Abstract
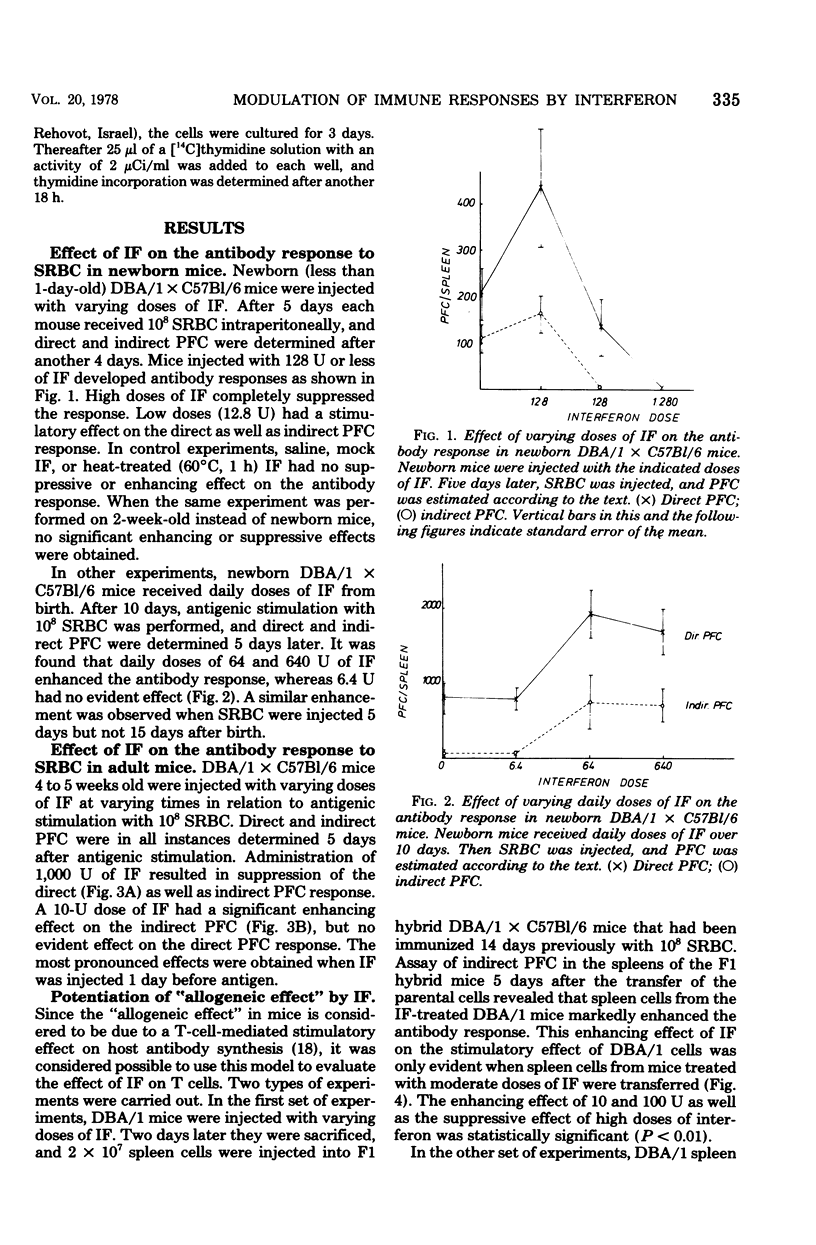
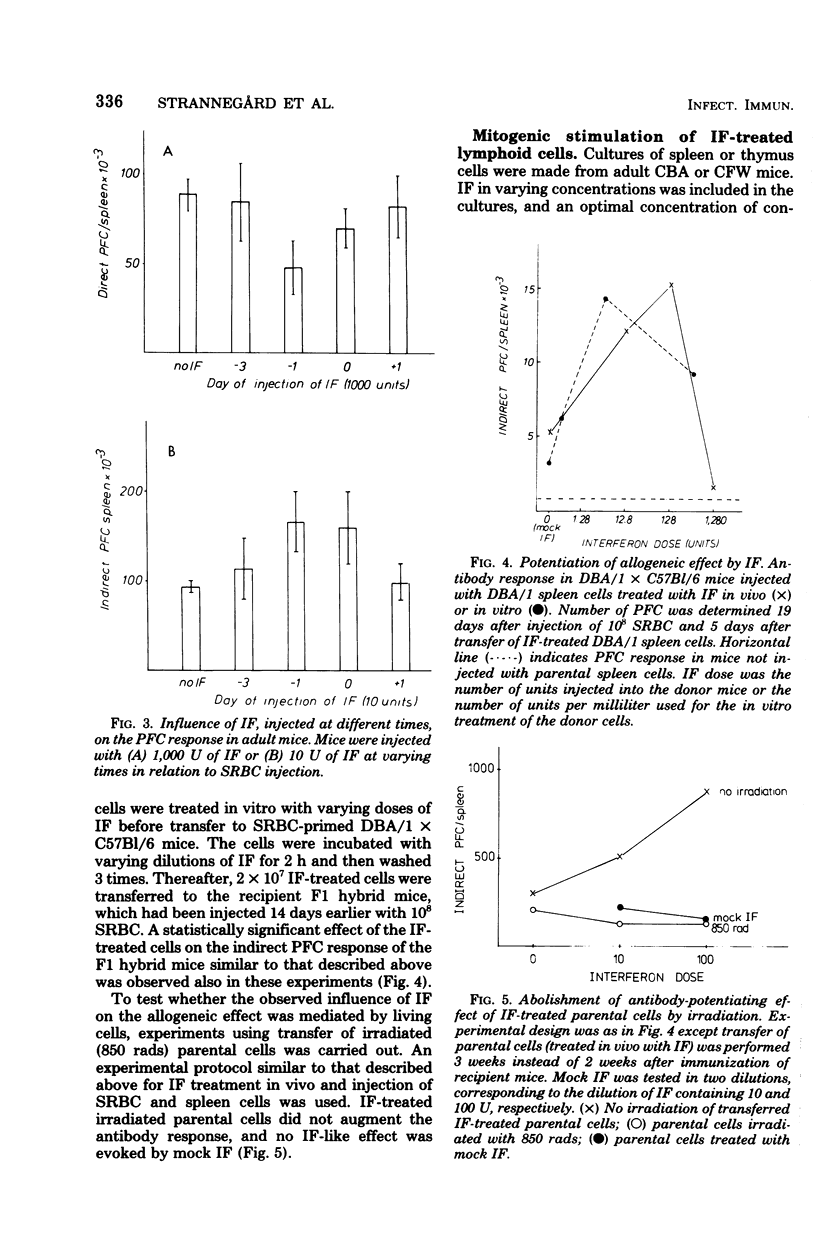
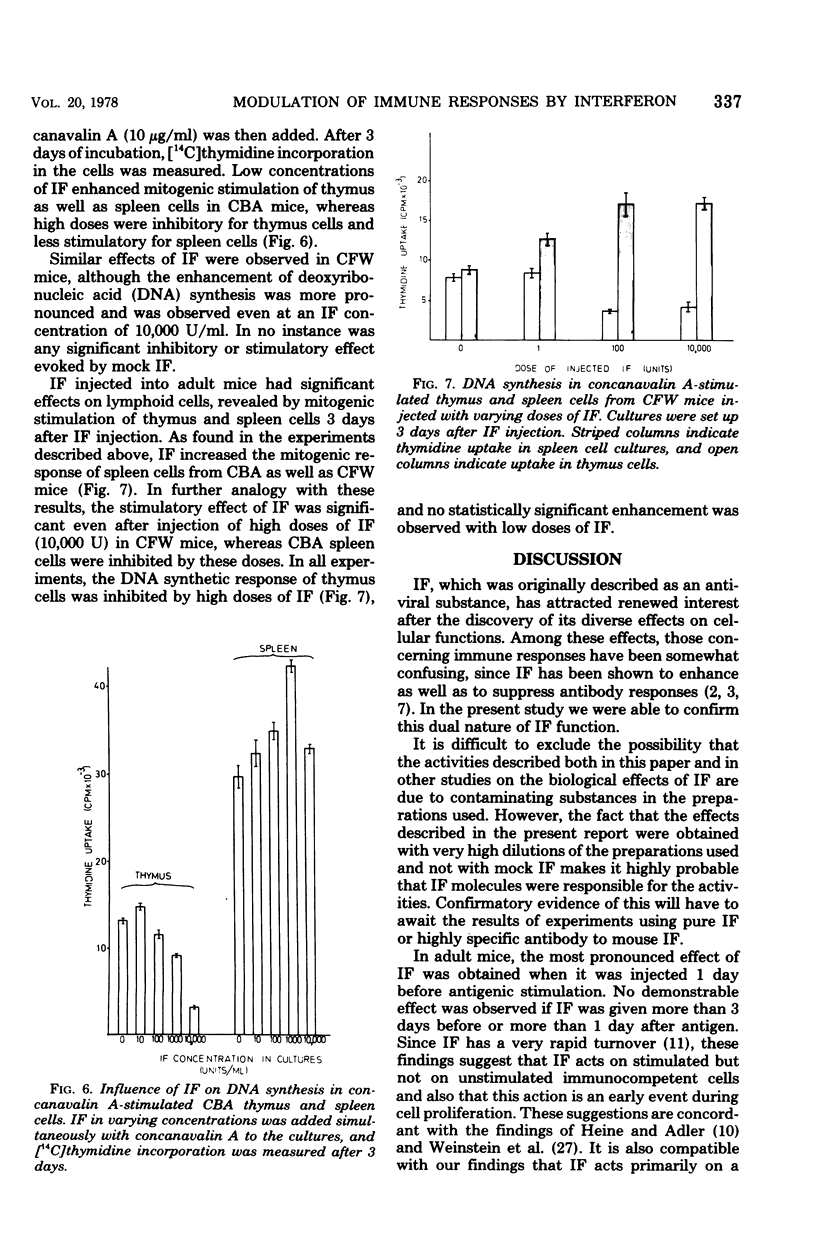
Interferon was found to have both suppressive and enhancing effects on the antibody response in newborn and adult mice. Evidence was obtained that these effects are primarily evoked during the initial steps controlling cell proliferation. Stimulation of thymus and spleen cells with a T-cell mitogen was enhanced by low doses and suppressed by high doses of interferon. Treatment of parental spleen cells with interferon before injecting them into immunized F1 hybrid mice resulted in an enhanced allogeneic effect. These results are compatible with the hypothesis that interferon affects T cells and has an immunoregulatory role, either by inhibiting the action of suppressor cells or by promoting immunological maturation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besancon F., Ankel H. Binding of interferon to gangliosides. Nature. 1974 Dec 6;252(5483):478–480. doi: 10.1038/252478a0. [DOI] [PubMed] [Google Scholar]
- Braun W., Levy H. B. Interferon preparations as modifiers of immune responses. Proc Soc Exp Biol Med. 1972 Dec;141(3):769–773. doi: 10.3181/00379727-141-36868. [DOI] [PubMed] [Google Scholar]
- Brodeur B. R., Merigan T. C. Mechanism of the suppressive effect of interferon on antibody synthesis in vivo. J Immunol. 1975 Apr;114(4):1323–1328. [PubMed] [Google Scholar]
- Brodeur B. R., Weinstein Y., Melmon K. L., Merigan T. C. Reciprocal changes in interferon production and immune responses of mouse spleen cells fractionated over columns of insolubilized conjugates of histamine. Cell Immunol. 1977 Mar 15;29(2):363–372. doi: 10.1016/0008-8749(77)90331-8. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Pastan I. Interferon and cyclic-3'5'-adenosine monophosphate: potentiation of antiviral activity. Biochem Biophys Res Commun. 1969 Aug 22;36(5):735–740. doi: 10.1016/0006-291x(69)90671-8. [DOI] [PubMed] [Google Scholar]
- Gisler R. H., Lindahl P., Gresser I. Effects of interferon on antibody synthesis in vitro. J Immunol. 1974 Aug;113(2):438–444. [PubMed] [Google Scholar]
- Havell E. A., Vilcek J. Production of high-titered interferon in cultures of human diploid cells. Antimicrob Agents Chemother. 1972 Dec;2(6):476–484. doi: 10.1128/aac.2.6.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J. W., Adler W. H. The kinetics of interferon production by mouse lymphocytes and its modulating effect of the virus plaqueforming cell assay as a quantitative method to determine activated lymphocytes. J Immunol. 1976 Sep;117(3):1045–1048. [PubMed] [Google Scholar]
- Huang K. Y., Donahoe R. M., Gordon F. B., Dressler H. R. Enhancement of phagocytosis by interferon-containing preparations. Infect Immun. 1971 Nov;4(5):581–588. doi: 10.1128/iai.4.5.581-588.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H. M., Baron S. The nature of the suppressive effect of interferon and interferon inducers on the in vitro immune response. Cell Immunol. 1976 Jul;25(1):106–115. doi: 10.1016/0008-8749(76)90100-3. [DOI] [PubMed] [Google Scholar]
- Johnson H. M. Cyclic AMP regulation of mitogen-induced interferon production and mitogen suppression of immune response. Nature. 1977 Jan 13;265(5590):154–155. doi: 10.1038/265154a0. [DOI] [PubMed] [Google Scholar]
- Killander D., Lindahl P., Lundin L., Leary P., Gresser I. Relationship between the enhanced expression of histocompatibility antigens on interferon-treated L 1210 cells and their position in the cell cycle. Eur J Immunol. 1976 Jan;6(1):56–59. doi: 10.1002/eji.1830060112. [DOI] [PubMed] [Google Scholar]
- Lindahl P., Leary P., Gresser I. Enhancement by interferon of the expression of surface antigens on murine leukemia L 1210 cells. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2785–2788. doi: 10.1073/pnas.70.10.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm L., Holmgren J., Lange S., Lönnroth I. Interaction of cholera toxin and toxin derivatives with lymphocytes. II. Modulating effects of cholera toxin on in vivo humoral and cellular immune responses. Int Arch Allergy Appl Immunol. 1976;50(5):555–573. doi: 10.1159/000231560. [DOI] [PubMed] [Google Scholar]
- Lindholm L., Rydberg L., Strannegård O. Development of host plasma cells during graft-versus-host reactions in mice. Eur J Immunol. 1973 Aug;3(8):511–515. doi: 10.1002/eji.1830030812. [DOI] [PubMed] [Google Scholar]
- Lindholm L., Strannegård O. Enhancement of the allogeneic effect by irradiation of transferred cells. Int Arch Allergy Appl Immunol. 1977;53(2):110–115. doi: 10.1159/000231740. [DOI] [PubMed] [Google Scholar]
- Miörner H., Landström L. E., Larner E., Larsson I., Lundgren E., Strannegård O. Regulation of mitogen-induced lymphocyte DNA synthesis by human interferon of different origins. Cell Immunol. 1978 Jan;35(1):15–24. doi: 10.1016/0008-8749(78)90122-3. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Johnson B. M. Ontogeny of mouse lymphocyte function. II. Development of the ability to produce antibody is modulated by T lymphocytes. J Exp Med. 1975 Jan 1;141(1):216–226. doi: 10.1084/jem.141.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid M. P., Hoffmann M. K., Komuro K., Hämmerling U., Abbott J., Boyse E. A., Cohen G. H., Hooper J. A., Schulof R. S., Goldstein A. L. Differentiation of T cells induced by preparations from thymus and by nonthymic agents. J Exp Med. 1973 Oct 1;138(4):1027–1032. doi: 10.1084/jem.138.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh U., Owen J. J. Studies on the effect of various agents on the maturation of thymus stem cells. Eur J Immunol. 1975 Apr;5(4):286–288. doi: 10.1002/eji.1830050414. [DOI] [PubMed] [Google Scholar]
- Teh H. S., Paetkau V. Regulation of immune responses. II. The cellular basis of cyclic AMP effects on humoral immunity. Cell Immunol. 1976 Jun 15;24(2):220–229. doi: 10.1016/0008-8749(76)90207-0. [DOI] [PubMed] [Google Scholar]
- Tovey M. G., Begon-Lours J., Gresser I. A method for the large scale production of potent interferon preparations. Proc Soc Exp Biol Med. 1974 Jul;146(3):809–815. doi: 10.3181/00379727-146-38196. [DOI] [PubMed] [Google Scholar]
- Vengris V. E., Reynolds F. H., Jr, Hollenberg M. D., Pitha P. M. Interferon action: role of membrane gangliosides. Virology. 1976 Jul 15;72(2):486–493. doi: 10.1016/0042-6822(76)90177-x. [DOI] [PubMed] [Google Scholar]
- Weber J. M., Stewart R. B. Cyclic AMP potentiation of interferon antiviral activity and effect of interferon on cellular cyclic AMP levels. J Gen Virol. 1975 Sep;28(3):363–372. doi: 10.1099/0022-1317-28-3-363. [DOI] [PubMed] [Google Scholar]
- Weinstein Y., Brodeur B. R., Melmon K. L., Merigan T. C. Interferon inhibition of lymphocyte mitogenesis. Immunology. 1977 Sep;33(3):313–319. [PMC free article] [PubMed] [Google Scholar]
- Yaron M., Yaron I., Gurari-Rotman D., Revel M., Lindner H. R., Zor U. Stimulation of prostaglandin E production in cultured human fibroblasts by poly(I)-poly(C) and human interferon. Nature. 1977 Jun 2;267(5610):457–459. doi: 10.1038/267457a0. [DOI] [PubMed] [Google Scholar]


