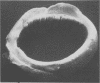
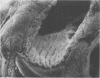
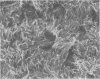
Abstract
Porcine tracheal rings and lung explants alone and in combination with monolayer cultures of porcine lung fibroblasts (PLF) were separately inoculated with virulent strains of Mycoplasma hyopneumoniae and incubated at various times. The preparations were observed by bright-field, phase-contrast, and scanning electron microscopy. In PLF cultures, the strains at initial concentrations of 10(1.3) colony-forming units/ml increased within 3 days to 10(6) colony-forming units/ml, showed progressive clustering on the cells, and caused some sloughing. Introduction of a tracheal ring or lung explant into these mycoplasma-infected PLF cultures caused the explant to lose its epithelial ciliary motility. Eventually parts or whole cells of the respective ciliated epithelium were lost. Without infected PLF monolayers, the explants inoculated with M. hyopneumoniae were less susceptible to infection. When uninfected explants were incubated for 18 days or kept in stock for 2 months, they did not show the above changes. With 5 h postinoculation, M. hyopneumoniae cultures became intimately associated with the PLF culture, but when epithelial cell sloughing occurred, the mycoplasmal cells became dependent on the introduction of a fresh PLF monolayer or a tracheal or lung explant for survival.
Full text
PDF

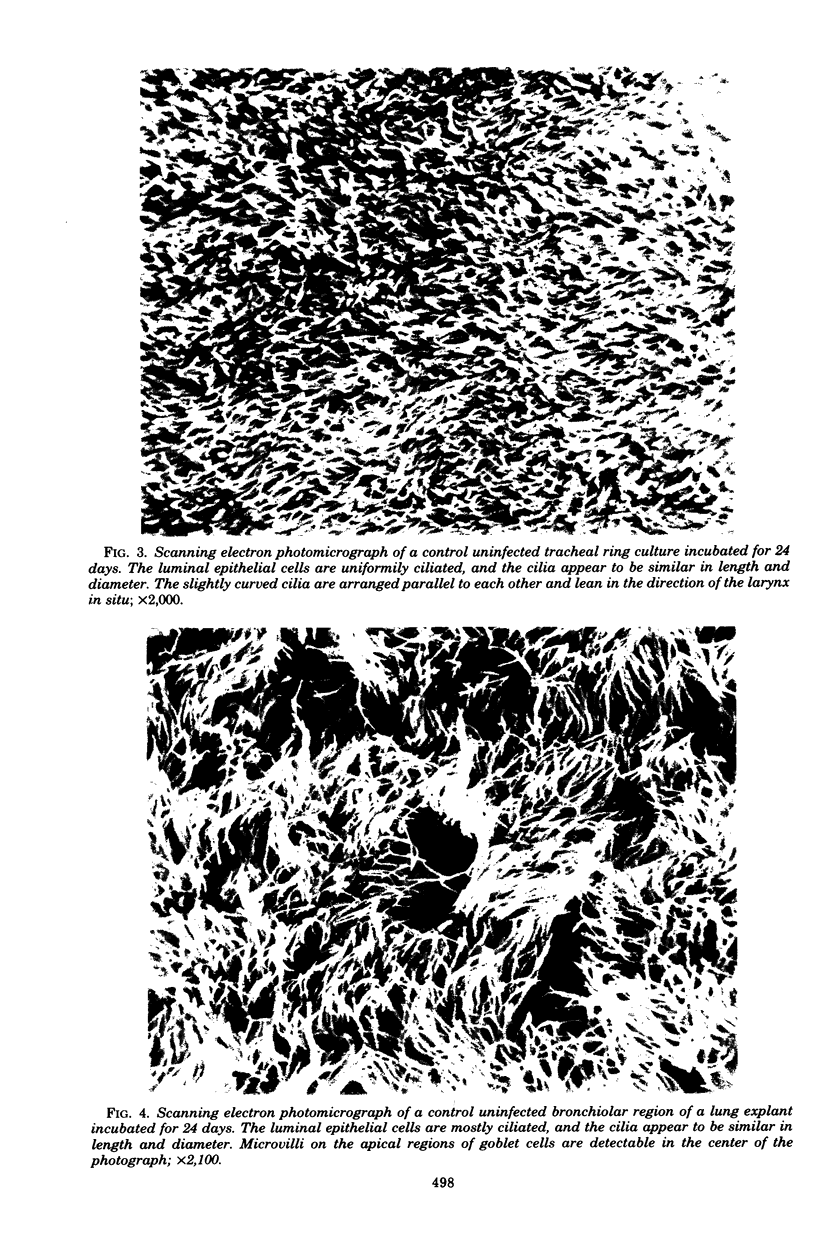
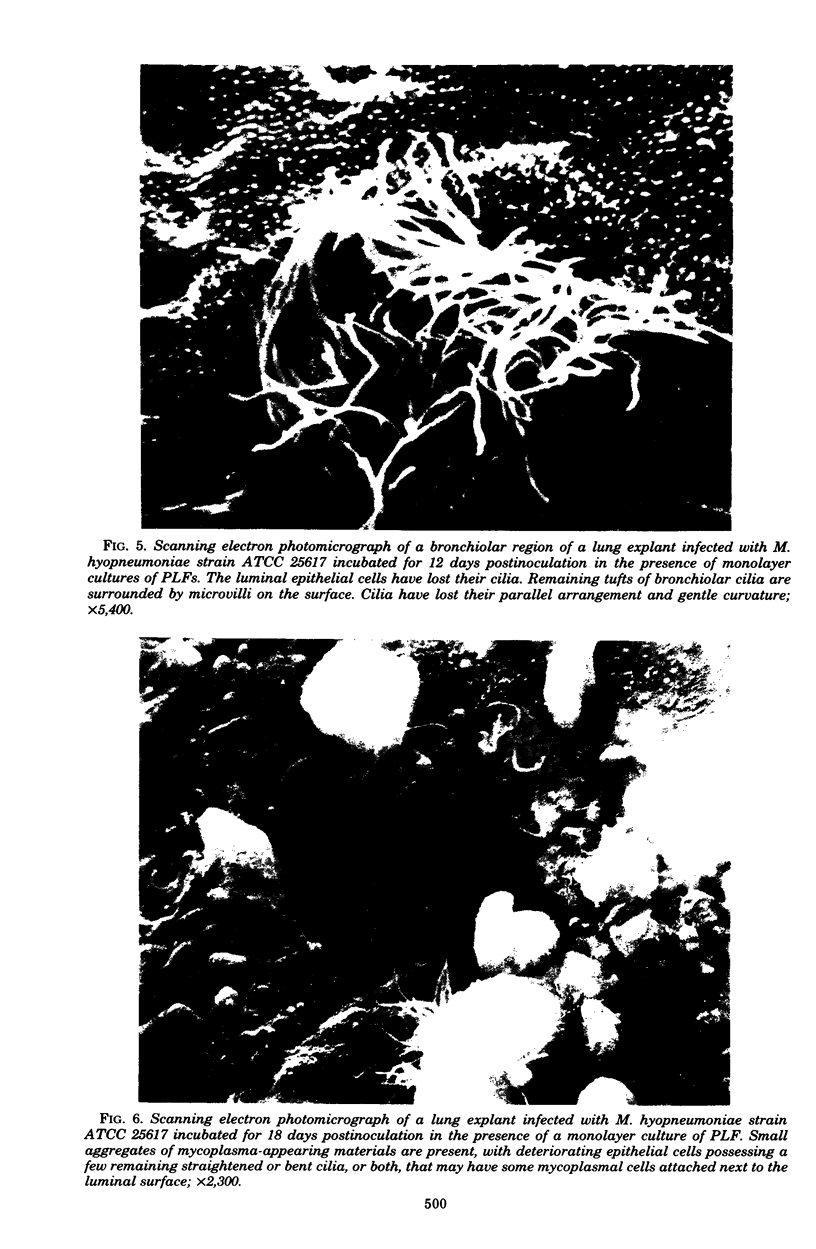


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. E. Mycoplasmosis in animals. Adv Vet Sci. 1965;10:205–244. [PubMed] [Google Scholar]
- Brown S., Teplitz M., Revel J. P. Interaction of mycoplasmas with cell cultures, as visualized by electron microscopy. Proc Natl Acad Sci U S A. 1974 Feb;71(2):464–468. doi: 10.1073/pnas.71.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis N. F. Some recommendations concerning primary isolation of Mycoplasma suipneumoniae and Mycoplasma flocculare a survey. Nord Vet Med. 1975 Jun;27(6):337–339. [PubMed] [Google Scholar]
- Gabridge M. G., Johnson C. K., Cameron A. M. Cytotoxicity of Mycoplasma pneumoniae Membranes. Infect Immun. 1974 Nov;10(5):1127–1134. doi: 10.1128/iai.10.5.1127-1134.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood M. F., Holland P. The mammalian respiratory tract surface. A scanning electron microscopic study. Lab Invest. 1972 Sep;27(3):296–304. [PubMed] [Google Scholar]
- Hoorn B., Tyrrell D. A. Organ cultures in virology. Prog Med Virol. 1969;11:408–450. [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Interaction of virulent Mycoplasma pneumoniae with hamster tracheal organ cultures. Infect Immun. 1976 Jul;14(1):217–224. doi: 10.1128/iai.14.1.217-224.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston C. W., Jr, Stair E. L., Underdahl N. R., Mebus C. A. Pathogenesis of mycoplasmal pneumonia in swine. Am J Vet Res. 1972 Nov;33(11):2249–2258. [PubMed] [Google Scholar]
- Pijoan C., Roberts D. H., Harding J. D. The effect of porcine mycoplasmas on pig tracheal organ cultures. J Appl Bacteriol. 1972 Sep;35(3):361–365. doi: 10.1111/j.1365-2672.1972.tb03710.x. [DOI] [PubMed] [Google Scholar]
- Pijoan C. The effects of Mycoplasma hyorhinis and of Mycoplasma hyopneumoniae on pig kidney primary tissue culture. Br Vet J. 1975 Sep-Oct;131(5):586–594. doi: 10.1016/s0007-1935(17)35192-8. [DOI] [PubMed] [Google Scholar]
- ROBERTS E. D., SWITZER W. P., L'ECUYER C. Influence of Pasteurella multocida and Mycoplasma hyorhinis (PPLO) on the histopathology of field case of swine pneumonia. Cornell Vet. 1962 Jul;52:306–327. [PubMed] [Google Scholar]
- Thomas L. Mechanisms of pathogenesis in Mycoplasma infection. Harvey Lect. 1969;63:73–98. [PubMed] [Google Scholar]
- Williams P. P. Collection and cultivation of and phagocytosis by pulmonary macrophages obtained from hysterectomy-derived pigs. Am J Vet Res. 1978 Mar;39(3):485–489. [PubMed] [Google Scholar]
- YOUNGNER J. S. Monolayer tissue cultures. I. Preparation and standardization of suspensions of trypsin-dispersed monkey kidney cells. Proc Soc Exp Biol Med. 1954 Feb;85(2):202–205. doi: 10.3181/00379727-85-20830. [DOI] [PubMed] [Google Scholar]