Abstract
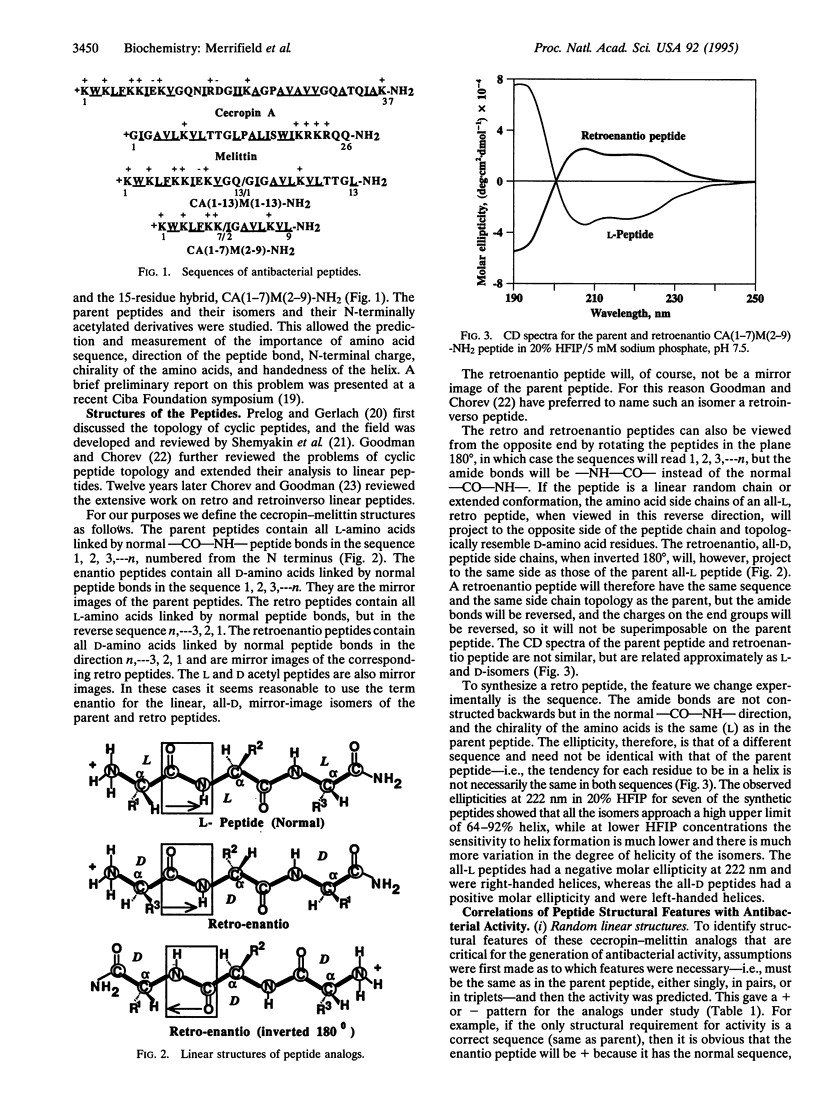
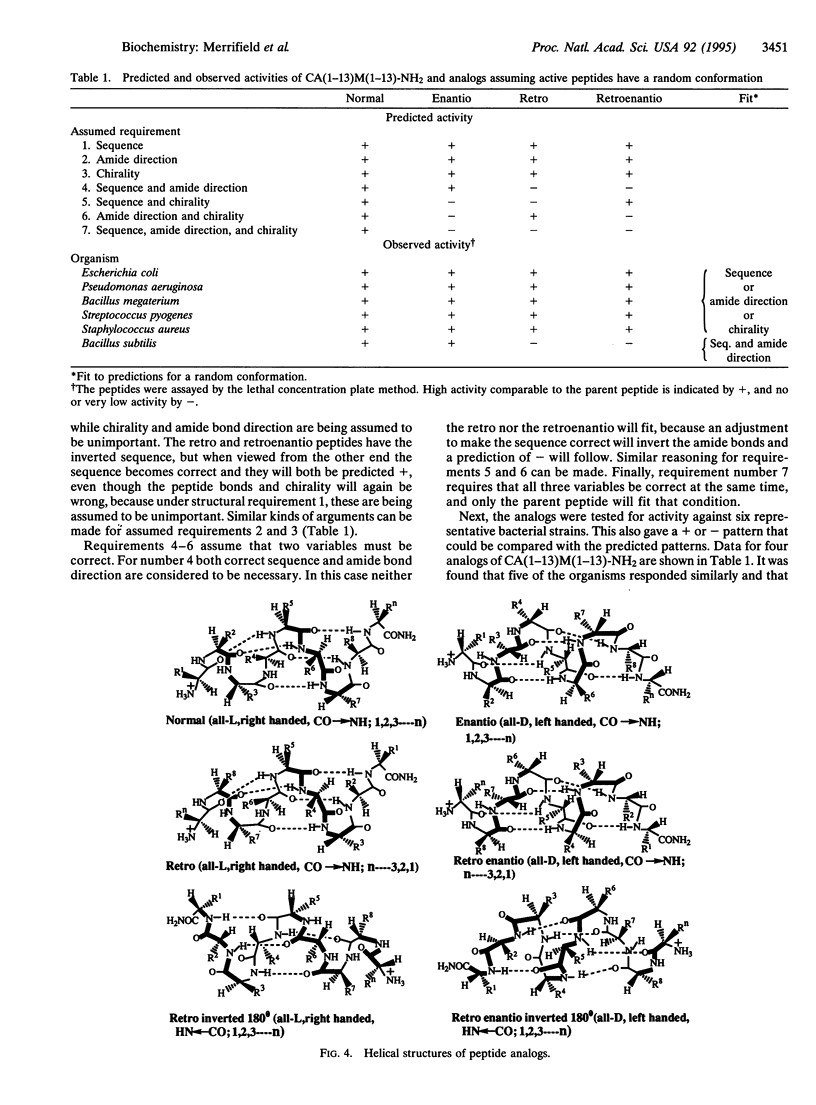
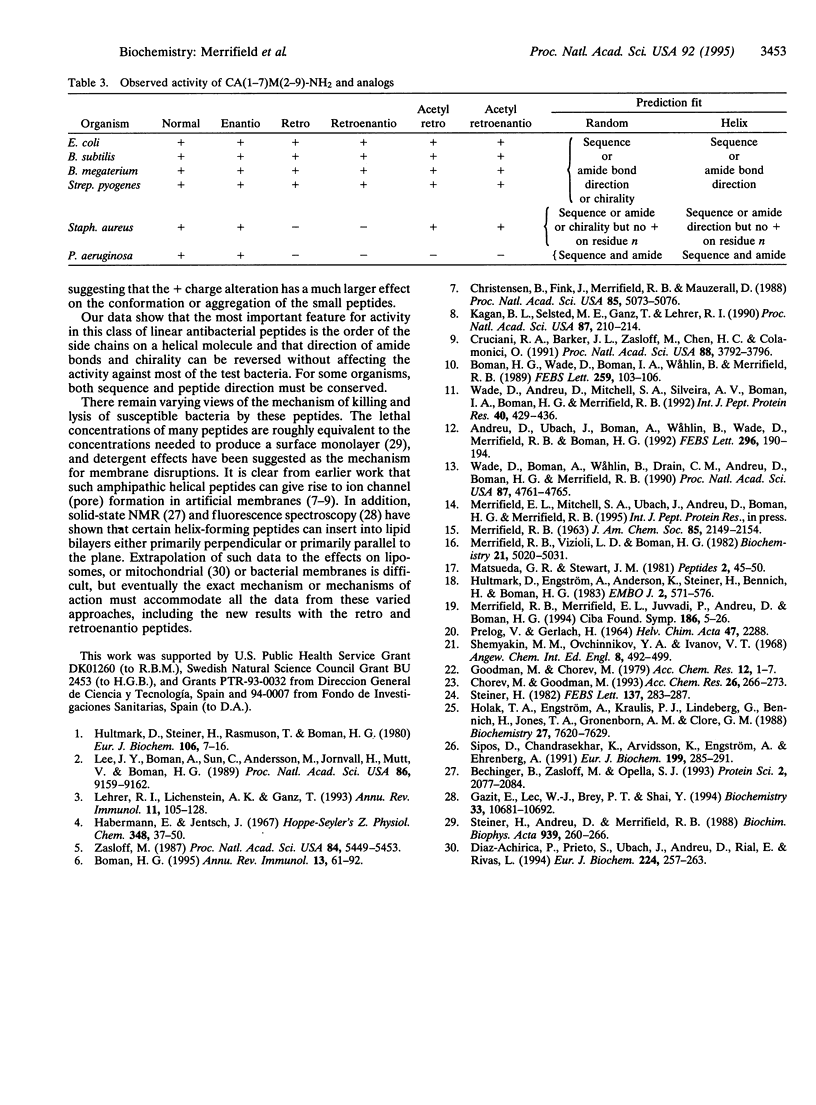
Hybrid analogs of cecropin A (CA) and melittin (M), which are potent antibacterial peptides, have been synthesized. To understand the structural requirements for this antibacterial activity, we have also synthesized the enantio, retro, and retroenantio isomers of two of the hybrids and their N-terminally acetylated derivatives. All analogs of CA(1-13)M(1-13)-NH2 were as active as the parent peptide against five test bacterial strains, but one bacterial strain was resistant to the retro and retroenantio derivatives. Similarly, all analogs of CA(1-7)M(2-9)-NH2 were active against four strains, while two strains were resistant to the retro and retroenantio analogs containing free NH3+ end groups, but acetylation restored activity against one of them. From these data it was concluded that chirality of the peptide was not a critical feature, and full activity could be achieved with peptides containing either all L- or all D-amino acids in their respective right-handed or left-handed helical conformations. For most of the bacterial strains, the sequence of these peptides or the direction of the peptide bonds could be critical but not both at the same time. For some strains, both needed to be conserved.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreu D., Ubach J., Boman A., Wåhlin B., Wade D., Merrifield R. B., Boman H. G. Shortened cecropin A-melittin hybrids. Significant size reduction retains potent antibiotic activity. FEBS Lett. 1992 Jan 20;296(2):190–194. doi: 10.1016/0014-5793(92)80377-s. [DOI] [PubMed] [Google Scholar]
- Bechinger B., Zasloff M., Opella S. J. Structure and orientation of the antibiotic peptide magainin in membranes by solid-state nuclear magnetic resonance spectroscopy. Protein Sci. 1993 Dec;2(12):2077–2084. doi: 10.1002/pro.5560021208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman H. G. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Wade D., Boman I. A., Wåhlin B., Merrifield R. B. Antibacterial and antimalarial properties of peptides that are cecropin-melittin hybrids. FEBS Lett. 1989 Dec 18;259(1):103–106. doi: 10.1016/0014-5793(89)81505-4. [DOI] [PubMed] [Google Scholar]
- Christensen B., Fink J., Merrifield R. B., Mauzerall D. Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5072–5076. doi: 10.1073/pnas.85.14.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruciani R. A., Barker J. L., Zasloff M., Chen H. C., Colamonici O. Antibiotic magainins exert cytolytic activity against transformed cell lines through channel formation. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3792–3796. doi: 10.1073/pnas.88.9.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Achirica P., Prieto S., Ubach J., Andreu D., Rial E., Rivas L. Permeabilization of the mitochondrial inner membrane by short cecropin-A-melittin hybrid peptides. Eur J Biochem. 1994 Aug 15;224(1):257–263. doi: 10.1111/j.1432-1033.1994.tb20019.x. [DOI] [PubMed] [Google Scholar]
- Gazit E., Lee W. J., Brey P. T., Shai Y. Mode of action of the antibacterial cecropin B2: a spectrofluorometric study. Biochemistry. 1994 Sep 6;33(35):10681–10692. doi: 10.1021/bi00201a016. [DOI] [PubMed] [Google Scholar]
- Habermann E., Jentsch J. Sequenzanalyse des Melittins aus den tryptischen und peptischen Spaltstücken. Hoppe Seylers Z Physiol Chem. 1967 Jan;348(1):37–50. [PubMed] [Google Scholar]
- Holak T. A., Engström A., Kraulis P. J., Lindeberg G., Bennich H., Jones T. A., Gronenborn A. M., Clore G. M. The solution conformation of the antibacterial peptide cecropin A: a nuclear magnetic resonance and dynamical simulated annealing study. Biochemistry. 1988 Oct 4;27(20):7620–7629. doi: 10.1021/bi00420a008. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Engström A., Andersson K., Steiner H., Bennich H., Boman H. G. Insect immunity. Attacins, a family of antibacterial proteins from Hyalophora cecropia. EMBO J. 1983;2(4):571–576. doi: 10.1002/j.1460-2075.1983.tb01465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultmark D., Steiner H., Rasmuson T., Boman H. G. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur J Biochem. 1980 May;106(1):7–16. doi: 10.1111/j.1432-1033.1980.tb05991.x. [DOI] [PubMed] [Google Scholar]
- Kagan B. L., Selsted M. E., Ganz T., Lehrer R. I. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc Natl Acad Sci U S A. 1990 Jan;87(1):210–214. doi: 10.1073/pnas.87.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. Y., Boman A., Sun C. X., Andersson M., Jörnvall H., Mutt V., Boman H. G. Antibacterial peptides from pig intestine: isolation of a mammalian cecropin. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9159–9162. doi: 10.1073/pnas.86.23.9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Lichtenstein A. K., Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- Matsueda G. R., Stewart J. M. A p-methylbenzhydrylamine resin for improved solid-phase synthesis of peptide amides. Peptides. 1981 Spring;2(1):45–50. doi: 10.1016/s0196-9781(81)80010-1. [DOI] [PubMed] [Google Scholar]
- Merrifield R. B., Merrifield E. L., Juvvadi P., Andreu D., Boman H. G. Design and synthesis of antimicrobial peptides. Ciba Found Symp. 1994;186:5–26. [PubMed] [Google Scholar]
- Merrifield R. B., Vizioli L. D., Boman H. G. Synthesis of the antibacterial peptide cecropin A (1-33). Biochemistry. 1982 Sep 28;21(20):5020–5031. doi: 10.1021/bi00263a028. [DOI] [PubMed] [Google Scholar]
- Shemyakin M. M., Ovchinnikov Y. A., Ivanov V. T. Topochemical investigations of peptide systems. Angew Chem Int Ed Engl. 1969 Jul;8(7):492–499. doi: 10.1002/anie.196904921. [DOI] [PubMed] [Google Scholar]
- Sipos D., Chandrasekhar K., Arvidsson K., Engström A., Ehrenberg A. Two-dimensional proton-NMR studies on a hybrid peptide between cecropin A and melittin. Resonance assignments and secondary structure. Eur J Biochem. 1991 Jul 15;199(2):285–291. doi: 10.1111/j.1432-1033.1991.tb16122.x. [DOI] [PubMed] [Google Scholar]
- Steiner H., Andreu D., Merrifield R. B. Binding and action of cecropin and cecropin analogues: antibacterial peptides from insects. Biochim Biophys Acta. 1988 Apr 7;939(2):260–266. doi: 10.1016/0005-2736(88)90069-7. [DOI] [PubMed] [Google Scholar]
- Steiner H. Secondary structure of the cecropins: antibacterial peptides from the moth Hyalophora cecropia. FEBS Lett. 1982 Jan 25;137(2):283–287. doi: 10.1016/0014-5793(82)80368-2. [DOI] [PubMed] [Google Scholar]
- Wade D., Andreu D., Mitchell S. A., Silveira A. M., Boman A., Boman H. G., Merrifield R. B. Antibacterial peptides designed as analogs or hybrids of cecropins and melittin. Int J Pept Protein Res. 1992 Nov;40(5):429–436. doi: 10.1111/j.1399-3011.1992.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Wade D., Boman A., Wåhlin B., Drain C. M., Andreu D., Boman H. G., Merrifield R. B. All-D amino acid-containing channel-forming antibiotic peptides. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4761–4765. doi: 10.1073/pnas.87.12.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]


