Abstract
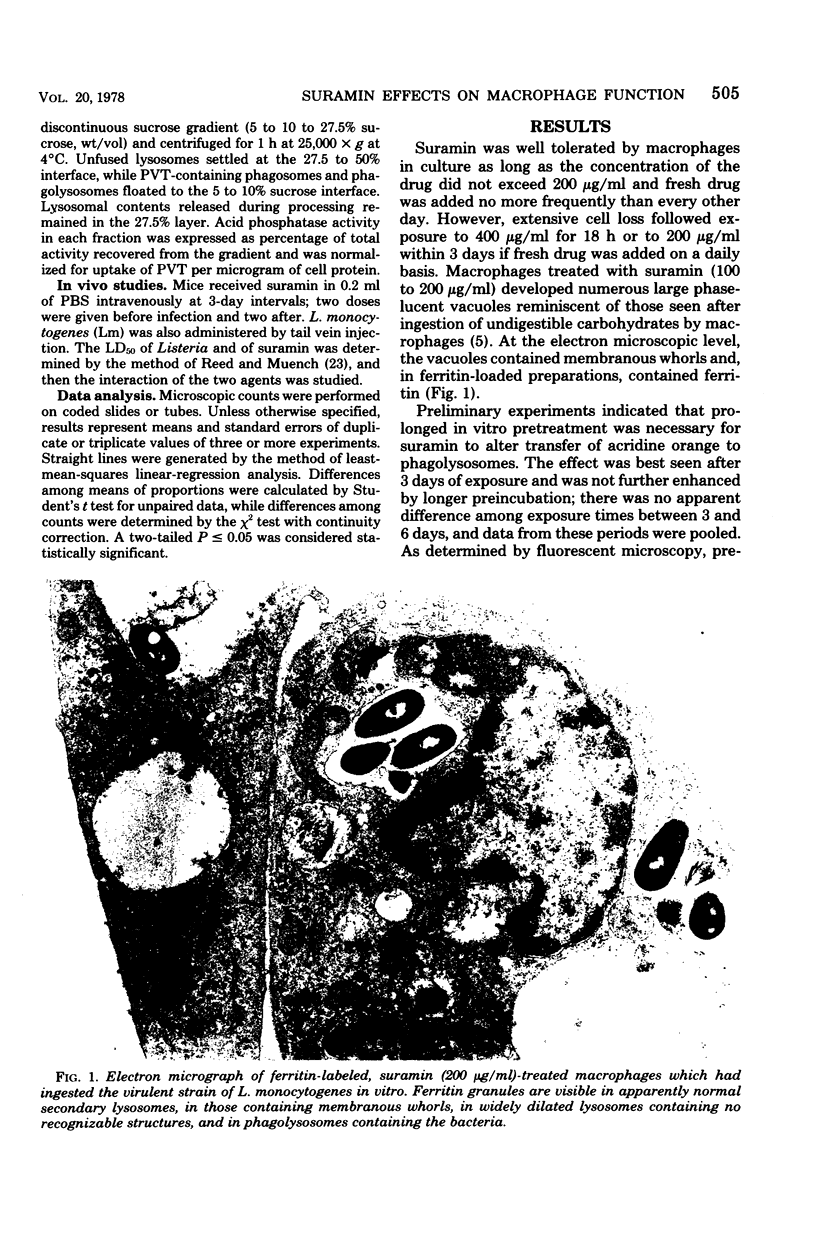
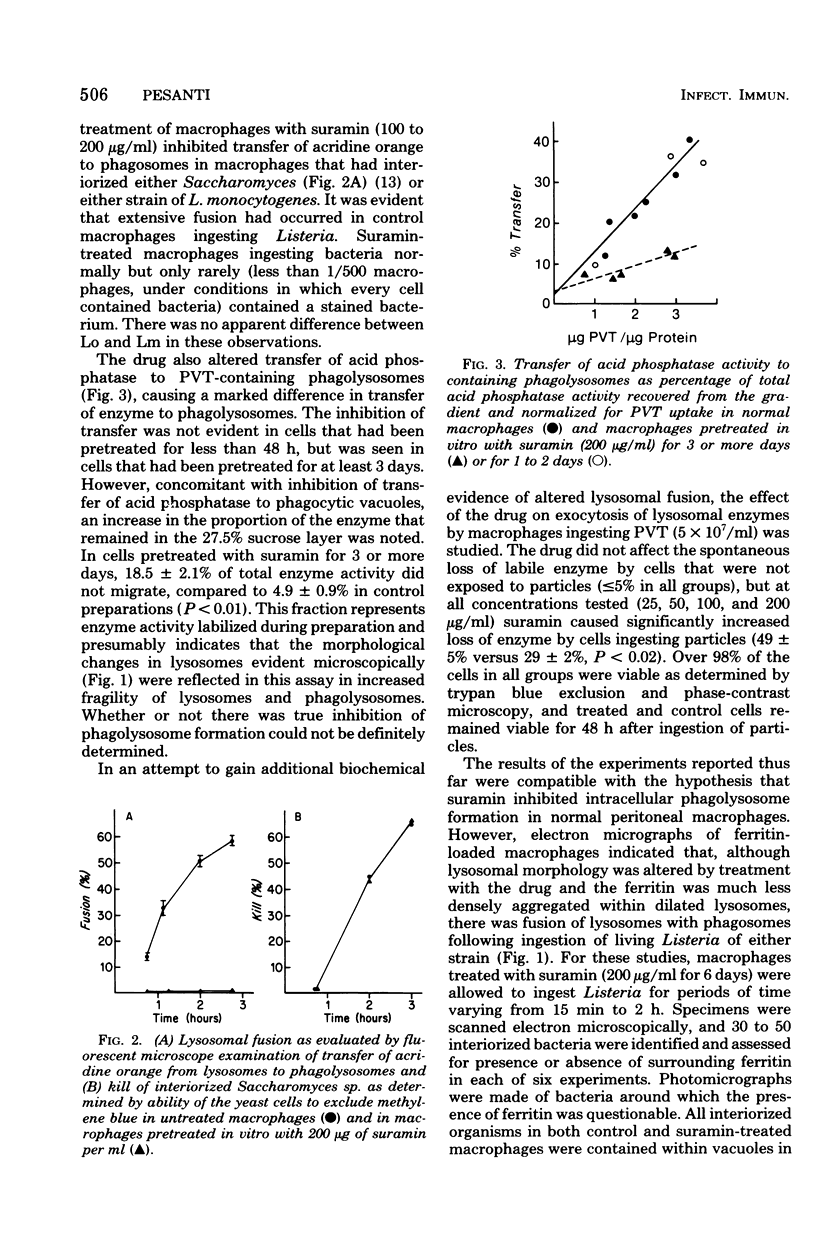
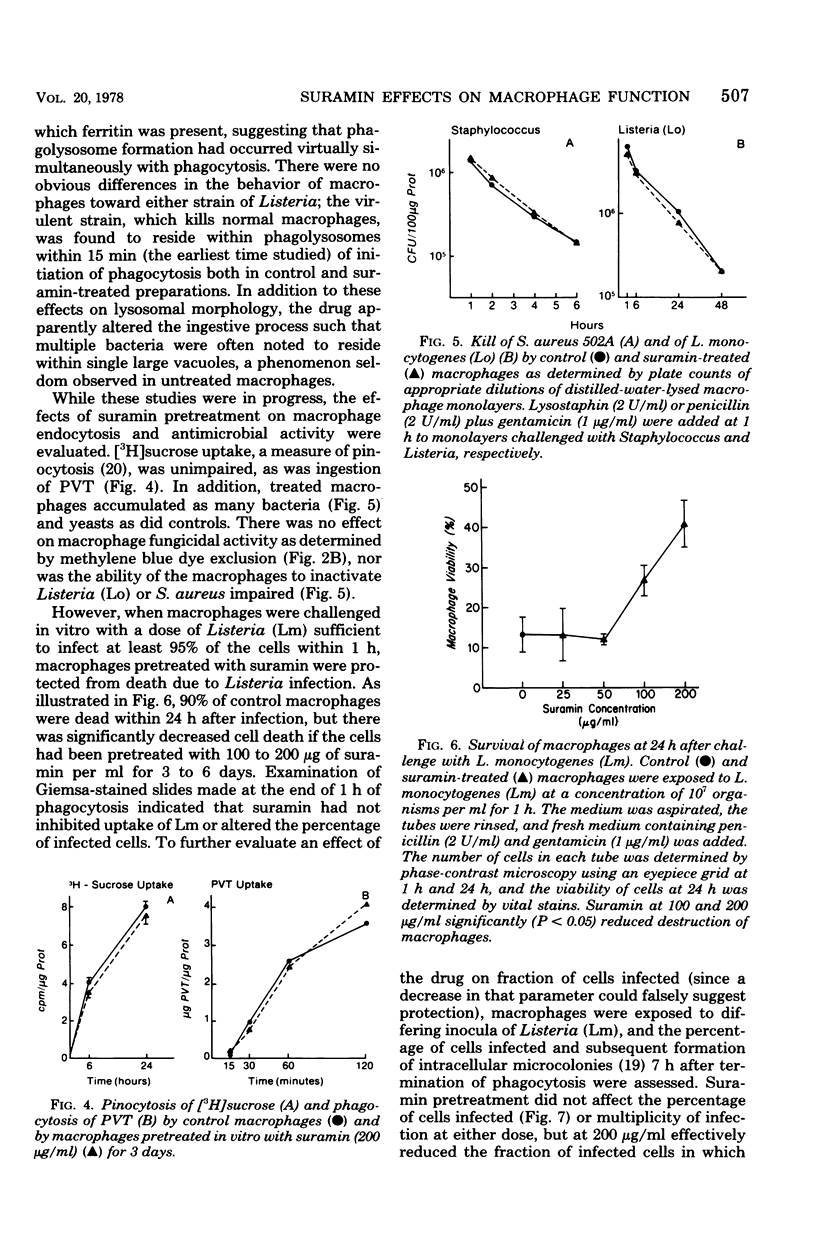
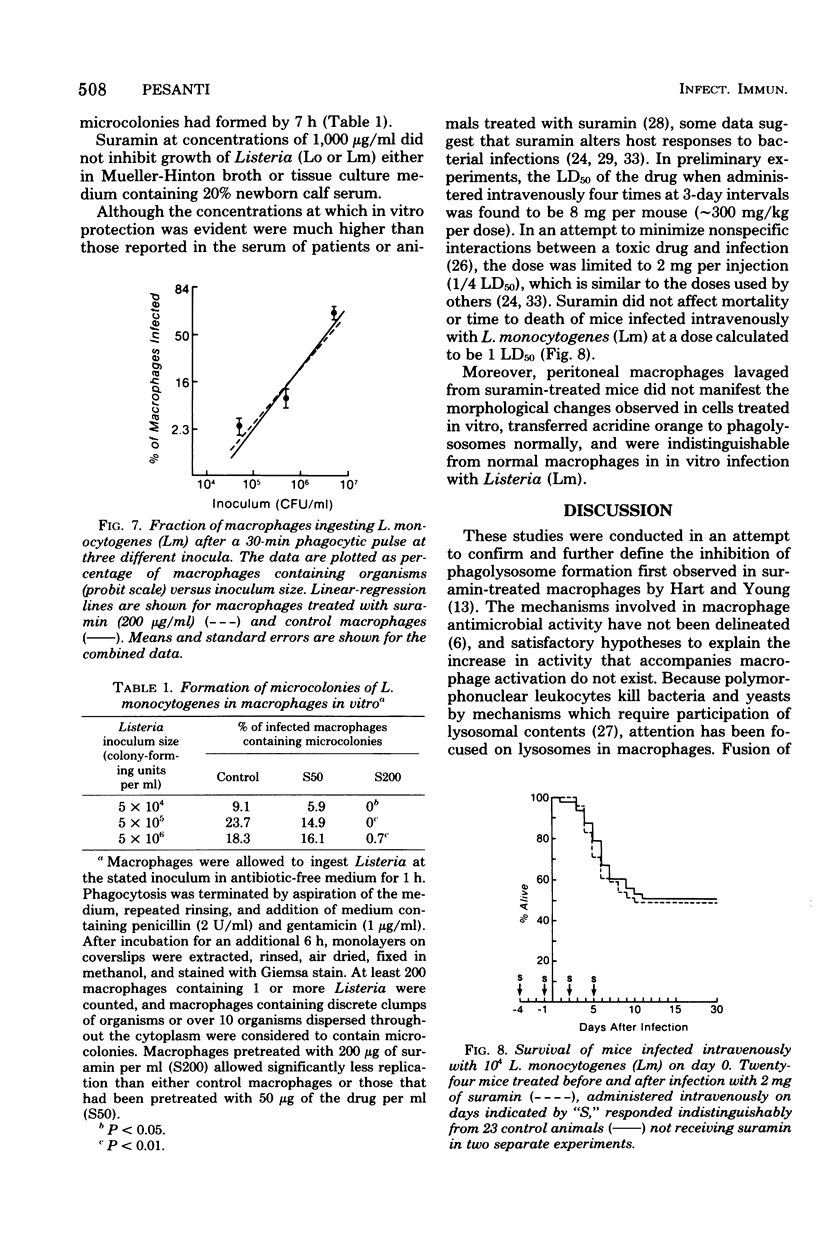
The effects of suramin on phagolysosome formation and antimicrobial activity of mouse peritoneal macrophages cultivated in vitro have been studied. Prolonged in vitro pretreatment of macrophages with high concentrations of suramin caused macrophages to form large fragile phagolysosomes in which the concentrations of the various lysosomal enzymes were inferred to be diminished. In addition, suramin-treated macrophages demonstrated enhanced exocytosis of acid phosphatase during phagocytosis of polyvinyl toluene spherules. However, suramin was found not to inhibit formation of phagolysosomes in macrophages that had ingested Listeria monocytogenes when those cells were examined by the electron microscope. Suramin pretreatment did not alter the ingestion or intracellular killing of Staphylococcus aureus or of a strain of L. monocytogenes that was essentially avirulent for mice, but did protect macrophages from destruction by virulent L. monocytogenes ingested in vitro, an effect that appeared to have been mediated through enhancement of the bacteriostatic potential of the macrophages. However, at a single dosage level, the drug did not alter the mortality of mice challenged with virulent L. monocytogenes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Hart P. D. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med. 1975 Jul 1;142(1):1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. P., Dwyer D. M. Multiplication of a human parasite (Leishmania donovani) in phagolysosomes of hamster macrophages in vitro. Science. 1976 Aug 20;193(4254):678–680. doi: 10.1126/science.948742. [DOI] [PubMed] [Google Scholar]
- Cohn Z. A., Ehrenreich B. A. The uptake, storage, and intracellular hydrolysis of carbohydrates by macrophages. J Exp Med. 1969 Jan 1;129(1):201–225. doi: 10.1084/jem.129.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'ARCY HART P., REES R. J. Enchancement of experimental tuberculosis in the mouse by suramin. Tubercle. 1956 Oct;37(5):327–332. doi: 10.1016/s0041-3879(56)80078-0. [DOI] [PubMed] [Google Scholar]
- Edelson P. J., Cohn Z. A. Effects of concanavalin A on mouse peritoneal macrophages. I. Stimulation of endocytic activity and inhibition of phago-lysosome formation. J Exp Med. 1974 Nov 1;140(5):1364–1386. doi: 10.1084/jem.140.5.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folds J. D., Orlando G., Spitznagel J. K. Immunosuppression by hydroxystilbamidine isethionate, a lysosome-stabilizing, anti-proteolytic, antifungal drug. Infect Immun. 1975 Mar;11(3):441–444. doi: 10.1128/iai.11.3.441-444.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth R. V., Jones T. C. Effect of glucocorticosteroids on phagosome-lysosome interaction. Infect Immun. 1975 Oct;12(4):888–890. doi: 10.1128/iai.12.4.888-890.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren M. B., D'Arcy Hart P., Young M. R., Armstrong J. A. Prevention of phagosome-lysosome fusion in cultured macrophages by sulfatides of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2510–2514. doi: 10.1073/pnas.73.7.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A., Brown C. A., Draper P. Ultrastructural study of the behavior of macrophages toward parasitic mycobacteria. Infect Immun. 1972 May;5(5):803–807. doi: 10.1128/iai.5.5.803-807.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. D. Mycobacterium tuberculosis in macrophages: effect of certain surfactants and other membrane-active compounds. Science. 1968 Nov 8;162(3854):686–689. doi: 10.1126/science.162.3854.686. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Young M. R. Interference with normal phagosome-lysosome fusion in macrophages, using ingested yeast cells and suramin. Nature. 1975 Jul 3;256(5512):47–49. doi: 10.1038/256047a0. [DOI] [PubMed] [Google Scholar]
- JANCSO N., JANCSO-GABOR A. Suramin (Bayer 205) in animal tissues; demonstration of Bayer 205 (suramin) in tissues and its cellular distribution. Nature. 1952 Oct 4;170(4327):567–571. doi: 10.1038/170567a0. [DOI] [PubMed] [Google Scholar]
- Kress Y., Bloom B. R., Wittner M., Rowen A., Tanowitz H. Resistance of Trypanosoma cruzi to killing by macrophages. Nature. 1975 Oct 2;257(5525):394–396. doi: 10.1038/257394a0. [DOI] [PubMed] [Google Scholar]
- Kripke M. L., Norbury K. C., Gruys E., Hibbs J. B., Jr Effects of trypan blue treatment on the immune responses of mice. Infect Immun. 1977 Jul;17(1):121–129. doi: 10.1128/iai.17.1.121-129.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesanti E. L., Axline S. G. Colchicine effects on lysosomal enzyme induction and intracellular degradation in the cultivated macrophage. J Exp Med. 1975 May 1;141(5):1030–1046. doi: 10.1084/jem.141.5.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesanti E. L., Axline S. G. Phagolysosome formation in normal and colchicine-treated macrophages. J Exp Med. 1975 Oct 1;142(4):903–913. doi: 10.1084/jem.142.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS E., MARCUS P. I. DYNAMICS OF ACRIDINE ORANGE-CELL INTERACTION. I. INTERRELATIONSHIPS OF ACRIDINE ORANGE PARTICLES AND CYTOPLASMIC REDDENING. J Cell Biol. 1963 Aug;18:237–250. doi: 10.1083/jcb.18.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. M., Hazard E. C. Anomalous results of high dose chemotherapy in experimental peritonitis. Surg Gynecol Obstet. 1970 Jan;130(1):94–98. [PubMed] [Google Scholar]
- TOWN B. W., WILLS E. D., WILSON E. J., WORMALL A. Studies on suramin; the action of the drug on enzymes and some other proteins. General considerations. Biochem J. 1950 Aug;47(2):149–158. doi: 10.1042/bj0470149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Meer C., Hofhuis F. M., Willers J. M. Killed Listeria monocytogenes vaccine becomes protective on addition of polyanions. Nature. 1977 Oct 13;269(5629):594–595. doi: 10.1038/269594a0. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON J. Chemotherapy and chemoprophylaxis of African trypanosomiasis. Exp Parasitol. 1962 Oct;12:323–367. doi: 10.1016/0014-4894(62)90046-2. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON J. Chemotherapy and chemoprophylaxis of African trypanosomiasis. Exp Parasitol. 1962 Aug;12:274–322. doi: 10.1016/0014-4894(62)90075-9. [DOI] [PubMed] [Google Scholar]
- WONG P. C., MA L. Effect of suramin on serum protein in Mycobacterium lepraemurium-infected mice. J Trop Med Hyg. 1963 Apr;66:99–101. [PubMed] [Google Scholar]
- Zurier R. B., Weissmann G., Hoffstein S., Kammerman S., Tai H. H. Mechanisms of lysosomal enzyme release from human leukocytes. II. Effects of cAMP and cGMP, autonomic agonists, and agents which affect microtubule function. J Clin Invest. 1974 Jan;53(1):297–309. doi: 10.1172/JCI107550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]



