Abstract
Visit-to-visit variability (VVV) of blood pressure (BP) has been associated with cardiovascular disease (CVD) and mortality in some but not all studies. We conducted a systematic review and meta-analysis to examine the association between VVV of BP and CVD and all-cause mortality. Medical databases were searched through June 4, 2014 for studies meeting the following eligibility criteria: adult participants; BP measurements at ≥3 visits; follow-up for CVD, coronary heart disease (CHD), stroke, or mortality outcomes; events confirmed via database, death certificate, and/or event ascertainment committee; and adjustment for confounders. Data were extracted by two reviewers and pooled using a random-effects model. Overall, 8,870 abstracts were identified of which 37 studies, representing 41 separate cohorts, met inclusion criteria. Across studies, VVV of systolic BP (SBP) and diastolic BP showed significant associations with outcomes in 181 of 312 (58.0%) and 61 of 188 (32.4%) analyses, respectively. Few studies provided sufficient data for pooling risk estimates. For each 5 mmHg higher SD of SBP, the pooled hazard ratios for stroke across seven cohorts was 1.17 (95% CI:1.07–1.28), for CHD across four cohorts was 1.27 (95% CI:1.07–1.51), for CVD across five cohorts was 1.12 (95% CI:0.98–1.28), for CVD mortality across five cohorts was 1.22 (95% CI:1.09–1.35), and for all-cause mortality across four cohorts was 1.20 (95% CI:1.05–1.36). In summary, modest associations between VVV of BP and CVD and all-cause mortality are present in published studies. However, these findings are limited by the small amount of data available for meta-analysis.
Keywords: blood pressure, blood pressure variability, cardiovascular disease, mortality, systematic review, meta-analysis
INTRODUCTION
In many individuals, blood pressure (BP) varies between clinic visits conducted days, weeks, or months apart. Although long thought to be artifact, recent data suggest that visit-to-visit variability (VVV) of BP is associated with an increased risk for incident coronary heart disease (CHD), stroke, and mortality, independent of mean BP.1–3 Most noteworthy was a series of publications in Lancet and Lancet Neurology in 2010 by Rothwell and colleagues who showed that VVV of BP was a strong risk factor for stroke, independent of mean BP.3–5 These publications stimulated a great deal of interest in VVV of BP as a novel risk factor for cardiovascular disease (CVD). More recent findings, however, have yielded mixed results regarding the association between VVV of BP and risk for future cardiovascular events.6,7 Given the uncertainty of the association between VVV of BP and CVD risk, we conducted a systematic review and meta-analysis. Our primary objective was to document the association between VVV of BP and CVD, including stroke and CHD, and all-cause mortality. Our secondary objective was to document the methodology (e.g., number of visits, time interval between visits, etc.) used to estimate VVV of BP in published studies.
METHODS
Search Strategy and Selection Criteria
Studies were included if they met the following criteria: (1) adult participants aged 18 years and over, (2) measurement of BP at three or more visits on different days, (3) follow-up for outcomes of incident CVD, CHD, stroke, or mortality, (4) events confirmed via database, death certificate, and/or event ascertainment committee, and (5) adjustment for confounders undertaken in the design or analysis stages of the study. We excluded studies that only assessed BP variability via ambulatory monitoring, did not use a comparison or referent group, or that were reported exclusively in letters to the editor, commentaries, meeting abstracts, editorials, or review articles. There was no restriction on language.
The following databases were searched through June 4, 2014: MEDLINE, Database of Abstracts of Reviews of Effects (DARE), Health Technology Assessment Database (HTA), Cumulative Index to Nursing and Allied Health Literature (CINAHL), Scopus, ProQuest Dissertations & Theses (PQDT), and ClinicalTrials.gov. The MEDLINE search strategy is described in the online-only Data Supplement. Terms for the other databases were adapted accordingly. To supplement the database searches, a PubMed related articles search and a cited reference search through ISI Web of Science were conducted using the included articles identified from the first set of search results. A manual search was also performed using the reference lists from the included articles and the reference lists from review articles produced by the electronic database searches.
Two investigators (KMD and RMT) independently reviewed all identified articles for eligibility using the above criteria. The title and abstract of identified articles were reviewed and those deemed ineligible were excluded. The full-text for the remainder of articles were retrieved and reviewed. Discrepancies on whether to include a study were resolved by discussion including a third investigator (PM).
Data Extraction
Data were abstracted from all articles by two separate investigators (KMD and RMT), independently, using a standardized instrument. Study characteristics (cohort name, sample size, population characteristics, country of origin, outcomes, and follow-up period), VVV measurement methodology (number of visits used to derive VVV, number of BP readings taken at each visit, time interval between visits, BP measurement device, BP indexes assessed, VVV metrics quantified, and whether VVV was analyzed as a continuous and/or categorical variable), and results (e.g., hazard ratios) from fully adjusted models were abstracted for the overall study population and for all subgroups reported. The quality of data abstraction was controlled by comparing the forms of the data abstractors. Discrepancies in data abstraction were resolved by discussion and by a third investigator (PM), when needed. When potentially relevant data were not reported, two attempts were made to contact the corresponding author via email. Any data that were not reported in the full-text and were not provided by the corresponding author are described as “not reported” or “NR”. For this manuscript we describe all included articles as “studies”. As several studies reported results from multiple populations, the term “cohort” is used to describe each unique population. Finally, for many cohorts, the results from multiple analyses of VVV of SBP or VVV of DBP with an outcome were reported. Therefore, each result is referred to as an “analysis”.
Statistical Analyses
Meta-analyses were conducted for VVV of SBP and VVV of DBP modeled as continuous variables. Analyses were restricted to cohorts in which VVV was quantified as the standard deviation (SD) of BP, the most commonly used VVV metric. Pooled hazard ratios (HR) and 95% confidence intervals (CI) were calculated per 5 mmHg higher SD of BP using a random-effects model. Tests for heterogeneity were not conducted because of the small amount of data available for pooling each outcome (range: 2 to 7 cohorts). Publication bias was assessed by funnel plots and with a regression asymmetry test8 for measures of VVV of BP with >10 analyses (pooling all outcomes).9 Data analyses were conducted using Stata V11 (Stata Inc., College Station, TX).
RESULTS
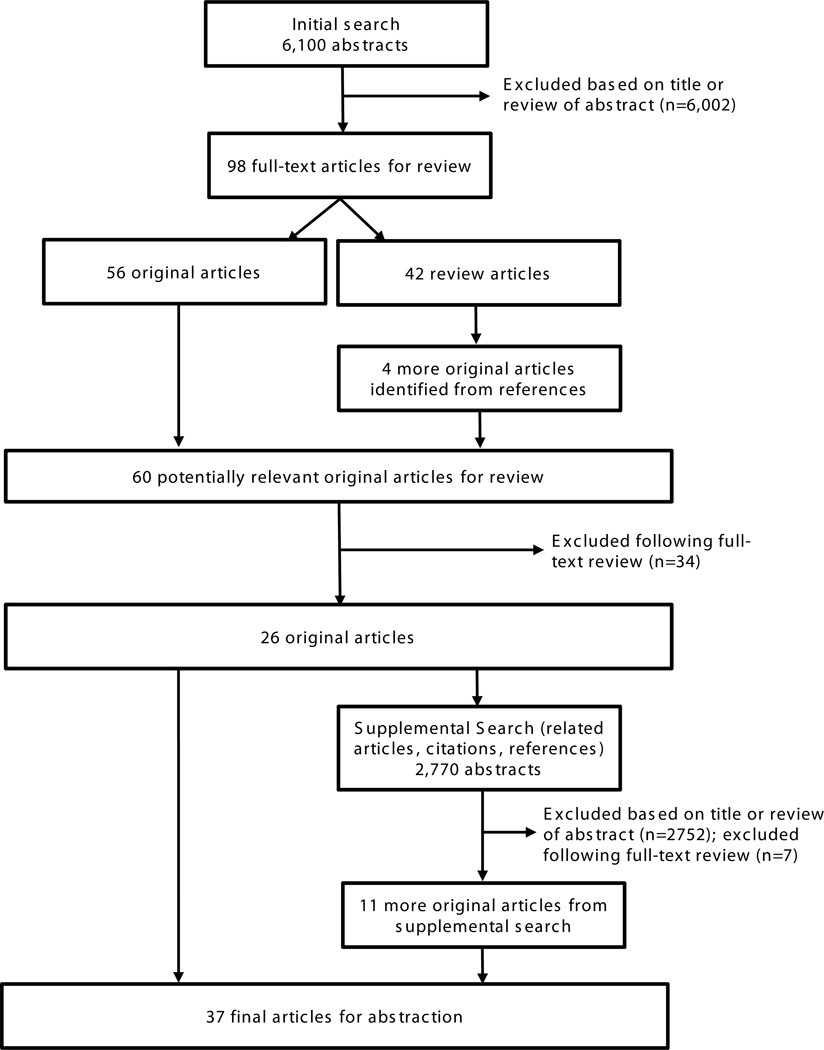
The original search identified 6,100 abstracts (Figure 1). Following review of the title and abstract, 6,002 abstracts were excluded. Of the 98 full-text articles retrieved, 42 were review articles, leaving 56 original articles. An additional 4 potentially relevant articles were identified from a manual search of the reference lists from the 42 review articles. Of the 60 original articles reviewed, 34 were excluded leaving 26 original articles. An additional 11 original articles were identified from 2,770 abstracts identified in a supplemental search of other sources (reference lists, related articles search, citations). In total, of the 8,870 abstracts retrieved and reviewed from the original and supplemental searches, 37 studies met the inclusion criteria for abstraction.1–3,6,7,10–41
Figure 1.
Flow diagram of article selection for the systematic review.
Study Characteristics
The earliest study identified was published in 1983 with 28 studies (32 cohorts) published between 2010 and June 2014 (Table 1). Two studies reported data from multiple cohorts. The study by Rothwell et. al. analyzed data from four cohorts.3 The study by Poortvliet et. al. reported results for cohorts at two different follow-up lengths: a short-term follow-up cohort (included countries: Ireland, Scotland, the Netherlands) and a long-term follow-up cohort that included the subset of individuals from Scotland.31 Overall, the 37 included studies comprised 41 different cohorts. Cohort sample sizes ranged from 144 to 58,228 participants. Cohorts included general population/community-based samples (4 cohorts), elderly populations (5 cohorts), individuals with hypertension (11 studies), a history of stroke (5 cohorts), on hemodialysis (8 cohorts), with chronic kidney disease not on hemodialysis (3 cohorts), individuals with type 2 diabetes (4 cohort), and post-menopausal women (1 cohort). A number of countries were represented including populations exclusively from Australia, Hong Kong, Italy, Japan, Korea, Taiwan, the Netherlands, the U.K., and the U.S., as well as aggregated populations from a number of countries.
Table 1.
Characteristics of included cohorts.
| Year | First Author | Cohort | Sample Size |
Population | Countries | Outcomes | Follow-up Period |
|---|---|---|---|---|---|---|---|
| 2010 | Brickman10 | WHICAP | 686 | Older adults 65+ years of age |
USA | Stroke | 4.5 ± 0.8 years (mean) |
| 2008 | Brunelli11 | ArMORR | 6,961 | Adult hemodialysis patients |
USA | All-cause mortality, CVD mortality |
185 days (median); range 181–365 days |
| 2012 | Carr12 | MRC Elderly Trial |
4,396 | Elderly hypertensive patients |
UK | CHD, stroke | 5.8 years (mean) |
| 2014 | Chang13 | HEMO | 1,844 | Adult hemodialysis patients |
USA | All-cause mortality, CVD mortality |
2.5 years (median); IQR 1.3–4.3 years |
| 2014 | Chowdhury14 | ANPB2 | In-trial: 5,880 Post-trial: 5,542 |
Elderly hypertensive patients |
Australia | In-trial: CVD (fatal and non-fatal), MI (fatal and non-fatal), stroke (fatal and non-fatal) Post-trial: Fatal CVD, fatal MI, fatal stroke |
In-trial: 4.1 years (median) Post-trial: 6.9 years post-trial (median) |
| 2012 | Di Iorio16 | N/A | 374 | Adult CKD patients |
Italy | All-cause mortality | 33 ± 21 months (mean) |
| 2013 | Di Iorio15 | N/A | 1,088 | Adult hemodialysis patients |
Italy | All-cause mortality, CVD mortality |
5 years (max) |
| 2012 | Eguchi17 | Karatsu- Nishiarita Study |
457 | Adult hypertensive patients |
Japan | Hard CVD (stroke, MI, sudden cardiac death) |
66 ± 27 months (mean) |
| 2014 | Gao7 | N/A | 2,906 | Elderly primary care patients |
USA | All-cause mortality, CHD mortality, stroke mortality, stroke or CHD mortality |
12.9 years (median); range 2–16 years |
| 1997 | Grove18 | Honolulu Heart |
1,433 | Middle-age men of Japanese ancestry living in Oahu, HI |
USA | CHD | 11.6 years (mean) |
| 2013 | Hastie1 | N/A | 14,522 | Adult hypertensive patients |
Scotland | All-cause mortality, CVD mortality, ischemic heart disease mortality, stroke mortality |
35 years (max) |
| 2013 | Hata, J.19 | ADVANCE Trial |
8,811 | Adult type 2 diabetic patients |
20 countries from Asia, Australasia, Europe, and North America |
All-cause mortality, CVD mortality, MI, stroke, major macrovascular events (composite of stroke, MI, CVD mortality) |
2.4 years (median) |
| 2000 | Hata, Y.20 | N/A | 521 | Elderly hypertensive patients |
Japan | Stroke | 1 year |
| 2002 | Hata, Y.21 | N/A | 419 | Elderly hypertensive patients |
Japan | MI | 1 year |
| 1983 | Hofman22 | Framingham | 3,350 | Adult general population |
USA | All-cause mortality, CHD, CVD |
26 years (max) |
| 2012 | Hsieh23 | N/A | 2,161 | Adult type 2 diabetic patients |
Taiwan | All-cause mortality, CVD mortality |
66.7 ± 7.5 months (mean); range 21–80 months |
| 2013 | Kawai24 | NOAH | 485 | Adult hypertensive patients |
Japan | CVD | 7.6 ± 2.6 years (mean) |
| 2013 | Kim25 | N/A | 2,174 | Adult hemodialysis patients |
Korea | All-cause Mortality | 46.5 months (mean) |
| 2013 | Kostis26 | SHEP | 4,736 | Elderly with isolated systolic hypertension |
USA | CVD Mortality | Range 11.7 – 15.0 years |
| 2013 | Lau27 | N/A | 281 | Patients with recent lacunar infarct |
Hong Kong | ACS, all-cause mortality, CVD mortality, stroke |
78 ± 18 months (mean) |
| 2014 | Lau28 | N/A | 632 | Patients with recent ischemic stroke |
Hong Kong | ACS, all-cause mortality, CVD mortality, stroke |
76 ± 18 months |
| 2013 | Mallamaci29 | N/A | 1,618 | Adult CKD patients |
Italy | Composite of all-cause mortality and fatal and non-fatal CVD |
37 months (median); range 0.3–110 months |
| 2012 | Mancia6 | ELSA | 1,521 | Adult hypertensive patients |
Europe (France, Germany, Greece, Italy, Spain, Sweden, UK |
CVD | 4 years (max) |
| 2013 | McMullan30 | AASK | 908 | Adult African Americans with CKD |
USA | All-cause mortality, CVD, CVD mortality |
52 months (median); 75 months (max) |
| 2011 | Muntner2 | NHANES III | 956 | Adult general population |
USA | All-cause mortality | 14 years (median) |
| 2012 | Poortvliet (Short Term)31 |
PROSPER | 4,819 | Elderly adults with or at risk for CVD |
Ireland, Scotland, The Netherlands |
All-cause mortality, coronary events, stroke (fatal and non-fatal), vascular mortality |
3 years (max); 2.3 years (mean) |
| 2012 | Poortvliet (Long Term)31 |
PROSPER | 1,808 | Elderly adults with or at risk for CVD |
Scotland | All-cause mortality, coronary events, stroke (fatal and non-fatal), vascular mortality |
7.1 years (mean); 9.3 years (max) |
| 2003 | Pringle32 | Syst-Eur | 744 | Elderly hypertensive patients |
Europe (23 countries) |
CVD, CVD mortality, stroke |
4.4 years (median); range 1–109 months |
| 2012 | Rossignol33 | FOSIDIAL | 388 | Hemodialysis patients with LVH |
France | CVD | 2 years (max) |
| 2010 | Rothwell3 | UK-TIA Aspirin Trial |
2,006 | Patients with recent TIA or stroke |
UK | Stroke | 10 follow-up visits, occurring every 4 months (median); range 1–20 visits |
| 2010 | Rothwell3 | ASCOT- BPLA Trial |
18,530 | Adult hypertensive patients |
Europe (7 countries) |
Coronary events, stroke, composite of coronary events and stroke |
10 follow-up visits, occurring every 6 months (median) |
| 2010 | Rothwell3 | ESPS-1 Study | 1,247 | Patients with recent TIA or stroke (Placebo group) |
Europe (6 countries) |
Stroke | NR |
| 2010 | Rothwell3 | Dutch-TIA Trial |
3,150 | Patients with recent TIA or stroke |
The Netherlands |
Stroke | NR |
| 2014 | Selvarajah34 | N/A | 203 | Adult hemodialysis patients |
England | All-cause mortality | 2.0 ± 1.3 years (mean) |
| 2014 | Shafi35 | DEcIDE- ESRD |
11,291 | Adult hemodialysis patients |
USA | All-cause mortality, CVD, CVD mortality |
22 months (median); 25th–75th percentile 13–36 months |
| 2012 | Shimbo36 | WHI | 58,228 | Post-menopausal women |
USA | Stroke | 5.4 years (median) |
| 2013 | Suchy-Dicey37 | CHS | 3,852 | Elderly general population |
USA | All-cause mortality, stroke, MI |
9.9 years (mean) |
| 1999 | Tozawa38 | OKIDS | 144 | Adult hemodialysis patients |
Japan | All-cause mortality, CVD mortality |
35.2 ± 8.1 months (mean) |
| 2013 | Yinon39 | HEALS | 11,153 | Adult general population |
Bangladesh | CVD mortality (all and major), CHD mortality, stroke mortality, all- cause mortality |
6.5 years (mean) |
| 2007 | Zoppini41 | Verona Diabetes Study |
1,128 | Adult type 2 diabetic patients |
Italy | All-cause mortality, cerebrovascular disease mortality, CVD mortality, ischemic heart disease mortality |
10 years (max) |
| 2008 | Zoppini40 | Verona Diabetes Study |
1,319 | Adult type 2 diabetic patients |
Italy | All-cause mortality | 10 years (max) |
ACS, acute coronary syndrome; CHD, coronary heart disease; CKD, chronic kidney disease; CVD, cardiovascular disease; IQR, interquartile range; LVH, left ventricular hypertrophy; MI, myocardial infarction; N/A, not available; NR, not reported; TIA, transient ischemic attack.
Data in table are sorted in alphabetical order.
Visit-to-Visit Variability Metrics
The number of visits used to derive VVV ranged from 3 visits to 156 visits with 13 cohorts using the same number of visits for each participant (Table 2). The number of BP readings per visit was 1 measure (9 cohorts), 2 measures (19 cohorts), 3 measures (8 cohorts), varied (2 cohorts), or was not reported (3 cohorts). Across the cohorts, the time-interval between visits ranged from 2 days to 3–4 years. The time-interval between visits was uniform for 26 cohorts, varied for 13 cohorts, and was not reported for 2 cohorts. Among the included cohorts, 22 reported one measure of VVV (e.g., SD) and 8 reported two measures of VVV (e.g., SD and coefficient of variation [CV]). The remaining cohorts reported more than 2 VVV measures with six different measures of VVV reported for one cohort. The most common measures used to quantify VVV were SD (23 cohorts) and CV (21 cohorts). Nineteen other VVV measures were reported. VVV of SBP was reported in 37 cohorts, VVV of DBP was reported in 21 cohorts, VVV of MAP was reported in 2 cohorts, and VVV of PP was reported in 7 cohorts. VVV of SBP and VVV of DBP were both reported in 20 cohorts.
Table 2.
Methodology for measurement of BP and calculation of visit-to-visit variability.
| Year | First Author |
Number of Visits to Derive VVV |
Number of BP Readings per Visit to Derive VVV |
Time Between Visits |
Method of BP Assessment |
VVV of SBP, DBP, MAP, or PP |
VVV Metrics |
VVV as continuous |
VVV as categorical |
|---|---|---|---|---|---|---|---|---|---|
| 2010 | Brickman | 3 | 1 (3 taken, but only the 3rd used to calculate VVV) |
~2 years (visit 1 to 2: 2.12 ± 0.71 yrs; visit 2 to 3: 2.45 ± 0.65 yrs) |
Automated (Dinamap Pro 100) |
MAP | SD | No | Yes; 4 groups based on the median split of the mean BP measurement and the median split of the SD across the study |
| 2008 | Brunelli | 35.9 ± 4.5; range 4–52 |
1 | 2 days (visits 3×/week on either Mon./Wed./Fri. or Tues./Thurs./Sat.) |
Manual | SBP, DBP | Average residual: intercept ratio |
Yes | No |
| 2012 | Carr | NR | 2 | 2 weeks for 1st month, monthly for 3 months, every 3 months thereafter |
Manual | SBP, DBP | Maximum BP, RSV, standard residual |
Yes | No |
| 2014* | Chang | 4.9 ± 1.2; range 3–13 |
1 | 8.0 ± 4.7 days; range 3–56 days |
Automated (varied devices) |
SBP | ARV, CV | Yes | No |
| 2014 | Chowdhury | 8 (median); range 2–19 |
2 (3 taken, but only last 2 used) |
6 months (mean); 5.5 months (median); IQR 4.5–6.5 months |
Manual | SBP | In-trial: ARV, SD Post-trial: SD |
Yes | Yes; deciles of VVV |
| 2012 | Di Iorio | 5 | 3 | ~1 month (5 visits over a 4–5 month period) |
Semi- automated oscillometric device |
SBP | CV | Yes | No |
| 2013 | Di Iorio | NR | 1 | Visits 3×/week | Manual | SBP | CV | Yes | Yes; quartiles of VVV |
| 2012 | Eguchi | 36.5 ± 22.6; range 1–78 |
2 (3 taken, but only last 2 used) |
1 month | Manual | SBP, DBP | SD | Yes | No |
| 2014 | Gao | 35 (median), 39.8 (mean); range 6–258 |
1 | NR (derived from electronic medical records; time interval varied for each participant) |
NR | SBP, DBP | Root mean square error |
Yes | Yes; 6 groups based on tertile of BP regression slope and quartile of VVV (lowest quartile or all other quartiles) |
| 1997 | Grove | 3 or 4 | 2 or 3 (3 at visits 1 and 2; 2 at visits 3 and 4) |
~3 years for visits 1–3; ~4 years for visit 4 |
Manual | SBP | Variance of the residuals |
Yes | Yes; quintiles of VVV |
| 2013 | Hastie | ≥3; Year 1 VVV: 3.6 ± 0.8; Years 2–5 VVV: 7.8 ± 3.2; Years 5–10 VVV: 7.9 ± 3.7 |
2 (3 taken, but only last 2 used) |
≥30 days; Year 1 VVV: 77.9 ± 37.1 days; Years 2–5 VVV: 157.5 ± 111.9 days; 204.1 ± 193.0 days |
Manual | SBP, DBP | ARV, CV, SD |
No | Yes; quartiles of VVV; 4 groups based on median split of VVV over Year 1 and median split of VVV over Years 2–5 |
| 2013 | Hata, J. | 6 | 2 | 1 month between visits 1 and 2, 2 months between visits 2 and 3, every 6 months thereafter |
Automated (Omron HEM- 705CP) |
SBP | CV, Maximum BP, SD |
Yes | Yes; deciles of VVV |
| 2000 | Hata, Y. | cases: 9.8 ± 2.4; controls: 10.3 ± 2.3 |
2 | ~1 month (all visits occurred over 1 year) |
Manual | SBP, DBP | BP range, CV, maximum BP change |
Yes | No |
| 2002 | Hata, Y. | 10 ± 2 | 2 | ~1 month (all visits occurred over 1 year) |
Manual | DBP | CV | Yes | No |
| 1983 | Hofman | range 5–7 | 2 | 2 years | Manual | SBP | Yearly change, conditional on initial BP level or attained BP level |
Yes | No |
| 2012 | Hsieh | 15.7 ± 3.4; range 9–23 |
2 | 2–6 months | Automated (Omron HEM- 1000) |
SBP, DBP, MAP, PP |
CV, SD | Yes | No |
| 2013 | Kawai | 6 | 2 | 1–2 months | Automated (Omron HEM- 705IT or HEM-711) |
SBP | SD | No | Yes; High vs. low VVV cut -off determined by ROC curve analysis |
| 2013 | Kim | NR | 2 (3 taken, but only highest and lowest used) |
NR (days between dialysis visits) |
NR | SBP, DBP | ARV | No | Yes; high vs. low VVV (cut- off determination NR) |
| 2013 | Kostis | 15 mean; range 9–33 |
2 | 1 month, visits 1–4; every 3 months for all remaining visits |
Random zero sphygmomano meter |
SBP | rSSR, VABS2, VIM |
Yes | No |
| 2013 | Lau | 12 ± 6; range 3–36 |
3 | 3–4 months | Automated (Dinamap PRO100) |
SBP, DBP | SD | Yes | Yes, tertiles of VVV |
| 2014 | Lau | 12 ± 6; range 3–36 |
3 | 3–4 months | Automated (Dinamap PRO 100) |
SBP, DBP | CV | Yes | Yes, quartiles of VVV |
| 2013 | Mallamaci | range 2–7 | 3 | 8 ± 5 months | Manual | SBP, DBP | CV, SD | Yes | No |
| 2012 | Mancia | 7+ | 3 | 6 months | Manual | SBP, DBP | CV, SD | Yes | No |
| 2013 | McMullan | 5 | 2 (3 taken, but only last 2 used) |
2 months | Random zero sphygmomano meter |
SBP | SD | Yes | Yes; tertiles of VVV |
| 2011 | Muntner | 3 | 2 (3 taken, but only last 2 used) |
17 days (median); range 1–48 |
Manual | SBP, DBP | CV, SD | No | Yes; tertiles of VVV |
| 2012 | Poortvliet (Short Term) |
5 | NR | 3 months | Automated (Omron M4) |
SBP, DBP, PP |
SD | Yes | Yes; quartiles of VVV |
| 2012 | Poortvliet (Long Term) |
9 | NR | 3 months | Automated (Omron M4) |
SBP, DBP, PP |
SD | Yes | Yes; quartiles of VVV |
| 2003 | Pringle | 3 | 2 | ~1 month | Manual | SBP | SD | Yes | No |
| 2012 | Rossignol | 17 | 3 | 1 week for weeks 1–6; bi-weekly for weeks 6–8; 3 months for all subsequent visits |
Manual | SBP, DBP, PP |
ARV†, CV, CV of ARV†, residual of the linear fit between SD and mean BP, SD |
Yes | No |
| 2010 | Rothwell (UK-TIA Aspirin Trial) |
2, 4, 6, 8, 10 (separate analyses) |
1 | 4 months | Random zero sphygmomano meter |
SBP, DBP | CV, maximum BP, SD, VIM |
No | Yes; deciles of VVV |
| 2010 | Rothwell (ASCOT-B PLA Trial) |
NR | 2 (3 taken, but only last 2 used) |
6 months | Automated (Omron HEM-705CP) |
SBP, DBP | ARV†, CV, maximum BP, RSD, SD, VIM |
Yes | Yes; deciles of VVV |
| 2010 | Rothwell (ESPS-1 Study) |
NR | 2 (mean of right and left arms) |
3 months | Manual | SBP | CV, SD, VIM |
No | Yes; deciles of VVV |
| 2010 | Rothwell (Dutch- TIA Trial) |
NR | 1 | 4 months | Manual | SBP | CV, SD, VIM |
No | Yes; deciles of VVV |
| 2014 | Selvarajah | 25.00 ± 1.63 | NR | 2–5 days | Automated oscillometric device (Fresenius 4008S or Nikisso DBB-05) |
SBP, DBP | CV, SD, VIM |
Yes | Yes; median split |
| 2014 | Shafi | 32.8 ± 9.3 | 1 | 2–3 days | Automated oscillometric device |
SBP | SD of residuals from modeled average BP over time |
Yes | Yes; tertiles of VVV |
| 2012 | Shimbo | 7.9 ± 1.8 | 2 | 1 year | Manual | SBP | SD, SDreg | Yes | Yes; quartiles of VVV |
| 2013 | Suchy-Dicey | 5 | 2 (3 taken, but only last 2 used) |
1 year | Random zero sphygomanometer for visit 1; manual for visits 2–5 |
SBP, DBP, PP |
SDreg | Yes | No |
| 1999 | Tozawa | 156 | 1 | visits 3x/week over 1 year |
Manual | SBP | ΔBP (maximum minus minimum), CV |
Yes | Yes; median split |
| 2013 | Yinon | 3.84 (mean); range 2–4 |
1, 3 if BP ≥ 140/90 mmHg at 1st measurement (lowest reading of 3 used) |
2.2 years (mean) |
Automated (Omron HEM 712-C) |
SBP | SD | Yes | Yes; tertiles of VVV |
| 2007 | Zoppini | 6+ | 3 | NR | Manual | PP | CV | Yes | No |
| 2008 | Zoppini | 7 (median); range 3–31 |
3 | NR | Manual | PP | CV | No | Yes; tertiles of VVV |
ARV, average real variability; BP, blood pressure; CV, coefficient of variation; DBP, diastolic blood pressure; IQR, interqua MAP, mean arterial pressure; NR, not reported; PP, pulse pressure; ROC, receiver operating characteristic; rSSR, sum deviations between daily average blood pressure value and the trend-predicted blood pressure; RSD, residual standard successive variance; SBP, systolic blood pressure; SD, standard deviation; SDreg, standard deviation about regression pressure regressed across visits; VABS2, variance of the absolute values of the second differences between successive pressure values; VIM, variance independent of the mean; VVV, visit-to-visit variability.
The study by Chang et. al. initially appeared online in 2013.
described as average successive variation (ASV) in original publication.
Data in table are sorted in alphabetical order.
VVV of SBP and Outcomes
SD of SBP, modeled as a continuous variable, was associated with an increased risk for stroke in 3 of 9 analyses, stroke mortality in 0 of 1 analyses, CHD in 4 of 6 analyses, CHD mortality in 0 of 1 analyses, CVD in 3 of 8 analyses, CVD mortality in 5 of 9 analyses, all-cause mortality in 4 of 7 analyses, and a composite outcome of all-cause mortality/CVD in 1 of 1 analyses (Table 3, left panel). Modeled as a categorical variable, increased risk was present in the highest versus lowest SD of SBP category in the majority of analyses for stroke, CVD, CVD mortality, and all-cause mortality, but not stroke mortality, CHD, or CHD mortality (Table 4, left panel). Mean BP was included as a covariate in 22 of the 24 cohorts (91.7%) which examined SD of SBP and outcomes.
Table 3.
Results reported for continuous analysis of standard deviation and coefficient of variation of systolic blood pressure and outcomes.
| Standard deviation | Coefficient of variation | |||
|---|---|---|---|---|
| Study | HR/OR/RR (95% CI) | Units* | HR/OR/RR (95% CI) | Units* |
| Stroke | ||||
| Chowdhury et al, 2014 (In-trial) | 1.07 (1.04 – 1.10) | 1 mmHg | - | - |
| Hata, J. et al, 2013 | 1.08 (0.93 – 1.25) | 5 mmHg | 1.08 (0.93 – 1.25) | 3.4% |
| Hata, Y. et al, 2000 | - | - | 1.15 (1.03 – 1.29) | 2% |
| Lau et al, 2013 | 1.13 (0.83 – 1.52) | 6 mmHg | - | - |
| Lau et al, 2014 | - | - | 1.02 (0.97 – 1.07) | 4% |
|
Poortvliet et al, 2012 (Short- term follow-up cohort) |
Not statistically sig. (Data NR) |
NR | - | - |
|
Poortvliet et al, 2012 (Long- term follow-up cohort) |
1.1 (1.0 – 1.3) | 4.88 mmHg | - | - |
|
Pringle et al, 2003 (Treatment group) |
1.50 (0.93 – 2.41) | 5 mmHg | - | - |
|
Pringle et al, 2003 (Placebo group) |
0.84 (0.50 – 1.39) | 5 mmHg | - | - |
|
Rothwell et al, 2010 (ASCOT-BPLA ABPM Substudy) |
1.69 (1.34 – 2.11) | 1 SD | 1.78 (1.40 – 2.26) | 1 SD |
| Shimbo et al, 2012 | 1.16 (1.08 – 1.24) | 5 mmHg | - | - |
| Stroke Mortality | ||||
| Yinon et al, 2013 | 1.51 (0.93 – 2.44) | 1 SD of Log | - | - |
| CHD | ||||
| Chowdhury et al, 2014 (In-trial) | 1.09 (1.05 – 1.12) | 1 mmHg | - | - |
| Hata, J. et al, 2013 | 1.32 (1.11 – 1.55) | 5 mmHg | 1.29 (1.10 – 1.52) | 3.4% |
| Lau et al, 2013 | 1.14 (0.75 – 1.73) | 6 mmHg | - | - |
| Lau et al, 2014 | - | - | 0.95 (0.85 – 1.06) | 4% |
|
Poortvliet et al, 2012 (Short- term follow-up cohort) |
Not statistically sig. (Data NR) |
NR | - | - |
|
Poortvliet et al, 2012 (Long- term follow-up cohort) |
1.1 (1.0 – 1.3) | 5 mmHg | - | - |
|
Rothwell et al, 2010 (ASCOT-BPLA ABPM Substudy) |
1.43 (1.23 – 1.67) | 1 SD | 1.49 (1.27 – 1.75) | 1 SD |
| CHD Mortality | ||||
| Yinon et al, 2013 | 0.78 (0.56 – 1.08) | 1 SD of Log | - | - |
| CVD | ||||
| Chowdhury et al, 2014 (In-trial) | 1.05 (1.04 – 1.06) | 1 mmHg | - | - |
| Eguchi et al, 2012 | 0.75 (0.48 – 1.17) | 5 mmHg | - | - |
| Hata, J. et al, 2013 | 1.18 (1.07 – 1.30) | 5 mmHg | 1.18 (1.07 – 1.29) | 3.4% |
| Mancia et al, 2012 | 0.999 (0.952 – 1.048) | NR | 0.976 (0.906 – 1.051) | NR |
|
Pringle et al, 2003 (Treatment group) |
0.88 (0.64 – 1.20) | 5 mmHg | - | - |
|
Pringle et al, 2003 (Placebo group) |
1.04 (0.74 – 1.47) | 5 mmHg | - | - |
| Rossignol et al, 2012 | Not statistically sig. (Data NR) |
NR | 1.08 (1.03 – 1.14) | NR |
|
Rothwell et al, 2010 (ASCOT-BPLA ABPM Substudy) |
1.50 (1.31 – 1.72) | 1 SD | 1.57 (1.37 – 1.80) | 1 SD |
| CVD Mortality | ||||
| Chang et al, 2014 | - | - | 1.10 (0.89 – 1.37) | 10% |
| Di Iorio et al, 2013 | - | - | 1.21 (1.05 – 1.33) | NR |
| Hata, J. et al, 2013 | 1.30 (1.13 – 1.50) | 5 mmHg | 1.29 (1.12 – 1.48) | 3.4% |
| Hsieh et al, 2012 | 1.05 (0.96 – 1.14) | NR | 1.08 (0.95 – 1.22) | NR |
| Lau et al, 2013 | 1.53 (1.05 – 2.25) | 6 mmHg | - | - |
| Lau et al, 2014 | - | - | 1.25 (0.99 – 1.57) | 4% |
|
Poortvliet et al, 2012 (Short- term follow-up cohort) |
Not statistically sig. (Data NR) |
NR | - | - |
|
Poortvliet et al, 2012 (Long- term follow-up cohort) |
1.2 (1.1 – 1.4) | 5 mmHg | - | - |
|
Pringle et al, 2003 (Treatment group) |
1.15 (0.76 – 1.74) | 5 mmHg | - | - |
|
Pringle et al, 2003 (Placebo group) |
0.82 (0.49 – 1.38) | 5 mmHg | - | - |
| Tozawa et al, 1999 | - | - | 1.78 (0.94 – 3.37) | 1% |
|
Yinon et al, 2013 (All CVD Mortality) |
1.41 (1.04 – 1.92) | 1 SD of Log | - | - |
|
Yinon et al, 2013 (Major CVD Mortality) |
1.84 (1.27 – 2.66) | 1 SD of Log | - | - |
| All-Cause Mortality | ||||
| Chang et al, 2014 | - | - | 1.18 (1.02 – 1.36) | 10% |
|
Di Iorio et al, 2012 (Before Dialysis Entry) |
- | - | 1.06 (1.02 – 1.09) | NR |
|
Di Iorio et al, 2012 (Including Time After Dialysis Inception) |
- | - | 1.05 (1.03 – 1.09) | NR |
| Di Iorio et al, 2013 | - | - | 1.02 (0.95 – 1.06) | NR |
| Hata, J. et al, 2013 | 1.29 (1.17 – 1.43) | 5 mmHg | 1.28 (1.16 – 1.40) | 3.4% |
| Hsieh et al, 2012 | 1.05 (1.01 – 1.09) | NR | 1.06 (1.00 – 1.12) | NR |
| Lau et al, 2013 | 1.20 (0.96 – 1.51) | 6 mmHg | - | - |
| Lau et al, 2014 | - | - | 1.23 (1.07 – 1.41) | 4% |
|
Poortvliet et al, 2012 (Short- term follow-up cohort) |
Not statistically sig. (Data NR) |
NR | - | - |
|
Poortvliet et al, 2012 (Long- term follow-up cohort) |
1.1 (1.1 – 1.2) | 5 mmHg | - | - |
| Selvarajah et al, 2014 | 1.08 (1.01 – 1.16) | 1 mmHg | 1.13 (1.02 – 1.24) | 1% |
| Tozawa et al, 1999 | - | - | 1.63 (1.05 – 2.53) | 1% |
| Yinon et al, 2013 | 0.99 (0.82 – 1.74) | 1 SD of Log | - | - |
|
Composite of all-cause mortality and fatal and non- fatal CVD |
||||
| Mallamaci et al, 2013 | 1.15 (1.03 – 1.27) | 5 mmHg | 1.17 (1.02 – 1.34) | 5% |
CHD, coronary heart disease; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; MI, myocardial infarction; NR, not reported; OR, odds ratio; RR, relative risk; SD, standard deviation; VVV, visit-to-visit variability.
Units represented by the measure of association
Dash indicates data were not examined.
For each outcome, data in table are sorted in alphabetical order by first author’s last name.
Table 4.
Results reported for categorical analysis of standard deviation and coefficient of variation of systolic blood pressure and outcomes.
| Standard deviation | Coefficient of variation | |||||
|---|---|---|---|---|---|---|
| Study |
HR/OR/RR (95% CI) |
Levels | Comparison |
HR/OR/RR (95% CI) |
Levels | Comparison |
| Stroke | ||||||
|
Chowdhury et al, 2014 (In-trial) |
2.78 (1.28 – 6.05) | Deciles | Top decile (≥19.72 mmHg) vs. bottom decile (≤7.07 mmHg) |
- | - | - |
| Hata, J. et al, 2013 | 1.06 (0.53 – 2.10) | Deciles | Top decile (≥18.1 for placebo group; ≥16.8 for active treatment group) vs. bottom decile (≤5.2 for placebo group; ≤5.0 for active treatment group) |
- | - | - |
| Lau et al, 2013 | 1 (ref) 0.95 (0.41 – 2.19) 1.14 (0.51 – 2.56) |
Tertiles | <13.0 13.0 – 17.5 >17.5 |
- | - | - |
| Lau et al, 2014 | - | - | - | 1 (ref) 0.90 (0.49 – 1.66) 0.68 (0.35 – 1.30) 1.08 (0.60 – 1.93) |
Quartiles | <8.6 (ref) 8.6–10.9 11.0–14.2 >14.2 |
|
Poortvliet et al, 2012 (Short-term follow-up cohort) |
1 (ref) 1.0 (0.6 – 1.6) 1.1 (0.7 – 1.8) 1.2 (0.8 – 1.9) |
Quartiles | ≤9 (ref) >9 – 12.5 >12.5 – ≤17 >17 |
- | - | - |
|
Poortvliet et al, 2012 (Long-term follow-up cohort) |
1 (ref) 1.0 (0.7 – 1.5) 1.3 (0.9 – 2.0) 1.3 (0.9 – 1.8) |
Quartiles | <10.5 (ref) >10.5 – ≤13 >13 – ≤16.5 >16.5 |
- | - | - |
|
Rothwell et al, 2010 (UK-TIA Aspirin Trial) |
4.37 (2.73 – 6.99) | Deciles | Top vs. bottom decile | 3.82 (2.54 – 5.73) | Deciles | Top vs. bottom decile |
|
Rothwell et al, 2010 (ASCOT-BPLA Trial, all participants) |
2.57 (1.59 – 4.15) | Deciles | Top vs. bottom decile | 2.06 (1.28 – 3.31) | Deciles | Top vs. bottom decile |
|
Rothwell et al, 2010 (ASCOT- BPLA Trial, treatment cohorts: amlodipine and atenolol treatment groups combined) |
2.54 (1.28 – 5.04) | Deciles | Top vs. bottom decile | 2.28 (1.13 – 4.60) | Deciles | Top vs. bottom decile |
|
Rothwell et al, 2010 (ASCOT- BPLA Trial, Amlodipine Treatment Group) |
3.80 (1.67 – 8.65) | Deciles | Top vs. bottom decile | 3.01 (1.39 – 6.52) | Deciles | Top vs. bottom decile |
|
Rothwell et al, 2010 (ASCOT- BPLA Trial, Atenolol Treatment Group) |
4.06 (2.17 – 7.60) | Deciles | Top vs. bottom decile | 3.30 (1.83 – 5.94) | Deciles | Top vs. bottom decile |
|
Rothwell et al, 2010 (ESPS-1 Study) |
1.78 (1.21 – 2.62) | Deciles | Top vs. bottom decile | 2.22 (1.52 – 3.22) | Deciles | Top vs. bottom decile |
|
Rothwell et al 2010 (Dutch TIA Trial) |
3.35 (1.63 – 6.87) | Deciles | Top vs. bottom decile | 3.41 (1.62 – 7.19) | Deciles | Top vs. bottom decile |
| Shimbo et al, 2012 | 1 (ref) 1.39 (1.03 – 1.89) 1.52 (1.13 – 2.03) 1.72 (1.28 – 2.32) |
Quartiles | <6 (ref) 6.0 – 8.9 9.0 – 12.9 ≥13.0 |
- | - | - |
| Stroke Mortality | ||||||
|
Chowdhury et al, 2014 (Post-trial) |
1.90 (0.50 – 7.21) | Deciles | Top decile (≥19.72 mmHg) vs. bottom decile (≤7.07 mmHg) |
- | - | - |
|
Hastie et al, 2013 (Year 1) |
1 (ref) 1.01 (0.67 – 1.53) 0.80 (0.53 – 1.20) 1.28 (0.87 – 1.88) |
Quartiles | <13.87 (ref) 13.87 – 18.20 18.21 – 23.14 >23.15 |
1 (ref) 0.94 (0.66 – 1.35) 0.99 (0.70 – 1.40) 1.00 (0.71 – 1.42) |
Quartiles | <11.0 (ref) 11.0 – 13.0 13.1 – 18.0 >18.0 |
|
Hastie et al, 2013 (Years 2–5) |
1 (ref) 0.91 (0.55 – 1.52) 0.98 (0.61 – 1.58) 1.65 (1.04 – 2.62) |
Quartiles | <12.98 (ref) 12.98 – 17.07 17.08 – 21.80 >21.81 |
1 (ref) 0.93 (0.58 – 1.49) 1.12 (0.71 – 1.75) 1.49 (0.95 – 2.31) |
Quartiles | <9.6 (ref) 9.6 – 10.8 10.9 – 12.3 >12.4 |
|
Hastie et al, 2013 (Years 5–10) |
1 (ref) 1.19 (0.58 – 2.44) 1.45 (0.75 – 2.83) 1.40 (0.71 – 2.76) |
Quartiles | <13.04 (ref) 13.04 – 16.99 17.00 – 21.52 >21.53 |
1 (ref) 0.96 (0.48 – 1.91) 1.21 (0.62 – 2.36) 1.31 (0.66 – 2.56) |
Quartiles | <9.8 (ref) 9.8 – 10.9 11.0 – 12.4 >12.5 |
|
Hastie et al, 2013 (Years 10+) |
1 (ref) 0.72 (0.21 – 2.54) 1.06 (0.33 – 3.46) 2.39 (0.84 – 6.77) |
Quartiles | <13.15 (ref) 13.15 – 17.59 17.60 – 21.81 >21.82 |
1 (ref) 1.09 (0.34 – 3.48) 1.65 (0.53 – 5.09) 2.29 (0.78 – 6.72) |
Quartiles | <9.5 (ref) 9.5 – 11.4 11.5 – 12.6 >12.7 |
| Yinon et al, 2013 | 1 (ref) 0.70 (0.30 – 1.63) 1.43 (0.73 – 2.79) |
Tertiles | <7.36 (ref) 7.36 – 11.49 >11.49 |
- | - | - |
| CHD | ||||||
|
Chowdhury et al, 2014 (In-trial) |
4.11 (1.87 – 9.06) | Deciles | Top decile (≥19.72 mmHg) vs. bottom decile (≤7.07 mmHg) |
- | - | - |
| Hata, J. et al, 2013 | 1.55 (0.75 – 3.20) | Deciles | Top decile (≥18.1 for placebo group; ≥16.8 for active treatment group) vs. bottom decile (≤5.2 for placebo group; ≤5.0 for active treatment group) |
- | - | - |
| Lau et al, 2013 | 1 (ref) 1.16 (0.31 – 4.37) 2.13 (0.62 – 7.35) |
Tertiles | <13.0 13.0 – 17.5 >17.5 |
- | - | - |
| Lau et al, 2014 | - | - | - | 1 (ref) 1.06 (0.36 – 3.14) 0.34 (0.08 – 1.51) 0.60 (0.18 – 2.02) |
Quartiles | <8.6 (ref) 8.6–10.9 11.0–14.2 >14.2 |
|
Poortvliet et al, 2012 (Short-term follow-up cohort) |
1 (ref) 0.8 (0.6 – 1.1) 1.0 (0.7 – 1.3) 1.0 (0.8 – 1.3) |
Quartiles | ≤9 (ref) >9 – 12.5 >12.5 – ≤17 >17 |
- | - | - |
|
Poortvliet et al, 2012 (Long-term follow-up cohort) |
1 (ref) 0.9 (0.6 – 1.3) 1.3 (0.9 – 1.9) 1.2 (0.8 – 1.7) |
Quartiles | <10.5 (ref) >10.5 – ≤13 >13 – ≤16.5 >16.5 |
- | - | - |
| CHD Mortality | ||||||
|
Chowdhury et al, 2014 (Post-trial) |
4.35 (1.18 – 16.06) | Deciles | Top decile (≥19.72 mmHg) vs. bottom decile (≤7.07 mmHg) |
- | - | - |
|
Hastie et al, 2013 (Year 1) |
1 (ref) 1.24 (0.94 – 1.63) 1.33 (1.01 – 1.74) 1.26 (0.95 – 1.66) |
Quartiles | <13.87 (ref) 13.87 – 18.20 18.21 – 23.14 >23.15 |
1 (ref) 1.23 (0.97 – 1.57) 1.18 (0.93 – 1.50) 1.09 (0.85 – 1.40) |
Quartiles | <11.0 (ref) 11.0 – 13.0 13.1 – 18.0 >18.0 |
|
Hastie et al, 2013 (Years 2–5) |
1 (ref) 0.95 (0.69 – 1.30) 1.14 (0.85 – 1.54) 1.22 (0.90 – 1.65) |
Quartiles | <12.98 (ref) 12.98 – 17.07 17.08 – 21.80 >21.81 |
1 (ref) 1.25 (0.93 – 1.68) 1.28 (0.96 – 1.71) 1.35 (1.01 – 1.82) |
Quartiles | <9.6 (ref) 9.6 – 10.8 10.9 – 12.3 >12.4 |
|
Hastie et al, 2013 (Years 5–10) |
1 (ref) 0.83 (0.55 – 1.25) 0.92 (0.62 – 1.35) 1.12 (0.76 – 1.65) |
Quartiles | <13.04 (ref) 13.04 – 16.99 17.00 – 21.52 >21.53 |
1 (ref) 1.10 (0.73 – 1.64) 1.25 (0.84 – 1.86) 1.23 (0.81 – 1.86) |
Quartiles | <9.8 (ref) 9.8 – 10.9 11.0 – 12.4 >12.5 |
|
Hastie et al, 2013 (Years 10+) |
1 (ref) 1.28 (0.69 – 2.38) 1.52 (0.82 – 2.81) 1.28 (0.68 – 2.42) |
Quartiles | <13.15 (ref) 13.15 – 17.59 17.60 – 21.81 >21.82 |
1 (ref) 1.06 (0.56 – 2.02) 1.73 (0.93 – 3.21) 1.58 (0.83 – 3.02) |
Quartiles | <9.5 (ref) 9.5 – 11.4 11.5 – 12.6 >12.7 |
| Yinon et al, 2013 | 1 (ref) 0.78 (0.49 – 1.27) 0.89 (0.55 – 1.44) |
Tertiles | <7.36 (ref) 7.36 – 11.49 >11.49 |
- | - | - |
| CVD | ||||||
|
Chowdhury et al, 2014 (In-trial) |
2.18 (1.52 – 3.13) | Deciles | Top decile (≥19.72 mmHg) vs. bottom decile (≤7.07 mmHg) |
- | - | - |
| Hata, J. et al, 2013 | 1.54 (0.99 – 2.39) | Deciles | Top decile (≥18.1 for placebo group; ≥16.8 for active treatment group) vs. bottom decile (≤5.2 for placebo group; ≤5.0 for active treatment group) |
- | - | - |
| Kawai et al, 2013 | 1 (ref) 1.96 (1.05 – 4.10) |
High vs. low cut-off determined by ROC curve analysis |
<8.1 (ref) vs. ≥8.1 |
- | - | - |
| McMullan et al, 2013 | 1 (ref) 1.28 (0.71 – 2.29) 1.23 (0.65 – 2.34) |
Tertiles | 1.30 – 9.37 (ref) 9.40 – 15.47 15.51 – 55.56 |
- | - | - |
|
Rothwell et al, 2010 (ASCOT-BPLA Trial, all participants) |
1.80 (1.30 – 2.49) | Deciles | Top vs. bottom decile | 1.57 (1.14 – 2.16) | Deciles | Top vs. bottom decile |
|
Rothwell et al, 2010 (ASCOT- BPLA Trial, treatment cohorts: amlodipine and atenolol treatment groups combined) |
1.94 (1.16 – 3.24) | Deciles | Top vs. bottom decile | 1.84 (1.11 – 3.05) | Deciles | Top vs. bottom decile |
|
Rothwell et al, 2010 (ASCOT- BPLA Trial, Amlodipine Treatment Group) |
2.85 (1.56 – 5.21) | Deciles | Top vs. bottom decile | 3.36 (2.00 – 5.66) | Deciles | Top vs. bottom decile |
|
Rothwell et al, 2010 (ASCOT- BPLA Trial, Atenolol Treatment Group) |
1.99 (1.25 – 3.18) | Deciles | Top vs. bottom decile | 2.05 (1.32 – 3.19) | Deciles | Top vs. bottom decile |
| CVD Mortality | ||||||
|
Chowdhury et al, 2014 (Post-trial) |
2.41 (1.45 – 4.00) | Deciles | Top decile (≥19.72 mmHg) vs. bottom decile (≤7.07 mmHg) |
- | - | - |
|
Hastie et al, 2013 (Year 1) |
1 (ref) 1.16 (0.94 – 1.43) 1.21 (0.99 – 1.48) 1.28 (1.05 – 1.57) |
Quartiles | <13.87 (ref) 13.87 – 18.20 18.21 – 23.14 >23.15 |
1 (ref) 1.13 (0.94 – 1.35) 1.08 (0.91 – 1.29) 1.04 (0.87 – 1.25) |
Quartiles | <11.0 (ref) 11.0 – 13.0 13.1 – 18.0 >18.0 |
|
Hastie et al, 2013 (Years 2–5) |
1 (ref) 0.94 (0.74 – 1.18) 1.04 (0.83 – 1.30) 1.23 (0.98 – 1.54) |
Quartiles | <12.98 (ref) 12.98 – 17.07 17.08 – 21.80 >21.81 |
1 (ref) 1.07 (0.86 – 1.33) 1.11 (0.89 – 1.36) 1.23 (0.99 – 1.53) |
Quartiles | <9.6 (ref) 9.6 – 10.8 10.9 – 12.3 >12.4 |
|
Hastie et al, 2013 (Years 5–10) |
1 (ref) 0.95 (0.69 – 1.31) 1.02 (0.75 – 1.38) 1.16 (0.86 – 1.58) |
Quartiles | <13.04 (ref) 13.04 – 16.99 17.00 – 21.52 >21.53 |
1.04 (0.77 – 1.40) 1.12 (0.83 – 1.52) 1.19 (0.87 – 1.63) |
Quartiles | <9.8 (ref) 9.8 – 10.9 11.0 – 12.4 >12.5 |
|
Hastie et al, 2013 (Years 10+) |
1 (ref) 1.26 (0.78 – 2.02) 1.36 (0.84 – 2.20) 1.56 (0.98 – 2.50) |
Quartiles | <13.15 (ref) 13.15 – 17.59 17.60 – 21.81 >21.82 |
1 (ref) 1.27 (0.78–2.07) 1.79 (1.11–2.90) 1.69 (1.02–2.77) |
Quartiles | <9.5 (ref) 9.5 – 11.4 11.5 – 12.6 >12.7 |
| Hata, J. et al, 2013 | 2.49 (1.15 – 5.37) | Deciles | Top decile (≥18.1 for placebo group; ≥16.8 for active treatment group) vs. bottom decile (≤5.2 for placebo group; ≤5.0 for active treatment group) |
- | - | - |
| Lau et al, 2013 | 1 (ref) 2.00 (0.36 – 11.21) 7.64 (1.65 – 35.41) |
Tertiles | <13.0 13.0 – 17.5 >17.5 |
- | - | - |
| Lau et al, 2014 | - | - | - | 1 (ref) 1.69 (0.67 – 4.26) 1.64 (0.68 – 3.98) 2.36 (1.02 – 5.49) |
Quartiles | <8.6 (ref) 8.6–10.9 11.0–14.2 >14.2 |
|
Poortvliet et al, 2012 (Short-term follow-up cohort) |
1 (ref) 0.8 (0.5 – 1.2) 1.0 (0.6 – 1.5) 0.9 (0.6 – 1.3) |
Quartiles | ≤9 (ref) >9 – 12.5 >12.5 – ≤17 >17 |
- | - | - |
|
Poortvliet et al, 2012 (Long-term follow-up cohort) |
1 (ref) 1.1 (0.7 – 1.5) 1.5 (1.0 – 2.1) 1.6 (1.1 – 2.2) |
Quartiles | <10.5 (ref) >10.5 – ≤13 >13 – ≤16.5 >16.5 |
- | - | - |
|
Yinon et al, 2013 (All CVD Mortality) |
1 (ref) 0.56 (0.33 – 0.96) 1.27 (0.85 – 1.92) |
Tertiles | <7.36 (ref) 7.36 – 11.49 >11.49 |
- | - | - |
|
Yinon et al, 2013 (Major CVD Mortality) |
1 (ref) 0.69 (0.36 – 1.30) 1.70 (1.03 – 2.82) |
Tertiles | <7.36 (ref) 7.36 – 11.49 >11.49 |
- | - | - |
|
All-Cause Mortality |
||||||
|
Hastie et al, 2013 (Year 1) |
1 (ref) 1.09 (0.93 – 1.27) 1.17 (1.01 – 1.36) 1.22 (1.05 – 1.42) |
Quartiles | <13.87 (ref) 13.87 – 18.20 18.21 – 23.14 >23.15 |
1 (ref) 1.10 (0.96 – 1.26) 1.08 (0.95 – 1.24) 1.07 (0.94 – 1.23) |
Quartiles | <11.0 (ref) 11.0 – 13.0 13.1 – 18.0 >18.0 |
|
Hastie et al, 2013 (Years 2–5) |
1 (ref) 0.99 (0.82 – 1.18) 1.13 (0.95 – 1.33) 1.32 (1.11 – 1.56) |
Quartiles | <12.98 (ref) 12.98 – 17.07 17.08 – 21.80 >21.81 |
1 (ref) 1.12 (0.95 – 1.32) 1.13 (0.96 – 1.32) 1.27 (1.08 – 1.50) |
Quartiles | <9.6 (ref) 9.6 – 10.8 10.9 – 12.3 >12.4 |
|
Hastie et al, 2013 (Years 5–10) |
1 (ref) 0.95 (0.75 – 1.21) 1.02 (0.81 – 1.29) 1.26 (0.99 – 1.58) |
Quartiles | <13.04 (ref) 13.04 – 16.99 17.00 – 21.52 >21.53 |
1 (ref) 1.10 (0.87 – 1.38) 1.28 (1.02 – 1.61) 1.37 (1.09 – 1.73) |
Quartiles | <9.8 (ref) 9.8 – 10.9 11.0 – 12.4 >12.5 |
|
Hastie et al, 2013 (Years 10+) |
1 (ref) 1.07 (0.76 – 1.51) 1.12 (0.85 – 1.68) 1.32 (0.94 – 1.84) |
Quartiles | <13.15 (ref) 13.15 – 17.59 17.60 – 21.81 >21.82 |
1 (ref) 1.13 (0.80 – 1.60) 1.73 (1.24 – 2.44) 1.49 (1.04 – 2.13) |
Quartiles | <9.5 (ref) 9.5 – 11.4 11.5 – 12.6 >12.7 |
| Hata, J. et al, 2013 | 2.08 (1.30 – 3.31) | Deciles | Top decile (≥18.1 for placebo group; ≥16.8 for active treatment group) vs. bottom decile (≤5.2 for placebo group; ≤5.0 for active treatment group) |
- | - | - |
| Lau et al, 2013 | 1 (ref) 1.47 (0.74 – 2.90) 1.97 (1.02 – 3.80) |
Tertiles | <13.0 13.0 – 17.5 >17.5 |
- | - | - |
| Lau et al, 2014 | - | - | - | 1 (ref) 1.06 (0.60 – 1.87) 1.18 (0.69 – 2.01) 1.46 (0.88 – 2.43) |
Quartiles | <8.6 (ref) 8.6–10.9 11.0–14.2 >14.2 |
| McMullan et al, 2013 | 1 (ref) 0.77 (0.28 – 2.16) 2.82 (1.14 – 6.95) |
Tertiles | 1.30 – 9.37 (ref) 9.40 – 15.47 15.51 – 55.56 |
- | - | - |
| Muntner et al, 2011 | 1 (ref) 1.57 (1.07 – 2.18) 1.50 (1.03 – 2.18) |
Tertiles | <4.80 (ref) 4.80 – 8.34 ≥8.35 |
1 (ref) 1.55 (1.09 – 2.22) 1.49 (1.05 – 2.10) |
Tertiles | <3.9 3.9 – 6.7 ≥6.8 |
|
Poortvliet et al, 2012 (Short-term follow-up cohort) |
1 (ref) 1.0 (0.7 – 1.3) 1.1 (0.8 – 1.6) 1.0 (0.8 – 1.4) |
Quartiles | ≤9 (ref) >9 – 12.5 >12.5 – ≤17 >17 |
- | - | - |
|
Poortvliet et al, 2012 (Long-term follow-up cohort) |
1 (ref) 1.2 (1.0 – 1.5) 1.4 (1.1 – 1.7) 1.5 (1.2 – 1.8) |
Quartiles | <10.5 (ref) >10.5 – ≤13 >13 – ≤16.5 >16.5 |
- | - | - |
| Selvarajah et al, 2014 | 1.48 (0.75 – 2.91) | Median split |
NR | 2.08 (1.04 – 1.16) | Median split |
NR |
| Yinon et al, 2013 | 1 (ref) 0.57 (0.42 – 0.78) 1.00 (0.78 – 1.31) |
Tertiles | <7.36 (ref) 7.36 – 11.49 >11.49 |
- | - | - |
CHD, coronary heart disease; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; MI, myocardial infarction; NR, not reported; OR, odds ratio; ROC, receiver operating characteristic; RR, relative risk; VVV, visit-to-visit variability.
Dash indicates data were not examined.
For each outcome, data in table are sorted in alphabetical order by first author’s last name.
CV of SBP modeled as a continuous variable was associated with each outcome except stroke and CVD mortality in the majority of analyses (Table 3, right panel). Modeled as a categorical variable, increased risk was present in the highest versus lowest category of CV of SBP in the majority of analyses for stroke, CVD, and all-cause mortality, but not stroke mortality, CHD, or CHD mortality (Table 4, right panel). Results for VVV of SBP using measures other than SD or CV modeled as a continuous variable are presented in Supplemental Table S1 and as categorical variable in Supplemental Table S2.
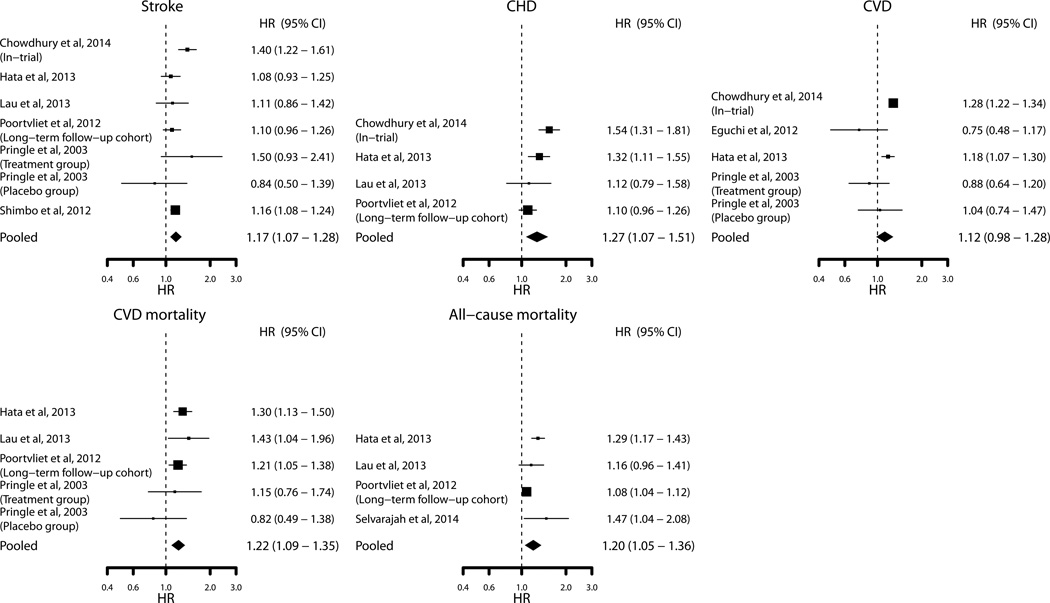
In total, 181 of 312 (58.0%) analyses showed a positive significant association between VVV of SBP and outcomes (Table 5). Results were similar after excluding studies among hemodialysis patients and studies that quantified VVV of SBP using visits separated by >1 year (Supplemental Table S3). At least one positive significant association was reported in 31 of the 37 cohorts that reported data for VVV of SBP. Meta-analyses of SD of SBP modeled as a continuous variable showed positive significant associations for stroke, CHD, CVD mortality, and all-cause mortality, but not CVD (Figure 2). Funnel plots and regression testing found no evidence of publication bias among the pooled studies for VVV of SBP (p=0.698).
Table 5.
Summary of significant positive associations reported for VVV of systolic blood pressure, diastolic blood pressure, pulse pressure, mean arterial pressure, and outcomes across all analyses.
| SBP | DBP | PP | MAP | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
VVV Metrics |
SD | CV | Other | Total | SD | CV | Other | Total | SD | CV | Other | Total | SD | CV | Other | Total |
| Modeled as Continuous Variable | ||||||||||||||||
| Stroke | 3 of 9 | 2 of 4 | 8 of 23 | 13 of 36 | 0 of 3 | 1 of 2 | 2 of 16 | 3 of 21 | 1 of 2 | - | 0 of 2 | 1 of 4 | - | - | - | - |
| Stroke Mortality | 0 of 1 | - | 0 of 1 | 0 of 2 | - | - | 0 of 1 | 0 of 1 | - | 0 of 1 | - | 0 of 1 | - | - | - | - |
| CHD | 4 of 6 | 2 of 3 | 9 of 22 | 15 of 31 | 1 of 3 | 1 of 2 | 1 of 14 | 3 of 19 | 0 of 2 | - | 0 of 2 | 0 of 4 | - | - | - | - |
| CHD Mortality | 0 of 1 | - | 0 of 1 | 0 of 2 | - | - | 0 of 1 | 0 of 1 | - | 0 of 1 | - | 0 of 1 | - | - | - | - |
| CVD | 3 of 8 | 3 of 4 | 7 of 10 | 13 of 22 | 1 of 3 | 0 of 1 | 0 of 2 | 1 of 6 | 0 of 1 | 0 of 1 | - | 0 of 2 | - | - | - | - |
| CVD Mortality | 5 of 9 | 2 of 6 | 4 of 14 | 11 of 29 | 1 of 4 | 0 of 2 | 1 of 1 | 2 of 7 | 2 of 3 | 1 of 2 | - | 3 of 5 | 0 of 1 | 0 of 1 | - | 0 of 2 |
| All-Cause Mortality | 4 of 7 | 8 of 9 | 11 of 12 | 23 of 28 | 2 of 5 | 2 of 3 | 3 of 5 | 7 of 13 | 1 of 3 | 0 of 2 | 1 of 2 | 2 of 7 | 1 of 1 | 1 of 1 | - | 2 of 2 |
| Composite Outcome: All-Cause Mortality and CVD | 1 of 1 | 1 of 1 | - | 2 of 2 | 0 of 1 | 0 of 1 | - | 0 of 2 | - | - | - | - | - | - | - | - |
| Sub-total | 20 of 42 | 18 of 27 | 39 of 83 | 77 of 152 | 5 of 19 | 4 of 11 | 7 of 40 | 16 of 70 | 4 of 11 | 1 of 7 | 1 of 6 | 6 of 24 | 1 of 2 | 1 of 2 | - | 2 of 4 |
| Modeled as Categorical Variable | ||||||||||||||||
| Stroke | 9 of 13 | 7 of 8 | 18 of 20 | 34 of 41 | 3 of 7 | 3 of 5 | 9 of 13 | 15 of 25 | 1 of 2 | - | - | 1 of 2 | 1 of 1 | - | - | 1 of 1 |
| Stroke Mortality | 1 of 6 | 0 of 4 | 1 of 5 | 2 of 15 | 0 of 4 | 0 of 4 | 1 of 5 | 1 of 13 | - | - | - | - | - | - | - | - |
| CHD | 1 of 5 | 0 of 1 | 2 of 2 | 3 of 8 | 2 of 3 | 0 of 1 | - | 2 of 4 | 0 of 2 | - | - | 0 of 2 | - | - | - | - |
| CHD Mortality | 1 of 6 | 1 of 4 | 5 of 5 | 7 of 15 | 0 of 4 | 0 of 4 | 0 of 5 | 0 of 13 | - | - | - | - | - | - | - | - |
| CVD | 6 of 8 | 4 of 4 | 14 of 15 | 24 of 27 | 3 of 4 | 4 of 4 | 9 of 12 | 16 of 20 | - | - | - | - | - | - | - | - |
| CVD Mortality | 6 of 11 | 2 of 5 | 7 of 7 | 15 of 23 | 1 of 7 | 0 of 5 | 2 of 5 | 3 of 17 | 1 of 2 | - | - | 1 of 2 | - | - | - | - |
| All-Cause Mortality | 7 of 12 | 5 of 7 | 7 of 12 | 19 of 31 | 3 of 9 | 1 of 7 | 4 of 10 | 8 of 26 | 1 of 2 | 1 of 2 | - | 2 of 4 | - | - | - | - |
| Composite Outcome: All-Cause Mortality/CVD | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Sub-total | 31 of 61 | 19 of 33 | 54 of 66 | 104 of 160 | 12 of 38 | 8 of 30 | 25 of 50 | 45 of 118 | 3 of 8 | 1 of 2 | - | 4 of 10 | 1 of 1 | - | - | 1 of 1 |
| Total | 51 of 103 | 37 of 60 | 93 of 149 | 181 of 312 | 17 of 57 | 12 of 41 | 32 of 90 | 61 of 188 | 7 of 19 | 2 of 9 | 1 of 6 | 10 of 34 | 2 of 3 | 1 of 2 | - | 3 of 5 |
CHD, coronary heart disease; CV, coefficient of variation; CVD, cardiovascular disease; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure; VVV, visit-to-visit variability.
Data are presented as the total number of analyses that showed significant positive associations of VVV with outcomes out of the total number of analyses that were reported. For example, ‘1 of 3’ indicates that one out of a total of three analyses reported higher VVV to be associated with an increased risk for outcomes.
Figure 2.
Association of the standard deviation of systolic blood pressure with outcomes. Sizes of the squares are proportional to the number of events in each study. Vertical lines denote 95% confidence intervals. The width of the diamond shapes represents the 95% confidence intervals in pooled analyses.
VVV of DBP and Outcomes
SD of DBP was associated with outcomes in 5 of 19 analyses when modeled as a continuous variable (Supplemental Table S4, left panel) and in 12 of 38 analyses when modeled as a categorical variable (Supplemental Table S5, left panel). CV of DBP was associated with outcomes in 12 of 41 analyses (Supplemental Table S4, right panel and Supplemental Table S5, right panel). Results for VVV of DBP metrics other than SD or CV are provided in Supplemental Table S6 for continuous and Supplemental Table S7 for categorical analyses.
In total, 61 of 188 (32.4%) analyses showed a significant positive association between VVV of DBP and outcomes (Table 5). Results were similar after excluding studies among hemodialysis patients and studies that quantified VVV of DBP using visits separated by >1 year (Supplemental Table S3). At least one significant positive association was reported in 11 of the 21 cohorts that reported data for VVV of DBP. A significant negative association was reported in one cohort. Meta-analyses of SD of DBP modeled as a continuous variable showed significant associations for CHD and CVD mortality, but not stroke or all-cause mortality (Supplemental Figure S1).
VVV of PP, VVV of MAP, and Outcomes
Modeled as continuous or categorical variables, VVV of PP metrics (SD, CV, and other) were associated with increased risk in less than 50% of reported analyses (Supplemental Tables S8, S9, and S10). Only two studies evaluated VVV of MAP and outcomes (Supplemental Tables S11 and S12). In total, 10 of 34 (29.4%) analyses showed a significant association between VVV of PP and outcomes and 3 of 5 analyses showed a significant association between VVV of MAP and outcomes (Table 5). Summary results excluding studies among hemodialysis patients and studies that quantified VVV of PP or MAP using visits separated by >1 year are reported in Supplemental Table S3.
DISCUSSION
In this systematic review, we identified 41 cohorts that evaluated the association of VVV of BP with cardiovascular outcomes and/or all-cause mortality. A rigorous meta-analysis to summarize all published data was not possible because of the large heterogeneity in quantifying, defining and reporting VVV. Pooling the available data, statistically significant associations, albeit modest in magnitude, were observed between VVV of SBP and outcomes including stroke, CHD, CVD mortality, and all-cause mortality.
The vast majority of studies we identified reported an increased risk for outcomes with higher VVV of BP in at least one analysis. In many cases, the positive findings within a cohort were accompanied by additional analyses wherein no association was observed. For example, the study by Hastie et al. reported 104 different analyses wherein 24 of 52 (46.1%) analyses for VVV of SBP and 10 of 52 (19.2%) analyses for VVV of DBP showed significant associations with outcomes.1 The mixed findings within studies underscores a need to more carefully consider negative results. Chance findings as a result of inflation of type I error rates with multiple analyses may also need more rigorous consideration. Nonetheless, the significant associations reported for many different outcomes (stroke, CHD, CVD, all-cause mortality) across many different populations (general population, chronic kidney disease, hypertension, diabetes, hemodialysis patients, etc.) suggests a potential role for VVV of BP as a CVD risk factor. It should be acknowledged that, given the rising and falling fluid volumes in hemodialysis patients, VVV of BP may be a different clinical entity in this population.
This review highlights a need for researchers to use standardized approaches when defining VVV of BP. The number of visits, time interval between visits, and the BP measurement protocols varied widely across studies. For example, the number of visits used to quantify VVV ranged from as few as 3 visits to as many as 156 visits and the time interval between visits ranged from 2 days to 3–4 years. It has been reported that VVV of BP is influenced by the number of visits used to calculate it, the time interval between visits, the BP measurement device, and the number of BP measurements per visit.42,43 These factors may affect the VVV of BP – outcome associations observed between studies. It has thus been suggested that adjustments should be made for the number of visits used to calculate VVV of BP and the time-interval between visits.42 Although VVV of BP was derived using the same number of visits for all participants in 13 cohorts and the same time-interval between visits in 26 cohorts, only 2 of the remaining cohorts20,29 adjusted for these factors. Moreover, there was inadequate description of the methodology used to quantify VVV of BP for several cohorts as the number of visits used to quantify VVV of BP, the time interval between visits, and the number BP measurements per visits were not reported.
A standardized approach to calculating VVV of BP is also needed. A total of 21 different metrics were used to calculate VVV of BP, with many studies reporting multiple metrics. The reporting of multiple metrics has made it challenging to interpret evidence on the association of VVV of BP and outcomes. It has been reported that many of the metrics provide largely redundant information.44 Therefore, future studies of VVV of BP may benefit from only reporting three metrics: a measure of variation around an individual’s mean BP (SD, CV or SD independent of the mean), a measure of change in BP over time (average real variability or successive variation), and a measure of spikes in BP (peak BP).
VVV of SBP was more often investigated and reported in comparison to VVV of DBP. However, both showed associations with adverse outcomes in the meta-analysis we conducted. Although sparingly studied, VVV of PP and VVV of MAP were also associated with CVD and all-cause mortality in some studies.10,23,31 These data implicate VVV in all four BP indexes as having potential prognostic value. In the only study to analyze all four BP indexes, Hsieh et al showed VVV of SBP, DBP, and MAP, but not VVV of PP, to be associated with all-cause mortality. In contrast, VVV of PP was the only BP index associated with CVD mortality in this study. Future studies are, therefore, still needed to determine which BP index carries the greatest prognostic information.
Several limitations should be considered when interpreting our findings. First, because of the aforementioned methodological considerations, a meta-analysis including all 41 cohorts was not possible. Second, studies adjusted for different sets of confounders which could have contributed to the heterogeneity of results. Third, given the small number of studies available for pooling we could not perform meta-regression to evaluate factors associated with the heterogeneity of results across studies. Finally, the majority of studies included were secondary analyses of randomized controlled trials or observational studies. Methodological factors that influence VVV of BP (e.g., number of visits, time interval between visits) may have affected the VVV of BP to outcomes association that we report. Future studies using rigorous methodology should be conducted to provide a better assessment of the VVV of BP – outcome association and determine the clinical utility of measuring VVV of BP. As ambulatory BP is considered to have superior prognostic value to clinic BP,45 another important area for future studies is to determine the clinical relevance of VVV of ambulatory BP.
PERSPECTIVES
In the current systematic review, an association between VVV of BP and CVD and mortality outcomes was present in some but not all studies. When data were available to pool, VVV of SBP was associated with a modest increased risk for stroke, CHD, CVD mortality, and all-cause mortality and VVV of DBP was associated with an increased risk for CHD and CVD mortality. The associations observed across a variety of populations suggest that VVV of BP may be a risk factor for CVD. However, the association between VVV of BP and outcomes that we report is limited by the various number of methodologies used to quantify VVV of BP. Thus, the clinical relevance of VVV of BP should be interpreted cautiously and is still unclear. Additional studies using standardized approaches for estimating VVV of BP are needed to clarify its prognostic value.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new?
This is the first study to systematically review and meta-analyze the published literature on the association between visit-to-visit variability (VVV) of blood pressure (BP) and health outcomes.
What is relevant?
Pooled estimates showed that VVV of BP was associated with a modest increased risk for cardiovascular disease and all-cause mortality. This finding, however, is limited by the small amount of available data to pool and lack of a standardized approach for estimating VVV of BP. Therefore, caution should be used in intepreting it’s clinical relevance.
Summary
Data from published studies suggest that VVV of BP may be a novel cardiovscular risk factor. However, the modest associations from pooled estimates may limit its potential clinical relevance. Additional studies using standardized approaches for estimating VVV of BP are needed to clarfiy its prognositc value.
Acknowledgments
Sources of Funding: This work was partially supported by P01-HL047540 from the National Heart, Lung, and Blood Institute at the National Institutes of Health (NIH) (DS) and a NIH Diversity Supplement P01-HL047540-19S1 (KMD).
Footnotes
Disclosures: None
REFERENCES
- 1.Hastie CE, Jeemon P, Coleman H, McCallum L, Patel R, Dawson J, Sloan W, Meredith P, Jones GC, Muir S, Walters M, Dominiczak AF, Morrison D, McInnes GT, Padmanabhan S. Long-term and ultra long-term blood pressure variability during follow-up and mortality in 14,522 patients with hypertension. Hypertension. 2013;62:698–705. doi: 10.1161/HYPERTENSIONAHA.113.01343. [DOI] [PubMed] [Google Scholar]
- 2.Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension. 2011;57:160–166. doi: 10.1161/HYPERTENSIONAHA.110.162255. [DOI] [PubMed] [Google Scholar]
- 3.Rothwell PM, Howard SC, Dolan E, O'Brien E, Dobson JE, Dahlof B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 4.Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375:938–948. doi: 10.1016/S0140-6736(10)60309-1. [DOI] [PubMed] [Google Scholar]
- 5.Rothwell PM, Howard SC, Dolan E, O'Brien E, Dobson JE, Dahlof B, Poulter NR, Sever PS. Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9:469–480. doi: 10.1016/S1474-4422(10)70066-1. [DOI] [PubMed] [Google Scholar]
- 6.Mancia G, Facchetti R, Parati G, Zanchetti A. Visit-to-visit blood pressure variability, carotid atherosclerosis, and cardiovascular events in the European Lacidipine Study on Atherosclerosis. Circulation. 2012;126:569–578. doi: 10.1161/CIRCULATIONAHA.112.107565. [DOI] [PubMed] [Google Scholar]
- 7.Gao S, Hendrie HC, Wang C, Stump TE, Stewart JC, Kesterson J, Clark DO, Callahan CM. Redefined Blood Pressure Variability Measure and Its Association With Mortality in Elderly Primary Care Patients. Hypertension. 2014;64:45–52. doi: 10.1161/HYPERTENSIONAHA.114.03576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egger M, Davey-Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0. [Accessed June 12, 2014]; [updated March 2011]. http://handbook.cochrane.org. [Google Scholar]
- 10.Brickman AM, Reitz C, Luchsinger JA, Manly JJ, Schupf N, Muraskin J, DeCarli C, Brown TR, Mayeux R. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol. 2010;67:564–569. doi: 10.1001/archneurol.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunelli SM, Thadhani RI, Lynch KE, Ankers ED, Joffe MM, Boston R, Chang Y, Feldman HI. Association between long-term blood pressure variability and mortality among incident hemodialysis patients. Am J Kidney Dis. 2008;52:716–726. doi: 10.1053/j.ajkd.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 12.Carr MJ, Bao Y, Pan J, Cruickshank K, McNamee R. The predictive ability of blood pressure in elderly trial patients. J Hypertens. 2012;30:1725–1733. doi: 10.1097/HJH.0b013e3283568a73. [DOI] [PubMed] [Google Scholar]
- 13.Chang TI, Flythe JE, Brunelli SM, Muntner P, Greene T, Cheung AK, Chertow GM. Visit-to-visit systolic blood pressure variability and outcomes in hemodialysis. J Hum Hypertens. 2014;28:18–24. doi: 10.1038/jhh.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chowdhury EK, Owen A, Krum H, Wing L, Nelson MR, Reid CM. Systolic blood pressure variability is an important predictor of cardiovascular outcomes in elderly hypertensive patients. J Hypertens. 2014;31:525–533. doi: 10.1097/HJH.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 15.Di Iorio B, Di Micco L, Torraca S, Sirico ML, Guastaferro P, Chiuchiolo L, Nigro F, De Blasio A, Romano P, Pota A, Rubino R, Morrone L, Lopez T, Casino FG. Variability of blood pressure in dialysis patients: a new marker of cardiovascular risk. J Nephrol. 2013;26:173–182. doi: 10.5301/jn.5000108. [DOI] [PubMed] [Google Scholar]
- 16.Di Iorio B, Pota A, Sirico ML, Torraca S, Di Micco L, Rubino R, Guastaferro P, Bellasi A. Blood pressure variability and outcomes in chronic kidney disease. Nephrol Dial Transplant. 2012;27:4404–4410. doi: 10.1093/ndt/gfs328. [DOI] [PubMed] [Google Scholar]
- 17.Eguchi K, Hoshide S, Schwartz JE, Shimada K, Kario K. Visit-to-visit and ambulatory blood pressure variability as predictors of incident cardiovascular events in patients with hypertension. Am J Hypertens. 2012;25:962–968. doi: 10.1038/ajh.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grove JS, Reed DM, Yano K, Hwang LJ. Variability in systolic blood pressure--a risk factor for coronary heart disease? Am J Epidemiol. 1997;145:771–776. doi: 10.1093/oxfordjournals.aje.a009169. [DOI] [PubMed] [Google Scholar]
- 19.Hata J, Arima H, Rothwell PM, Woodward M, Zoungas S, Anderson C, Patel A, Neal B, Glasziou P, Hamet P, Mancia G, Poulter N, Williams B, Macmahon S, Chalmers J, Group AC. Effects of visit-to-visit variability in systolic blood pressure on macrovascular and microvascular complications in patients with type 2 diabetes mellitus: the ADVANCE trial. Circulation. 2013;128:1325–1334. doi: 10.1161/CIRCULATIONAHA.113.002717. [DOI] [PubMed] [Google Scholar]
- 20.Hata Y, Kimura Y, Muratani H, Fukiyama K, Kawano Y, Ashida T, Yokouchi M, Imai Y, Ozawa T, Fujii J, Omae T. Office blood pressure variability as a predictor of brain infarction in elderly hypertensive patients. Hypertens Res. 2000;23:553–560. doi: 10.1291/hypres.23.553. [DOI] [PubMed] [Google Scholar]
- 21.Hata Y, Muratani H, Kimura Y, Fukiyama K, Kawano Y, Ashida T, Yokouchi M, Imai Y, Ozawa T, Fujii J, Omae T. Office blood pressure variability as a predictor of acute myocardial infarction in elderly patients receiving antihypertensive therapy. J Hum Hypertens. 2002;16:141–146. doi: 10.1038/sj.jhh.1001301. [DOI] [PubMed] [Google Scholar]
- 22.Hofman A, Feinleib M, Garrison RJ, van Laar A. Does change in blood pressure predict heart disease? BMJ. 1983;287:267–269. doi: 10.1136/bmj.287.6387.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsieh YT, Tu ST, Cho TJ, Chang SJ, Chen JF, Hsieh MC. Visit-to-visit variability in blood pressure strongly predicts all-cause mortality in patients with type 2 diabetes: a 5.5-year prospective analysis. Eur J Clin Invest. 2012;42:245–253. doi: 10.1111/j.1365-2362.2011.02574.x. [DOI] [PubMed] [Google Scholar]
- 24.Kawai T, Ohishi M, Ito N, Onishi M, Takeya Y, Yamamoto K, Kamide K, Rakugi H. Alteration of vascular function is an important factor in the correlation between visit-to-visit blood pressure variability and cardiovascular disease. J Hypertens. 2013;31:1387–1395. doi: 10.1097/HJH.0b013e328360f796. [DOI] [PubMed] [Google Scholar]
- 25.Kim HY, Kang YU, Kim CS, Choi JS, Bae EH, Ma SK, Kim SW. Association of age and BP variability with long-term mortality in hemodialysis patients. Kidney Blood Press Res. 2013;38:172–180. doi: 10.1159/000355765. [DOI] [PubMed] [Google Scholar]
- 26.Kostis JB, Sedjro JE, Cabrera J, Cosgrove NM, Pantazopoulos JS, Kostis WJ, Pressel SL, Davis BR. Visit-to-Visit Blood Pressure Variability and Cardiovascular Death in the Systolic Hypertension in the Elderly Program. J Clin Hypertens. 2014;16:34–40. doi: 10.1111/jch.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lau KK, Wong YK, Chang RS, Teo KC, Hon SF, Chan KH, Wat KL, Cheung RT, Li LS, Siu CW, Ho SL, Tse HF. Visit-to-visit systolic blood pressure variability predicts all-cause and cardiovascular mortality after lacunar infarct. Eur J Neurol. 2013;21:319–325. doi: 10.1111/ene.12310. [DOI] [PubMed] [Google Scholar]
- 28.Lau KK, Wong YK, Teo KC, Chang RS, Chan KH, Hon SF, Wat KL, Cheung RT, Li LS, Siu CW, Tse HF. Long-Term Prognostic Implications of Visit-to-Visit Blood Pressure Variability in Patients With Ischemic Stroke. [Accessed June 6, 2014];Am J Hypertens. doi: 10.1093/ajh/hpu070. [published online ahead of print May 18, 2014]. http://ajh.oxfordjournals.org/content/early/2014/05/18/ajh.hpu070.short. [DOI] [PubMed] [Google Scholar]
- 29.Mallamaci F, Minutolo R, Leonardis D, D'Arrigo G, Tripepi G, Rapisarda F, Cicchetti T, Maimone I, Enia G, Postorino M, Santoro D, Fuiano G, De Nicola L, Conte G, Zoccali C. Long-term visit-to-visit office blood pressure variability increases the risk of adverse cardiovascular outcomes in patients with chronic kidney disease. Kidney Int. 2013;84:381–389. doi: 10.1038/ki.2013.132. [DOI] [PubMed] [Google Scholar]
- 30.McMullan CJ, Bakris GL, Phillips RA, Forman JP. Association of BP variability with mortality among African Americans with CKD. Clin J Am Soc Nephrol. 2013;8:731–738. doi: 10.2215/CJN.10131012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poortvliet RK, Ford I, Lloyd SM, Sattar N, Mooijaart SP, de Craen AJ, Westendorp RG, Jukema JW, Packard CJ, Gussekloo J, de Ruijter W, Stott DJ. Blood pressure variability and cardiovascular risk in the PROspective Study of Pravastatin in the Elderly at Risk (PROSPER) PloS One. 2012;7:e52438. doi: 10.1371/journal.pone.0052438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pringle E, Phillips C, Thijs L, Davidson C, Staessen JA, de Leeuw PW, Jaaskivi M, Nachev C, Parati G, O'Brien ET, Tuomilehto J, Webster J, Bulpitt CJ, Fagard RH. Systolic blood pressure variability as a risk factor for stroke and cardiovascular mortality in the elderly hypertensive population. J Hypertens. 2003;21:2251–2257. doi: 10.1097/00004872-200312000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Rossignol P, Cridlig J, Lehert P, Kessler M, Zannad F. Visit-to-visit blood pressure variability is a strong predictor of cardiovascular events in hemodialysis: insights from FOSIDIAL. Hypertension. 2012;60:339–346. doi: 10.1161/HYPERTENSIONAHA.111.190397. [DOI] [PubMed] [Google Scholar]
- 34.Selvarajah V, Pasea L, Ojha S, Wilkinson IB, Tomlinson LA. Pre-Dialysis Systolic Blood Pressure-Variability Is Independently Associated with All-Cause Mortality in Incident Haemodialysis Patients. PLoS One. 2014;9(1):e86514. doi: 10.1371/journal.pone.0086514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shafi T, Sozio SM, Bandeen-Roche KJ, Ephraim PL, Luly JR, St Peter WL, McDermott A, Scialla JJ, Crews DC, Tangri N, Miskulin DC, Michels WM, Jaar BG, Herzog CA, Zager PG, Meyer KB, Wu AW, Boulware LE. J Am Soc Nephrol. 2014;25:799–809. doi: 10.1681/ASN.2013060667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimbo D, Newman JD, Aragaki AK, LaMonte MJ, Bavry AA, Allison M, Manson JE, Wassertheil-Smoller S. Association between annual visit-to-visit blood pressure variability and stroke in postmenopausal women: data from the Women's Health Initiative. Hypertension. 2012;60:625–630. doi: 10.1161/HYPERTENSIONAHA.112.193094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suchy-Dicey AM, Wallace ER, Mitchell SV, Aguilar M, Gottesman RF, Rice K, Kronmal R, Psaty BM, Longstreth WT., Jr Blood pressure variability and the risk of all-cause mortality, incident myocardial infarction, and incident stroke in the cardiovascular health study. Am J Hypertens. 2013;26:1210–1217. doi: 10.1093/ajh/hpt092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tozawa M, Iseki K, Yoshi S, Fukiyama K. Blood pressure variability as an adverse prognostic risk factor in end-stage renal disease. Nephrol Dial Transplant. 1999;14:1976–1981. doi: 10.1093/ndt/14.8.1976. [DOI] [PubMed] [Google Scholar]
- 39.Yinon L, Chen Y, Parvez F, Bangalore S, Islam T, Ahmed A, Rakibuz-Zaman M, Hasan R, Sarwar G, Ahsan H. A prospective study of variability in systolic blood pressure and mortality in a rural Bangladeshi population cohort. Prev Med. 2013;57:807–812. doi: 10.1016/j.ypmed.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zoppini G, Verlato G, Targher G, Bonora E, Trombetta M, Muggeo M. Variability of body weight, pulse pressure and glycaemia strongly predict total mortality in elderly type 2 diabetic patients. The Verona Diabetes Study. Diabetes Metab Res Rev. 2008;24:624–628. doi: 10.1002/dmrr.897. [DOI] [PubMed] [Google Scholar]
- 41.Zoppini G, Verlato G, Zamboni C, Venturi C, Gennaro N, Biasi V, Bonora E, Muggeo M. Pulse pressure and mortality from cerebrovascular diseases in type 2 diabetic patients: the Verona Diabetes Study. Cerebrovasc Dis. 2007;23:20–26. doi: 10.1159/000095754. [DOI] [PubMed] [Google Scholar]
- 42.Levitan EB, Kaciroti N, Oparil S, Julius S, Muntner P. Blood pressure measurement device, number and timing of visits, and intra-individual visit-to-visit variability of blood pressure. J Clin Hypertens. 2012;14:744–750. doi: 10.1111/jch.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mancia G, Facchetti R, Parati G, Zanchetti A. Visit-to-visit blood pressure variability in the European Lacidipine Study on Atherosclerosis: methodological aspects and effects of antihypertensive treatment. J Hypertens. 2012;30:1241–1251. doi: 10.1097/HJH.0b013e32835339ac. [DOI] [PubMed] [Google Scholar]
- 44.Levitan EB, Kaciroti N, Oparil S, Julius S, Muntner P. Relationships between metrics of visit-to-visit variability of blood pressure. J Hum Hypertens. 2013;27:589–593. doi: 10.1038/jhh.2013.19. [DOI] [PubMed] [Google Scholar]
- 45.Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med. 2006;354:2368–2374. doi: 10.1056/NEJMra060433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.