian species, telomere length and lifespan are inversely correlated [44].
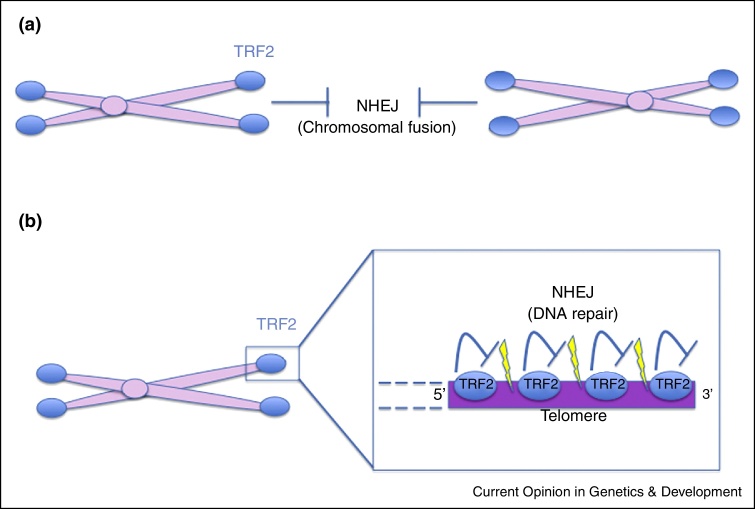
Figure 1.
Lack of DNA repair activity at telomeres. (a) Proteins belonging to shelterin complex inhibit DNA end joining (by NHEJ) at distal ends to prevent chromosomal fusions. (b) In the same manner, shelterin complex proteins prevent repair of DSBs (by NHEJ) occurred within telomeric repeats across the telomere length. NHEJ, non-homologous end joining.
Telomeric DNA damage in oncogene-induced senescence
In addition to its potential role in promoting ageing and age related disorders, telomere-initiated senescence, fuelled by oncogenic signals, plays a prominent role in suppressing malignant cancer progression in humans. In cells with functional DDR, oncogene expression usually results in cellular senescence after just a few population doublings [45]. This proliferative arrest is called oncogene-induced senescence (OIS) and, depending on cell type and oncogene expression levels, is caused by activation of a number of diverse pathways [46]. Thus, by preventing cancer onset, in addition to causing impairment of regenerative capacity during ageing, cellular senescence has been considered as an example of antagonistic pleiotropy, although this has recently put to question [47]. In some human cells, oncogene expression initially causes cells to hyper-proliferate, which leads to aberrantly increased DNA replication rates causing frequent DNA replication fork stalling events. As a consequence of this, DSBs are generated in the vicinity of collapsed replication forks and this activates a DDR and forces cells to undergo senescence [48, 49]. OIS not only functions as a tumour suppressing mechanism in animal model systems [50], but also cells with features of OIS, including abundant DDR foci formation, have been detected in a number of distinct benign neoplastic lesions in humans and not in the corresponding malignant cancers [51, 52, 53, 54••]. Given that initiation of aberrant cell proliferation in human tissues is often associated with oncogenic events, these data are strong evidence that OIS also suppresses cancer progression in humans.
Some chromosomal loci, called common fragile sites (CFS), appear to be hot-spots for DSB formation as a result of DNA replication stress. These sites are usually repetitive in nature and have a tendency to form secondary structures that can impede replication fork progression [55]. In addition, CFS belong to chromosomal regions poor of replication origins and thus unable to cope with stalled DNA replication forks [56]. Because of their repetitive nature, sensitivity to oxidative damage, and propensity to form secondary structures (called G quadruplexes), telomeres also pose a challenge to the replication machinery. In fact, telomeres share many other features of CFS [57, 58]. Not too surprisingly, therefore, recent results demonstrated that oncogene expression leads to DNA replication stress, replication fork stalling, and formation of DDR foci at increased rates at telomeres [54••]. However, non-telomeric DDR foci are also generated but are resolved over a period of several days in arrested oncogene-expressing cells. These telomeric DDR foci persist suggesting that also oncogene-induced telomeric lesions are not efficiently repaired. Does the persistence of the telomeric DDR foci cause oncogene-expressing cells to arrest stably? In support of this, overexpression of catalytically active telomerase prevents formation of telomeric DDR foci as a result of oncogene-induced and drug-induced DNA replication stresses. Consequently, telomerase destabilizes the proliferative arrest caused by aberrant oncogene signalling [54••]. Thus, OIS is a cellular stress response that can be enforced by telomere dysfunction.
Persistent telomeric DDR foci, or dysfunctional telomeres, can also be observed in most cells of benign human neoplasias and cancer precursor lesions before telomeres have become eroded. Foci form below a critical telomere length in most cells of benign human neoplasias and cancer precursor lesions such as melanocytic nevi, ductal breast hyperplasias, and colonic adenomas [54••]. Indeed, dysfunctional telomeres in cells comprising these benign lesions on average are not shorter compared to other telomeres in the same cells, supporting this conclusion. The irreparability of telomeric DSBs in this context might therefore act as cellular sensor of hyperproliferative signals. In cells expressing telomerase, such as those of invasive human cancers, we would anticipate that replication stresses would not result in telomeric DDR activation. Rather, they would and allow continuous cell proliferation. It is therefore likely that cancer cells re-activate telomerase expression not only to prevent telomere erosion, but also to cope with telomeric replication stress that would halt cell proliferation.
Irreparability of telomeres is evolutionary conserved in eukaryotes
The inherent characteristic of telomeres to be resistant to DNA repair is conserved in the yeast Saccharomyces cerevisiae and Schizoccharomyces pombe, whose natural chromosome ends do not join with each other or with random DNA breaks [59, 60, 61, 62]. Indeed, in a genetic system in S. cerevisiae, an endonuclease-induced DSB is generated immediately adjacent to a relatively short array of telomeric DNA repeats. The break inhibits the recruitment of DNA ligase IV and therefore prevents fusions by NHEJ [36••]. The presence of telomeric sequences at DNA ends can also prevent repair by HR, because it limits nucleolytic degradation and therefore the generation of single-stranded DNA (ssDNA). Moreover, it weakens the signalling activity of the Mec1 checkpoint kinase (ATR in mammals) [63, 64], which is recruited to RPA-coated ssDNA [65]. Interestingly, this phenomenon acts locally, as it inhibits checkpoint signalling from a nearby DSB devoid of telomeric repeats, but not from a DSB present on a different chromosome [63, 64].
In budding yeast, the ability of telomeric ends to resist NHEJ-mediated repair and nucleolytic degradation depends on at least three different protein complexes, which are conserved from yeast to mammals. One of them is the CST (Cdc13–Stn1–Ten1) complex, which binds to the telomeric single-stranded overhang and prevents nucleolytic degradation and therefore checkpoint activation at telomeres [66, 67]. A second complex, the Ku70-Ku80 heterodimer, blocks ssDNA formation specifically in the G1 phase of the cell cycle by inhibiting the action of the exonuclease Exo1 [68, 69, 70]. Finally, NHEJ inhibition at telomeres is controlled primarily by the Rap1 protein, which binds to the telomeric double-stranded DNA [71]. Rap1 prevents NHEJ by establishing two parallel inhibitory pathways through its interacting proteins Rif2 and Sir4 [72]. While it is currently unclear how these proteins prevent NHEJ, the observations that DSBs flanked by telomeric repeats show reduced DNA ligase IV binding [36••] suggest that they might function by counteracting the loading of NHEJ proteins. It has been recently shown that maintenance of NHEJ inhibition by Rap1 requires Uls1, which is both a Swi2/Snf2-related translocase and a Small Ubiquitin-related Modifier (SUMO)-Targeted Ubiquitin Ligase [73•]. Uls1 requirement is alleviated by inhibiting formation of SUMO chains and by rap1 mutations altering SUMOylation sites. Furthermore, Uls1 limits the accumulation of Rap1 poly-SUMO conjugates, suggesting that Uls1 ensures the efficiency of NHEJ inhibition by eliminating non-functional poly-SUMOylated Rap1 molecules from telomeres.
Removal of telomerase causes replicative senescence also in S. cerevisiae [74]. Interestingly, the presence of a single critically short telomere accelerates senescence in a telomerase-negative context [75, 76], suggesting that the length of the shortest telomere is a major determinant of the onset of senescence in this organism. The Mec1 checkpoint kinase is required for the accelerated loss of viability in the presence of a short telomere [75], indicating that, like in human fibroblasts, DDR is activated at the shortest telomere in cells undergoing senescence.
Conclusions
On the basis of the results described in this review, we can propose a unifying model, according to which telomeres play an essential role not only in replicative but also in DNA damage-induced and oncogene-induced cellular senescence (Figure 2). This provides a mechanism for DDR-mediated and senescence-mediated ageing of non-proliferating tissues, which could not be explained solely by telomeric shortening.
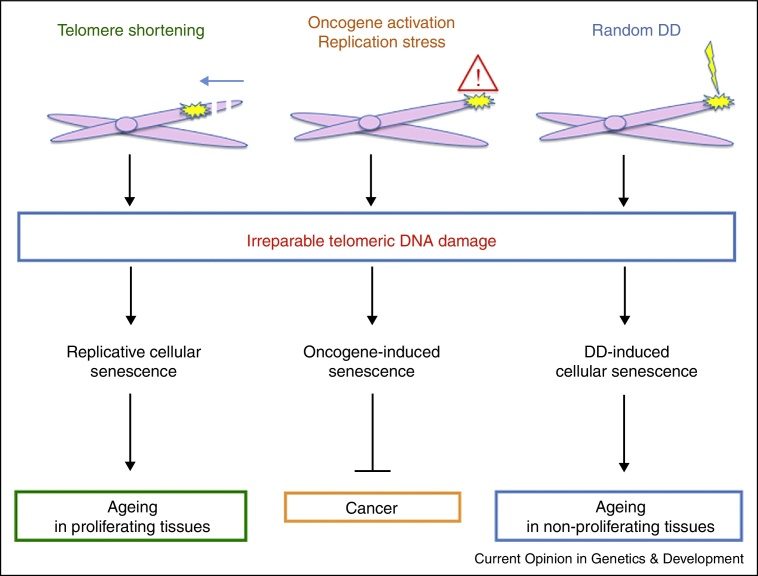
Figure 2.
Unifying model explaining the role of telomeres in replicative, DNA damage-induced and oncogene-induced senescence both at cellular and organismal levels. DNA damage at telomeres cannot be repaired, independently from the source that generated it, both endogenously (i.e. telomere shortening, replication stress) or exogenously (i.e. X-rays). Irreparable telomeres are, therefore, associated not only with replicative cellular senescence but also with oncogene-induced and DNA damage-induced cellular senescence. These events prevent cancer onset on the one side, but on the other side, cause impairment of regenerative capacity during ageing both in proliferating and non-proliferating tissues at organismal level. DD, DNA damage.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
We apologize to those whose work could not be discussed due to space limitations. We thank all Fd’AdF laboratory members for discussions. F.R. is supported by Fondazione Italiana per la Ricerca sul Cancro (FIRC, application number 12476). UH laboratory is supported by the NIH/NCI # R01CA136533. MPL laboratory is supported by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC, Grant Number IG11407) and Cofinanziamento 2010–2011 MIUR/Università di Milano-Bicocca. Fd’AdF laboratory is supported by FIRC, AIRC (application number 12971), AICR (14-1331), HFSP (Human Frontier Science Program; contract number: RGP 0014/2012), Cariplo Foundation (Grant Number 2010.0818), FP7 PEOPLE 2012 ITN (CodAge), Telethon (GGP12059), PRIN 2010–2011, European Research Council advanced grant (322726) and EPIGEN project (an initiative of the Italian Ministry of Education, University and Research and the National Research Council).
References
- 1.Polo S.E., Jackson S.P. Dynamics of DNA damage response proteins at DNA breaks: a focus on protein modifications. Genes Dev. 2011;25:409–433. doi: 10.1101/gad.2021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Francia S., Michelini F., Saxena A., Tang D., de Hoon M., Anelli V., Mione M., Carninci P., d’Adda di Fagagna F. Site-specific dicer and drosha rna products control the DNA-damage response. Nature. 2012;488:231–235. doi: 10.1038/nature11179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei W., Ba Z., Gao M., Wu Y., Ma Y., Amiard S., White C.I., Rendtlew Danielsen J.M., Yang Y.G., Qi Y. A role for small rnas in DNA double-strand break repair. Cell. 2012;149:101–112. doi: 10.1016/j.cell.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Karpenshif Y., Bernstein K.A. From yeast to mammals: recent advances in genetic control of homologous recombination. DNA Repair (Amst) 2012;11:781–788. doi: 10.1016/j.dnarep.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman J.R., Taylor M.R., Boulton S.J. Playing the end game: DNA double-strand break repair pathway choice. Mol Cell. 2012;47:497–510. doi: 10.1016/j.molcel.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 6.Hayflick L., Moorhead P.S. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 7.Watson J.D. Origin of concatemeric t7 DNA. Nat New Biol. 1972;239:197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- 8.d’Adda di Fagagna F., Reaper P.M., Clay-Farrace L., Fiegler H., Carr P., Von Zglinicki T., Saretzki G., Carter N.P., Jackson S.P. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 9.Herbig U., Jobling W.A., Chen B.P., Chen D.J., Sedivy J.M. Telomere shortening triggers senescence of human cells through a pathway involving atm, p53, and p21(cip1), but not p16(ink4a) Mol Cell. 2004;14:501–513. doi: 10.1016/s1097-2765(04)00256-4. [DOI] [PubMed] [Google Scholar]
- 10.Zou Y., Sfeir A., Gryaznov S.M., Shay J.W., Wright W.E. Does a sentinel or a subset of short telomeres determine replicative senescence? Mol Biol Cell. 2004;15:3709–3718. doi: 10.1091/mbc.E04-03-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazzerini Denchi E., de Lange T. Protection of telomeres through independent control of atm and atr by trf2 and pot1. Nature. 2007;448:1068–1071. doi: 10.1038/nature06065. [DOI] [PubMed] [Google Scholar]
- 12.de Lange T. How telomeres solve the end-protection problem. Science. 2009;326:948–952. doi: 10.1126/science.1170633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tumpel S., Rudolph K.L. The role of telomere shortening in somatic stem cells and tissue aging: lessons from telomerase model systems. Ann N Y Acad Sci. 2012;1266:28–39. doi: 10.1111/j.1749-6632.2012.06547.x. [DOI] [PubMed] [Google Scholar]
- 14.Dimri G.P., Lee X., Basile G., Acosta M., Scott G., Roskelley C., Medrano E.E., Linskens M., Rubelj I., Pereira-Smith O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sedelnikova O.A., Horikawa I., Zimonjic D.B., Popescu N.C., Bonner W.M., Barrett J.C. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat Cell Biol. 2004;6:168–170. doi: 10.1038/ncb1095. [DOI] [PubMed] [Google Scholar]
- 16.Herbig U., Ferreira M., Condel L., Carey D., Sedivy J.M. Cellular senescence in aging primates. Science. 2006;311:1257. doi: 10.1126/science.1122446. [DOI] [PubMed] [Google Scholar]
- 17.Nijnik A., Woodbine L., Marchetti C., Dawson S., Lambe T., Liu C., Rodrigues N.P., Crockford T.L., Cabuy E., Vindigni A., Enver T. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447:686–690. doi: 10.1038/nature05875. [DOI] [PubMed] [Google Scholar]
- 18.Rossi D.J., Bryder D., Seita J., Nussenzweig A., Hoeijmakers J., Weissman I.L. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 19.Wang C., Jurk D., Maddick M., Nelson G., Martin-Ruiz C., von Zglinicki T. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell. 2009;8:311–323. doi: 10.1111/j.1474-9726.2009.00481.x. [DOI] [PubMed] [Google Scholar]
- 20.Coppe J.P., Patil C.K., Rodier F., Sun Y., Munoz D.P., Goldstein J., Nelson P.S., Desprez P.Y., Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic ras and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodier F., Coppe J.P., Patil C.K., Hoeijmakers W.A., Munoz D.P., Raza S.R., Freund A., Campeau E., Davalos A.R., Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson G., Wordsworth J., Wang C., Jurk D., Lawless C., Martin-Ruiz C., von Zglinicki T. A senescent cell bystander effect: senescence-induced senescence. Aging Cell. 2012;11:345–349. doi: 10.1111/j.1474-9726.2012.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Acosta J.C., Banito A., Wuestefeld T., Georgilis A., Janich P., Morton J.P., Athineos D., Kang T.W., Lasitschka F., Andrulis M., Pascual G. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol. 2013;15:978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fumagalli M., d’Adda di Fagagna F. Saspense and ddrama in cancer and ageing. Nat Cell Biol. 2009;11:921–923. doi: 10.1038/ncb0809-921. [DOI] [PubMed] [Google Scholar]
- 25.Janzen V., Forkert R., Fleming H.E., Saito Y., Waring M.T., Dombkowski D.M., Cheng T., DePinho R.A., Sharpless N.E., Scadden D.T. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16ink4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 26.Krishnamurthy J., Ramsey M.R., Ligon K.L., Torrice C., Koh A., Bonner-Weir S., Sharpless N.E. P16ink4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443:453–457. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- 27.Molofsky A.V., Slutsky S.G., Joseph N.M., He S., Pardal R., Krishnamurthy J., Sharpless N.E., Morrison S.J. Increasing p16ink4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28••.Baker D.J., Wijshake T., Tchkonia T., LeBrasseur N.K., Childs B.G., van de Sluis B., Kirkland J.L., van Deursen J.M. Clearance of p16ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using an inducible mouse system in which p16-positive cells undergo cell death, in a BubR1 progeroid background, authors show that the absence of senescent cells in vivo leads to delay of ageing phenotype in different tissues and attenuated progression of age-related disorders.
- 29.Jeyapalan J.C., Ferreira M., Sedivy J.M., Herbig U. Accumulation of senescent cells in mitotic tissue of aging primates. Mech Ageing Dev. 2007;128:36–44. doi: 10.1016/j.mad.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudolph K.L., Chang S., Lee H.W., Blasco M., Gottlieb G.J., Greider C., DePinho R.A. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- 31.Ferron S., Mira H., Franco S., Cano-Jaimez M., Bellmunt E., Ramirez C., Farinas I., Blasco M.A. Telomere shortening and chromosomal instability abrogates proliferation of adult but not embryonic neural stem cells. Development. 2004;131:4059–4070. doi: 10.1242/dev.01215. [DOI] [PubMed] [Google Scholar]
- 32.Satyanarayana A., Greenberg R.A., Schaetzlein S., Buer J., Masutomi K., Hahn W.C., Zimmermann S., Martens U., Manns M.P., Rudolph K.L. Mitogen stimulation cooperates with telomere shortening to activate DNA damage responses and senescence signaling. Mol Cell Biol. 2004;24:5459–5474. doi: 10.1128/MCB.24.12.5459-5474.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sahin E., Depinho R.A. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–528. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choudhury A.R., Ju Z., Djojosubroto M.W., Schienke A., Lechel A., Schaetzlein S., Jiang H., Stepczynska A., Wang C., Buer J., Lee H.W. Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nat Genet. 2007;39:99–105. doi: 10.1038/ng1937. [DOI] [PubMed] [Google Scholar]
- 35••.Jaskelioff M., Muller F.L., Paik J.H., Thomas E., Jiang S., Adams A.C., Sahin E., Kost-Alimova M., Protopopov A., Cadinanos J., Horner J.W. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469:102–106. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report shows that reactivation of telomerase in telomerase knock out mice can revert ageing phenotypes in different tissues, suggesting an important role for telomere shortening in organismal ageing.
- 36••.Fumagalli M., Rossiello F., Clerici M., Barozzi S., Cittaro D., Kaplunov J.M., Bucci G., Dobreva M., Matti V., Beausejour C.M., Herbig U. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Biol. 2012;14:355–365. doi: 10.1038/ncb2466. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, it is shown that repair of telomeric double-strand breaks is inhibited by the telomere binding protein TRF2. The unrepaired DNA damage triggers a persistent DDR and cellular senescence. Consistently, accumulation of DDR at the telomeres is detected in non-proliferating tissues of aged baboons.
- 37••.Hewitt G., Jurk D., Marques F.D., Correia-Melo C., Hardy T., Gackowska A., Anderson R., Taschuk M., Mann J., Passos J.F. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun. 2012;3:708. doi: 10.1038/ncomms1708. [DOI] [PMC free article] [PubMed] [Google Scholar]; Authors show that persistent DDR foci localize preferentially at telomeres both in vitro, following exogenous DNA damage generation, and in vivo, in gut and liver tissues from old mice.
- 38.Bae N.S., Baumann P. A rap1/trf2 complex inhibits nonhomologous end-joining at human telomeric DNA ends. Mol Cell. 2007;26:323–334. doi: 10.1016/j.molcel.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 39.Sarthy J., Bae N.S., Scrafford J., Baumann P. Human rap1 inhibits non-homologous end joining at telomeres. EMBO J. 2009;28:3390–3399. doi: 10.1038/emboj.2009.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bombarde O., Boby C., Gomez D., Frit P., Giraud-Panis M.J., Gilson E., Salles B., Calsou P. Trf2/rap1 and DNA-pk mediate a double protection against joining at telomeric ends. EMBO J. 2010;29:1573–1584. doi: 10.1038/emboj.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Steensel B., Smogorzewska A., de Lange T. Trf2 protects human telomeres from end-to-end fusions. Cell. 1998;92:401–413. doi: 10.1016/s0092-8674(00)80932-0. [DOI] [PubMed] [Google Scholar]
- 42.Celli G.B., de Lange T. DNA processing is not required for atm-mediated telomere damage response after trf2 deletion. Nat Cell Biol. 2005;7:712–718. doi: 10.1038/ncb1275. [DOI] [PubMed] [Google Scholar]
- 43•.Sfeir A., de Lange T. Removal of shelterin reveals the telomere end-protection problem. Science. 2012;336:593–597. doi: 10.1126/science.1218498. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this report, authors dissect the role of the shelterin component and other proteins in suppressing DNA damage response activation and chromosomal fusions at the telomeres, revealing six different pathways of inhibition.
- 44.Gomes N.M., Ryder O.A., Houck M.L., Charter S.J., Walker W., Forsyth N.R., Austad S.N., Venditti C., Pagel M., Shay J.W., Wright W.E. Comparative biology of mammalian telomeres: Hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell. 2011;10:761–768. doi: 10.1111/j.1474-9726.2011.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serrano M., Lin A.W., McCurrach M.E., Beach D., Lowe S.W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16ink4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 46.Courtois-Cox S., Jones S.L., Cichowski K. Many roads lead to oncogene-induced senescence. Oncogene. 2008;27:2801–2809. doi: 10.1038/sj.onc.1210950. [DOI] [PubMed] [Google Scholar]
- 47.Giaimo S., d’Adda di Fagagna F. Is cellular senescence an example of antagonistic pleiotropy? Aging Cell. 2012;11:378–383. doi: 10.1111/j.1474-9726.2012.00807.x. [DOI] [PubMed] [Google Scholar]
- 48.Bartkova J., Rezaei N., Liontos M., Karakaidos P., Kletsas D., Issaeva N., Vassiliou L.V., Kolettas E., Niforou K., Zoumpourlis V.C., Takaoka M. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- 49.Di Micco R., Fumagalli M., Cicalese A., Piccinin S., Gasparini P., Luise C., Schurra C., Garre M., Nuciforo P.G., Bensimon A., Maestro R. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- 50.Collado M., Serrano M. Senescence in tumours: evidence from mice and humans. Nat Rev Cancer. 2010;10:51–57. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bartkova J., Bakkenist C.J., Rajpert-De Meyts E., Skakkebaek N.E., Sehested M., Lukas J., Kastan M.B., Bartek J. Atm activation in normal human tissues and testicular cancer. Cell Cycle. 2005;4:838–845. doi: 10.4161/cc.4.6.1742. [DOI] [PubMed] [Google Scholar]
- 52.Bartkova J., Horejsi Z., Koed K., Kramer A., Tort F., Zieger K., Guldberg P., Sehested M., Nesland J.M., Lukas C., Orntoft T. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]; Human cells expressing oncogenes show DDR markers. In the same way, in different human cancers, the tumoral cells, but not the normal tissues, activate the DDR.
- 53.Gorgoulis V.G., Vassiliou L.V., Karakaidos P., Zacharatos P., Kotsinas A., Liloglou T., Venere M., Ditullio R.A., Jr., Kastrinakis N.G., Levy B., Kletsas D. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]; Tumoral cells experience DNA replication stress that leads to DNA damage generation and DDR. Cancer progression is associated with inactivation of DDR signalling, allowing the cells to proliferate despite DNA damage.
- 54••.Suram A., Kaplunov J., Patel P.L., Ruan H., Cerutti A., Boccardi V., Fumagalli M., Di Micco R., Mirani N., Gurung R.L., Hande M.P. Oncogene-induced telomere dysfunction enforces cellular senescence in human cancer precursor lesions. EMBO J. 2012;31:2839–2851. doi: 10.1038/emboj.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that oncogenes also cause telomeric replication stress, which leads to telomere dysfunction and cellular senescence in cells with low telomerase activity. Cells with features of telomere dysfunction-induced cellular senescence were observed in benign human tumors suggesting that telomere based senescence functions as a tumor suppressing mechanism in humans.
- 55.Durkin S.G., Glover T.W. Chromosome fragile sites. Annu Rev Genet. 2007;41:169–192. doi: 10.1146/annurev.genet.41.042007.165900. [DOI] [PubMed] [Google Scholar]
- 56.Letessier A., Millot G.A., Koundrioukoff S., Lachages A.M., Vogt N., Hansen R.S., Malfoy B., Brison O., Debatisse M. Cell-type-specific replication initiation programs set fragility of the fra3b fragile site. Nature. 2011;470:120–123. doi: 10.1038/nature09745. [DOI] [PubMed] [Google Scholar]
- 57.Sfeir A., Kosiyatrakul S.T., Hockemeyer D., MacRae S.L., Karlseder J., Schildkraut C.L., de Lange T. Mammalian telomeres resemble fragile sites and require trf1 for efficient replication. Cell. 2009;138:90–103. doi: 10.1016/j.cell.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martinez P., Thanasoula M., Munoz P., Liao C., Tejera A., McNees C., Flores J.M., Fernandez-Capetillo O., Tarsounas M., Blasco M.A. Increased telomere fragility and fusions resulting from trf1 deficiency lead to degenerative pathologies and increased cancer in mice. Genes Dev. 2009;23:2060–2075. doi: 10.1101/gad.543509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferreira M.G., Cooper J.P. The fission yeast taz1 protein protects chromosomes from ku-dependent end-to-end fusions. Mol Cell. 2001;7:55–63. doi: 10.1016/s1097-2765(01)00154-x. [DOI] [PubMed] [Google Scholar]
- 60.DuBois M.L., Haimberger Z.W., McIntosh M.W., Gottschling D.E. A quantitative assay for telomere protection in saccharomyces cerevisiae. Genetics. 2002;161:995–1013. doi: 10.1093/genetics/161.3.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chan S.W., Blackburn E.H. Telomerase and atm/tel1p protect telomeres from nonhomologous end joining. Mol Cell. 2003;11:1379–1387. doi: 10.1016/s1097-2765(03)00174-6. [DOI] [PubMed] [Google Scholar]
- 62.Mieczkowski P.A., Mieczkowska J.O., Dominska M., Petes T.D. Genetic regulation of telomere-telomere fusions in the yeast saccharomyces cerevisae. Proc Natl Acad Sci U S A. 2003;100:10854–10859. doi: 10.1073/pnas.1934561100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Michelson R.J., Rosenstein S., Weinert T. A telomeric repeat sequence adjacent to a DNA double-stranded break produces an anticheckpoint. Genes Dev. 2005;19:2546–2559. doi: 10.1101/gad.1293805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ribeyre C., Shore D. Anticheckpoint pathways at telomeres in yeast. Nat Struct Mol Biol. 2012;19:307–313. doi: 10.1038/nsmb.2225. [DOI] [PubMed] [Google Scholar]
- 65.Zou L., Elledge S.J. Sensing DNA damage through atrip recognition of RPA-ssDNA complexes. Science. 2003;300:1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- 66.Garvik B., Carson M., Hartwell L. Single-stranded DNA arising at telomeres in cdc13 mutants may constitute a specific signal for the rad9 checkpoint. Mol Cell Biol. 1995;15:6128–6138. doi: 10.1128/mcb.15.11.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lydall D., Weinert T. Yeast checkpoint genes in DNA damage processing: implications for repair and arrest. Science. 1995;270:1488–1491. doi: 10.1126/science.270.5241.1488. [DOI] [PubMed] [Google Scholar]
- 68.Maringele L., Lydall D. Exo1-dependent single-stranded DNA at telomeres activates subsets of DNA damage and spindle checkpoint pathways in budding yeast yku70delta mutants. Genes Dev. 2002;16:1919–1933. doi: 10.1101/gad.225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bonetti D., Clerici M., Anbalagan S., Martina M., Lucchini G., Longhese M.P. Shelterin-like proteins and yku inhibit nucleolytic processing of saccharomyces cerevisiae telomeres. PLoS Genet. 2010;6:e1000966. doi: 10.1371/journal.pgen.1000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vodenicharov M.D., Laterreur N., Wellinger R.J. Telomere capping in non-dividing yeast cells requires yku and rap1. EMBO J. 2010;29:3007–3019. doi: 10.1038/emboj.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pardo B., Marcand S. Rap1 prevents telomere fusions by nonhomologous end joining. EMBO J. 2005;24:3117–3127. doi: 10.1038/sj.emboj.7600778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marcand S., Pardo B., Gratias A., Cahun S., Callebaut I. Multiple pathways inhibit nhej at telomeres. Genes Dev. 2008;22:1153–1158. doi: 10.1101/gad.455108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73•.Lescasse R., Pobiega S., Callebaut I., Marcand S. End-joining inhibition at telomeres requires the translocase and polysumo-dependent ubiquitin ligase uls1. EMBO J. 2013;32:805–815. doi: 10.1038/emboj.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that the Small Ubiquitin-related Modifier (SUMO)-Targeted Ubiquitin Ligase (STUbL) Usl1 ensures the continuous efficiency of NHEJ inhibition by removing non-functional poly-SUMOylated Rap1 molecules from telomeres.
- 74.Lundblad V., Szostak J.W. A mutant with a defect in telomere elongation leads to senescence in yeast. Cell. 1989;57:633–643. doi: 10.1016/0092-8674(89)90132-3. [DOI] [PubMed] [Google Scholar]
- 75.Abdallah P., Luciano P., Runge K.W., Lisby M., Geli V., Gilson E., Teixeira M.T. A two-step model for senescence triggered by a single critically short telomere. Nat Cell Biol. 2009;11:988–993. doi: 10.1038/ncb1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khadaroo B., Teixeira M.T., Luciano P., Eckert-Boulet N., Germann S.M., Simon M.N., Gallina I., Abdallah P., Gilson E., Geli V., Lisby M. The DNA damage response at eroded telomeres and tethering to the nuclear pore complex. Nat Cell Biol. 2009;11:980–987. doi: 10.1038/ncb1910. [DOI] [PubMed] [Google Scholar]