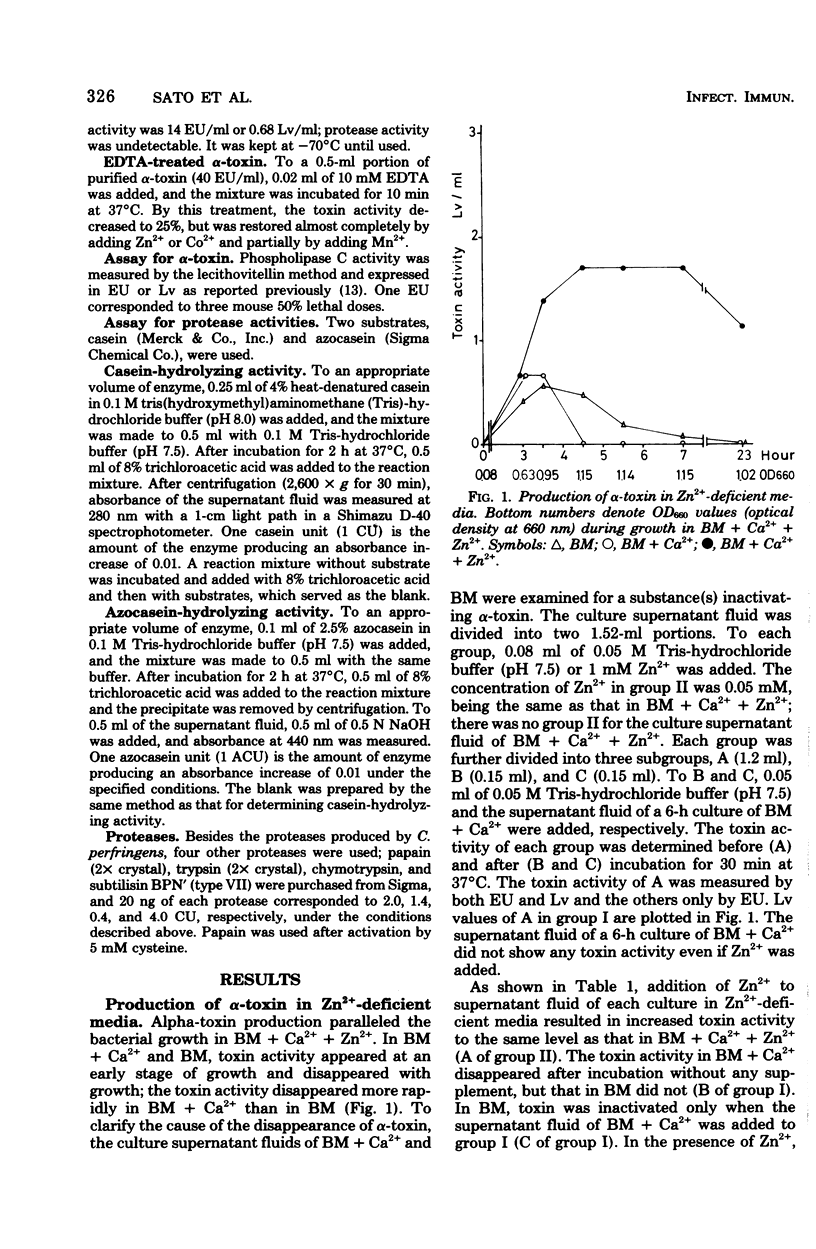
Abstract
Clostridium perfringens produced at least three distinct proteases in a synthetic medium containing calcium. Two of them, thiol and ethylenediaminetetraacetic acid disodium salt-sensitive proteases, appeared at an early stage of growth, but the other one, perhaps being identical to the one produced in a calcium-deficient medium, appeared at a late stage. The production of these proteases depended on Ca2+ but not on Zn2+ in the medium. Alpha-toxin, perhaps being a zinc-containing metalloenzyme, was rather resistant to the proteases, but toxin, produced in a zinc-deficient medium or deprived of zinc with ethylenediaminetetraacetic acid disodium salt, was very sensitive. By adding Zn2+, the toxin lacking zinc may have been converted to the zinc-containing metalloprotein that is resistant to proteases. This may explain why alpha-toxin activity increased progressively in a zinc-containing medium in spite of simultaneous production of potent proteases and why it disappeared rapidly in a zinc-deficient medium.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bissell M. J., Tosi R., Gorini L. Mechanism of excretion of a bacterial proteinase: factors controlling accumulation of the extracellular proteinase of a Sarcina strain (Coccus P). J Bacteriol. 1971 Mar;105(3):1099–1109. doi: 10.1128/jb.105.3.1099-1109.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman P. L., Iweibo I., Weiner H. Role of zinc in horse liver alcohol dehydrogenase. Influence on structure and conformational changes. Biochemistry. 1972 Mar 14;11(6):1010–1018. doi: 10.1021/bi00756a010. [DOI] [PubMed] [Google Scholar]
- Etherington D. J., Newman P. B., Dainty R. H., Partridge S. M. Purification and properties of the extracellular metallo-proteinases of Chromobacterium lividum (NCIB 10926). Biochim Biophys Acta. 1976 Oct 11;445(3):739–752. doi: 10.1016/0005-2744(76)90124-8. [DOI] [PubMed] [Google Scholar]
- Harris M. I., Coleman J. E. The biosynthesis of apo- and metalloalkaline phosphatases of Escherichia coli. J Biol Chem. 1968 Oct 10;243(19):5063–5073. [PubMed] [Google Scholar]
- Li E., Yousten A. A. Metalloprotease from Bacillus thuringiensis. Appl Microbiol. 1975 Sep;30(3):354–361. doi: 10.1128/am.30.3.354-361.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch K. L., Moskowitz M. Effects of chelates in chemotherapy of experimental gas-gangrene toxemia. J Bacteriol. 1968 Dec;96(6):1925–1930. doi: 10.1128/jb.96.6.1925-1930.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morihara K. Comparative specificity of microbial proteinases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):179–243. doi: 10.1002/9780470122860.ch5. [DOI] [PubMed] [Google Scholar]
- Murata R., Soda S., Yamamoto A., Ito A. Further investigations on the influence of inorganic cations on growth and toxin production by Clostridium perfringens PB6K. Jpn J Med Sci Biol. 1968 Feb;21(1):55–70. doi: 10.7883/yoken1952.21.55. [DOI] [PubMed] [Google Scholar]
- Murata R., Soda S., Yamamoto A., Sato H., Ito A. The effect of zinc on the production of various toxins of Clostridium perfringens. Jpn J Med Sci Biol. 1969 Jun;22(3):133–148. doi: 10.7883/yoken1952.22.133. [DOI] [PubMed] [Google Scholar]
- Nelbach M. E., Pigiet V. P., Jr, Gerhart J. C., Schachman H. K. A role for zinc in the quaternary structure of aspartate transcarbamylase from Escherichia coli. Biochemistry. 1972 Feb 1;11(3):315–327. doi: 10.1021/bi00753a002. [DOI] [PubMed] [Google Scholar]
- Oishi I., Okada T., Sakaguchi G. Responses of Clostridium botulinum type B and E progenitor toxins to some clostridial sulfhydryl-dependent proteases. Jpn J Med Sci Biol. 1975 Jun;28(3):157–164. doi: 10.7883/yoken1952.28.157. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Schlesinger M. J. Alterations in the structure and function of Escherichia coli alkaline phosphatase due to Zn2+ binding. Biochemistry. 1969 Feb;8(2):588–593. doi: 10.1021/bi00830a019. [DOI] [PubMed] [Google Scholar]
- Sarner N. Z., Bissell M. J., Di Girolamo M., Gorini L. Mechanism of excretion of a bacterial proteinase: demonstration of two proteolytic enzymes produced by a Sarcina strain (Coccus P). J Bacteriol. 1971 Mar;105(3):1090–1098. doi: 10.1128/jb.105.3.1090-1098.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Kameyama S., Murata R. Immunogenicity of highly purified -toxoid of Clostridium perfringens. Jpn J Med Sci Biol. 1972 Feb;25(1):53–56. [PubMed] [Google Scholar]
- Sato H., Murata R. Role of zinc in the production of Clostridium perfringens alpha toxin. Infect Immun. 1973 Sep;8(3):360–369. doi: 10.1128/iai.8.3.360-369.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Yamakawa Y., Ito A., Murata R. Effect of protease on the production of Clostridium perfringens alpha toxin. Jpn J Med Sci Biol. 1977 Feb;30(1):44–46. [PubMed] [Google Scholar]
- Soda S., Ito A., Yamamoto A. Production and properties of theta-toxin of Clostridium perfringens with special reference to lethal activity. Jpn J Med Sci Biol. 1976 Dec;29(6):335–349. [PubMed] [Google Scholar]
- Soda S., Sato H., Murata R. The effect of calcium on the production of various toxins of Clostridium perfringens. Jpn J Med Sci Biol. 1969 Jun;22(3):175–179. doi: 10.7883/yoken1952.22.175. [DOI] [PubMed] [Google Scholar]
- Szajn H., Csopak H. Metal ion-induced conformational changes in Escherichia coli alkaline phosphatase. Biochim Biophys Acta. 1977 Jan 11;480(1):143–153. doi: 10.1016/0005-2744(77)90329-1. [DOI] [PubMed] [Google Scholar]
- Trotman C. N., Greenwood C. Effects of zinc and other metal ions on the stability and activity of Escherichia coli alkaline phosphatase. Biochem J. 1971 Aug;124(1):25–30. doi: 10.1042/bj1240025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. P., Hamlin L. M. The role of Zn(II) on the folding of bovine carbonic anhydrase B. Arch Biochem Biophys. 1975 Sep;170(1):12–22. doi: 10.1016/0003-9861(75)90093-4. [DOI] [PubMed] [Google Scholar]


