Abstract
Long-term potentiation (LTP) at glutamatergic synapses is considered to underlie learning and memory and is associated with the enlargement of dendritic spines. Because the consolidation of memory and LTP require protein synthesis, it is important to clarify how protein synthesis affects spine enlargement. In rat brain slices, the repetitive pairing of postsynaptic spikes and two-photon uncaging of glutamate at single spines (a spike-timing protocol) produced both immediate and gradual phases of spine enlargement in CA1 pyramidal neurons. The gradual enlargement was strongly dependent on protein synthesis and brain-derived neurotrophic factor (BDNF) action, often associated with spine twitching, and was induced specifically at the spines that were immediately enlarged by the synaptic stimulation. Thus, this spike-timing protocol is an efficient trigger for BDNF secretion and induces protein synthesis–dependent long-term enlargement at the level of single spines.
The consolidation of memory and long-term potentiation (LTP) require protein synthesis (1, 2). Therefore, it is important to clarify whether protein synthesis can regulate synaptic plasticity at the level of a single synapse and how it affects synaptic structure. The spine enlargement associated with LTP can be immediately induced by intensive stimulation of postsynaptic N-methyl-d-aspartate (NMDA)–sensitive glutamate receptors (the conventional protocol) in CA1 pyramidal neurons (3–5). This spine enlargement can be induced even in the absence of postsynaptic spikes (3), although if synaptic stimulation is closely followed in time by postsynaptic spikes (a spike-timing protocol), a more robust form of LTP is induced that plays an important role in the development and learning of neuronal networks (6). In rat brain slices, we examined the structural plasticity of dendritic spines induced by the stimulation of single spines, using two-photon uncaging of glutamate (7) in the absence or presence of postsynaptic spikes in CA1 pyramidal neurons (uncaging is photorelease from a biologically inert precursor).
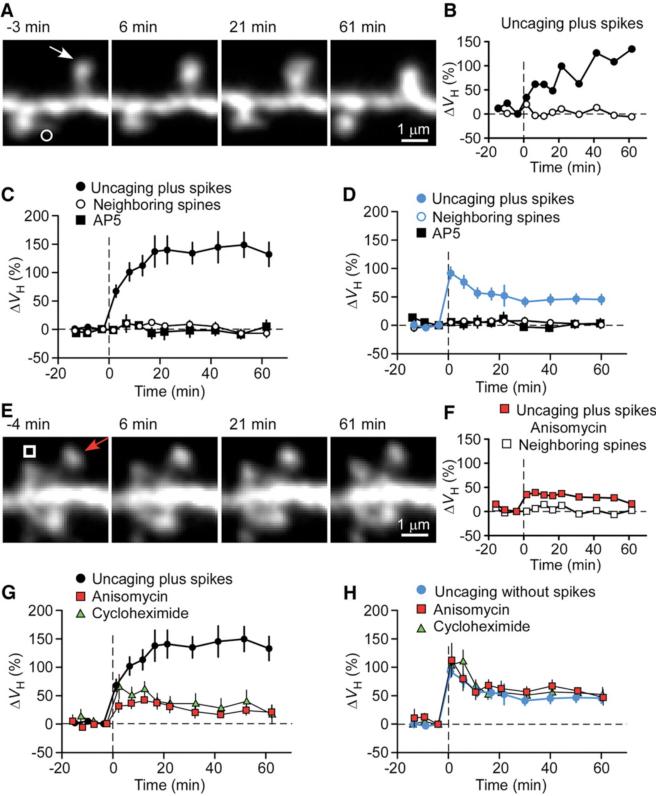
CA1 pyramidal neurons in slice culture were subjected to whole-cell perfusion with a solution containing the fluorescent dye Alexa594 (50 μM) and β-actin (5 μM) (8). The latter protein was included because we found that it delayed the washout of plasticity (3) (fig. S1 and supporting online text). We detected marked (>50%) increases in spine-head volume (ΔVH) in most (37 of 41) small spines stimulated by repetitive (80 times at 1 Hz) uncaging of 4-methoxy-7-nitroindolinyl (MNI)–glutamate paired with post-synaptic spikes within 20 ms (spike-timing protocol or uncaging plus spikes) (Fig. 1, A to C). Spine enlargement was not induced by repetitive glutamate uncaging (1.6 ± 6.6%, n =9 spines, in the presence of Mg2+) or spike application alone (–3.0 ± 2.6%, n = 54). It was also not induced when spikes were triggered >50 ms after uncaging (3.9 ± 3.4%, n = 10). Spine enlargement was restricted to stimulated spines; it did not spread to neighboring spines (Fig. 1, A to C). The NMDA receptor blocker d(–)-2-amino-5-phosphonopentanoic acid (AP5, 50 μM) prevented spine enlargement induced by uncaging plus spikes (n = 9) (Fig. 1C), as well as conventional enlargement (3) induced by uncaging without spikes in a Mg2+-free solution (Fig. 1D) (8). Subsequent experiments were performed with small spines (VH <0.1 μm3), most (95%) of which underwent enlargement in response to uncaging plus spikes (fig. S2). The enlargement was associated with an increase in postsynaptic glutamate sensitivity (fig. S3, A to D).
Fig. 1.
Spine-head enlargement induced by uncaging of glutamate with or without the application of postsynaptic spikes for single identified spines of CA1 pyramidal neurons in hippocampal slice culture. (A and E) Time-lapse z-integrated (z-stack) images of spines stimulated at time 0 by uncaging plus spikes in the absence (A) or presence (E) of anisomycin. Arrows indicate spots of two-photon uncaging of MNI-glutamate; open symbols indicate neighboring spines. (B and F) Time courses of changes in ΔVH for the stimulated (solid symbols) and neighboring (open symbols) spines shown in (A) and (E), respectively. (C and D) Averaged time courses of changes in ΔVH for spines stimulated by uncaging plus (C) or without (D) spikes in the absence (solid circles) or presence (solid squares) of AP5. Open circles represent data from neighboring spines in the absence of AP5 (open circles). Uncaging without spikes was performed in a Mg2+-free solution. Data are means ± SEM (n = 10 to 27 spines). The control trace shown in (C) and (G) was the average of 27 experiments performed in the same batches of slice preparations used for the test experiments. (G and H) Averaged time courses of changes in spine-head volume for spines stimulated by uncaging plus (G) or without (H) spikes in the absence (circles) or presence of anisomycin (red squares) or cycloheximide (green triangles). Data are means ± SEM (n = 7 to 27 spines).
We found critical differences between spine enlargement induced by uncaging plus spikes and that induced by uncaging without spikes (Figs. 1, 2, 3). First, the former protocol induced a secondary long-term phase (Fig. 1, B and C), unlike the latter one (Fig. 1D). The total ΔVH apparent after a 60-min recording period was 132 ± 22% (n = 27) (Fig. 1C) for uncaging plus spikes as compared with only 45.7 ± 9.6% for uncaging without spikes (n =20) (Fig. 1D). A similar difference was apparent in the increases in the amplitude of glutamate-induced currents evoked at the spines, which were 162 ± 38% (n = 12) (fig. S3B) and 36 ± 7% (n =9) for uncaging plus or without (3) spikes, respectively. The larger long-term enlargement was not simply ascribed to the strength of stimulation, because the immediate enlargement produced by uncaging plus spikes (67.5 ± 11.2%, mean ± SEM) (Fig. 1C) was smaller than that induced by uncaging without spikes (3) (92.4 ± 10.7%, P < 0.01) (Fig. 1D).
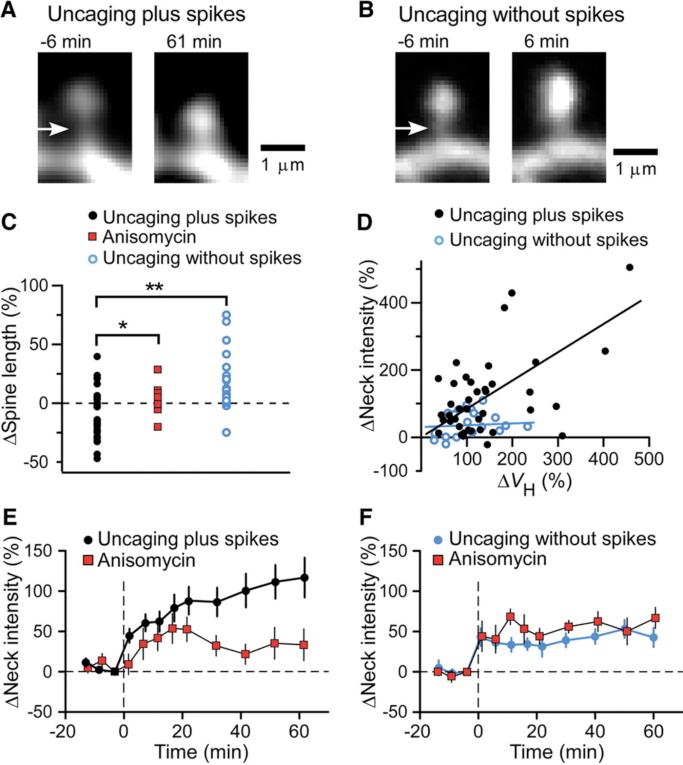
Fig. 2.
Spine-neck plasticity induced by glutamate uncaging plus or without postsynaptic spikes. (A and B) Images of spines before (left) and after (right) uncaging of MNI-glutamate with (A) or without (B) spikes. Arrows indicate the spine necks. (C) Changes in spine length induced either by uncaging plus spikes in the absence (black circles) or presence (red squares) of anisomycin or by uncaging without spikes (blue circles). *P < 0.05, **P < 0.01 (Mann-Whitney U test). (D) Correlation between the increases in spine-neck fluorescence intensity and ΔVH induced by uncaging plus (black circles; Spearman's correlation coefficient = 0.37, P = 0.013) or without (blue circles, P = 0.16) spikes. Data correspond to the increases observed at the time of maximal spine-head enlargement. (E and F) Time courses of changes in spine-neck fluorescence intensity for spines stimulated by uncaging plus (E) or without (F) spikes in the absence or presence of anisomycin. Data are means ± SEM (n = 10 to 41 spines).
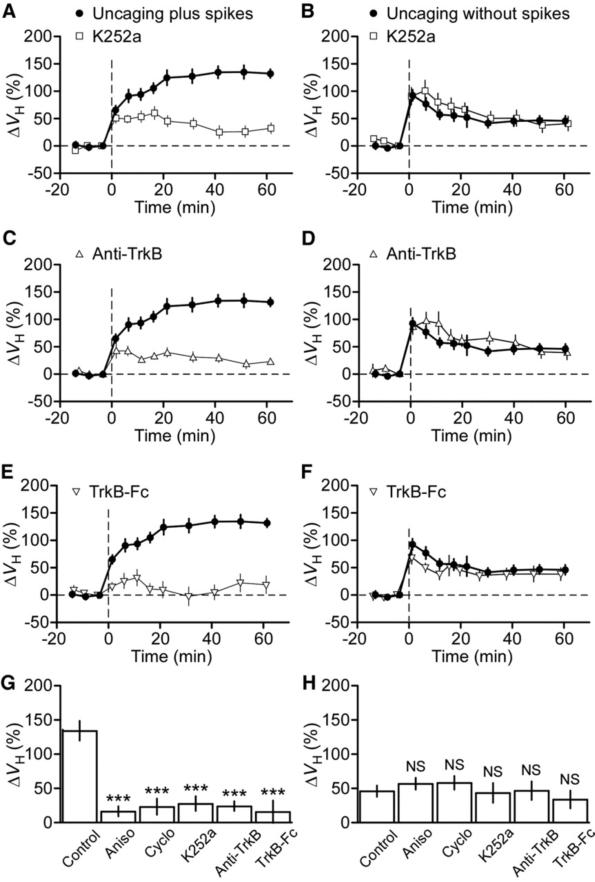
Fig. 3.
Dependence of the gradual long-term enlargement of spine heads on BDNF-TrkB signaling. (A to F) effects of inhibitors of BDNF-TrkB signaling, including K252a [(A) and (B]), an antibody to TrkB [(C) and (D)], and TrkB-Fc [(E) and (F)] on the time course of spine-head enlargement induced by glutamate uncaging plus [(A), (C), and (E)] or without [(B), (D), and (F)] postsynaptic spikes. The control traces are the average of 14 and 20 experiments for uncaging plus and without spikes, respectively, performed in the same batches of slice preparations used for the test experiments. (G and H) Mean enlargement of spine heads measured 40 to 60 min after the onset of uncaging plus (G) or without (H) spikes in the absence (control) or presence of inhibitors of protein synthesis or BDNFTrkB signaling. All data are means ± SEM (n = 8 to 20 spines). ***P < 0.001 versus corresponding control value (Mann-Whitney U test). NS, not significant.
The gradual long-term phase of spine-head enlargement was entirely dependent on protein synthesis. Pretreatment of hippocampal slices with the protein synthesis inhibitors anisomycin (n = 10 spines) or cycloheximide (n = 8) abolished this secondary phase (Fig. 1, E to G) without substantially affecting the immediate phase (Fig. 1, F and G). Anisomycin also blocked the gradual long-term increase in the size of glutamate-induced currents at the stimulated spines (fig. S3, E and F). In contrast, spine enlargement elicited by uncaging without spikes was unaffected by anisomycin (n = 11) (Fig. 1H and fig. S4, C and D) or cycloheximide (n =7). This was the case even when the number of repetitive stimulation was further increased (up to 80 times, n = 18).
Uncaging plus spikes often induced shortening of the spine length, or spine twitching (Figs. 1A and 2A). Apparent shortening of spines was detected in 23 out of 41 spines (mean= –9.9 ± 3.2%) (Fig. 2C), and in the remaining spines, it appeared to be canceled out by the enlargement of the spine heads. In contrast, spine twitching was rarely (2 out of 20) induced by uncaging without spikes (Fig. 2B and fig. S4A); rather, such spines often elongated as a result of spine-head enlargement (6.7 ± 4.9%) (Fig. 2C). To examine the spine-neck plasticity and its time course, we measured the fluorescence intensity of the spine neck (8), because it was difficult to delineate the spine-neck unambiguously, particularly when spines twitched. We detected pronounced increases in spine-neck fluorescence produced by uncaging plus spikes (128 ± 19%, mean ± SEM, n = 41) (Fig. 2D), but far less fluorescence was produced by uncaging without spikes (43 ± 12%, n = 20). The increase in spine-neck fluorescence was not correlated with that in ΔVH in uncaging without spikes (Fig. 2D), indicating that the increase in spine-neck fluorescence did not simply represent spine-head enlargement. The increase in spine-neck fluorescence occurred gradually in parallel with spine-head enlargement (Figs. 1A and 2E) and was inhibited by anisomycin (Figs. 1E and 2E), as was spine twitching (2.0 ± 1.9%) (Fig. 2C). In contrast, the smaller increase in neck fluorescence induced by uncaging without spikes was unaffected by anisomycin (Fig. 2F). The spine twitching may involve the spine apparatus (9) and translation machinery in the spine necks (10, 11) for protein synthesis-dependent spine-head enlargement.
A late phase of LTP has been shown to require BDNF (12–14), although the nature of this requirement varies depending on the induction protocol (15–18). We therefore investigated whether BDNF might play a role in the gradual long-term spine enlargement induced by uncaging plus spikes. K252a (200 nM), a tyrosine kinase inhibitor that blocks BDNF-TrkB signaling, abolished long-term enlargement of spine heads (n = 13) (Fig. 3, A and G), whereas it had no effect on the immediate enlargement induced by uncaging plus spikes (Fig. 3A) or without spikes (n = 15) (Fig. 3, B and H). The long-term enlargement of spine heads was also blocked by an antibody to TrkB (n = 9) (Fig. 3, C and G), whereas the same antibody did not affect the immediate enlargement induced by uncaging plus spikes (Fig. 3C) or without spikes (n =8) (Fig. 3, D and H). Activation of TrkB induced by uncaging plus spikes was dependent on the secretion of BDNF, given that the long-term enlargement of spine heads was also abolished by a TrkB-Fc fusion protein that acts as a scavenger of BDNF (n = 10) (Fig. 3, E and G). Again, the immediate spine-head enlargement induced by uncaging without spikes was largely unaffected by the scavenger (n = 10) (Fig. 3, F and H). Moreover, the increase in spine-neck fluorescence induced by uncaging plus spikes was abolished by K252a, the antibody to TrkB, or TrkB-Fc (fig. S5). The gradual long-term plasticity of spine structures was thus strongly dependent on the endogenous secretion of BDNF, and postsynaptic spikes were required for the secretion.
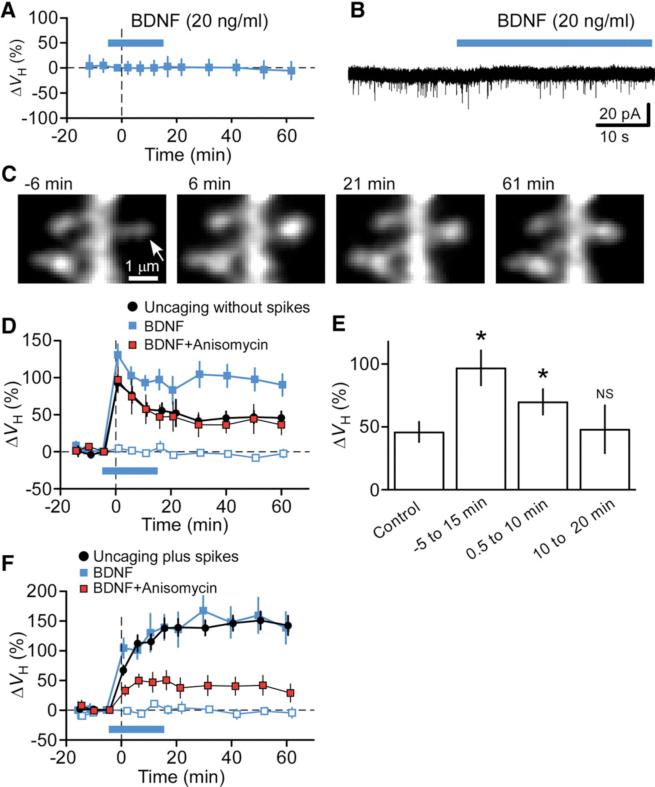
Finally, we examined whether exogenous BDNF might be able to replace postsynaptic spikes in the induction of protein synthesis–dependent spine-head enlargement. Bath application of a low concentration (20 ng/ ml) of BDNF did not by itself induce spine-head enlargement (n = 30) (Fig. 4A) or inward currents (0.5 ± 1.2 pA, n = 5) (Fig. 4B) (19, 20). However, uncaging without spikes in the presence of BDNF (20 ng/ml) resulted in marked enhancement in the long-term phase of spine-head enlargement (n = 16) as compared with that observed in the absence of BDNF (n = 20) (Fig. 4, C and D, and supporting online text). BDNF also induced gradual increases in spine-neck fluorescence (110 ± 21%, P < 0.05) (Fig. 4C). The effect of BDNF was selective for stimulated spines, was not observed for neighboring spines (n = 16) (Fig. 4D), and was blocked by anisomycin (n =11) (Fig. 4D). BDNF thus induced additional spine-head enlargement that was dependent on protein synthesis and specific to stimulated spines. BDNF could induce spine-head enlargement even when applied 0.5 min (n = 17) after the offset of stimulation, but it did not do so when applied 10 min after stimulation (n = 10) (Fig. 4E). Exogenous BDNF did not further augment the spine-head enlargement induced by uncaging plus spikes (n = 6) (Fig. 4F), suggesting that a sufficient amount of BDNF was released in response to synaptic stimulation paired with postsynaptic spikes. The spine-head enlargement induced by uncaging plus spikes was blocked by anisomycin even in the presence of BDNF (n = 9) (Fig. 4F), indicating that protein synthesis is required for the action of BDNF.
Fig. 4.
Effects of exogenous BDNF on spine plasticity. (A and B) Effects of BDNF (blue bar) on ΔVH (A) and whole-cell current (B) in the absence of stimulation. Data in (A) represent ΔVH relative to time 0 and are means ± SD (n = 30 spines). (C) Time-lapse z-stack images of a spine stimulated by glutamate uncaging without spikes in the presence of BDNF. The arrow indicates the spot of two-photon uncaging of MNI-glutamate. (D) Time courses of ΔVH for spines stimulated by uncaging without spikes in the absence (black circles) or presence of BDNF either alone (solid blue squares) or together with anisomycin (red squares). The effect of BDNF on neighboring spines (open blue squares) was also determined. Data are means ± SEM (n = 11 to 20 spines). (E) Spine-head enlargement induced by uncaging without spikes in the absence or presence of BDNF during the indicated time periods after the offset of synaptic stimulation (0 min). Data are means ± SEM (n = 10 to 20 spines). *P < 0.05 versus control value (Mann-Whitney U test). (F) Spine-head enlargement induced by uncaging plus spikes in the absence or presence of exogenous BDNF and anisomycin as in (D).
We have shown that synaptic stimulation paired with postsynaptic spikes induces a gradual long-term enlargement of spine heads that is mediated by BDNF and dependent on protein synthesis (fig. S7). In contrast, synaptic stimulation alone was not sufficient to trigger BDNF secretion (fig. S7), even though it induces a marked increase in the intracellular calcium concentration {[Ca2+]i} (>10 μM) of spines via NMDA receptors (21). Because BDNF secretion is not induced by a 1-Hz spike train alone (22, 23), our data suggest that the secretion of this neurotrophin is responsive to the synchrony of synaptic input and postsynaptic spikes. Given that such synchronous events result in only a short-lasting influx of Ca2+ through NMDA receptors during each spike (<2 μM) (24, 25), postsynaptic spikes must play the key role in the exocytosis of BDNF; for example, involving [Ca2+]i increases in the dendritic shaft via voltage-gated Ca2+ channels. Thus, BDNF secretion is finely regulated by correlated activities in a neuronal network and may consolidate nearby stimulated synapses by autocrine or paracrine mechanisms. BDNF action was selective on the spines that showed immediate enlargement, which may act as the structural tag for selective trapping (26) of the protein-synthetic machinery (11, 27) and the capture of plasticity proteins (2, 28) for long-term spine-head enlargement. Thus, BDNF acts as an associative messenger for the consolidation of synaptic plasticity, and the protein- synthetic process can regulate dendritic structures at the level of single spines.
Supplementary Material
Acknowledgments
This work was supported by grants-in-aid from MEXT of Japan (H.K. and M.M.), the Global COE Program (Integrative Life Science) of MEXT (H.K.), and NIH (GM053395 to G.C.R.E.-D. and GM065473 G.C.R.E.-D. & H.K.).
References and Notes
- 1.Squire LR. Memory and Brain. Oxford Univ. Press; New York: 1987. [Google Scholar]
- 2.Kelleher RJ, III, Govindarajan A, Tonegawa S. Neuron. 2004;44:59. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Nature. 2004;429:761. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Nat. Neurosci. 2004;7:1104. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- 5.Kopec CD, Li B, Wei W, Boehm J, Malinow R, Neurosci J. 2006;26:2000. doi: 10.1523/JNEUROSCI.3918-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dan Y, Poo MM. Physiol. Rev. 2006;86:1033. doi: 10.1152/physrev.00030.2005. [DOI] [PubMed] [Google Scholar]
- 7.Matsuzaki M, et al. Nat. Neurosci. 2001;4:1086. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Materials and methods are available as supporting material on Science Online.
- 9.Spacek J, Harris KM, Neurosci J. 1997;17:190. doi: 10.1523/JNEUROSCI.17-01-00190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steward O, Levy WB, Neurosci J. 1982;2:284. doi: 10.1523/JNEUROSCI.02-03-00284.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Job C, Eberwine J. Nat. Rev. Neurosci. 2001;2:889. doi: 10.1038/35104069. [DOI] [PubMed] [Google Scholar]
- 12.Patterson SL, et al. Neuron. 1996;16:1137. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 13.Minichiello L, et al. Neuron. 1999;24:401. doi: 10.1016/s0896-6273(00)80853-3. [DOI] [PubMed] [Google Scholar]
- 14.Poo MM. Nat. Rev. Neurosci. 2001;2:24. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 15.Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Nature. 1996;381:706. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 16.Kang H, Welcher AA, Shelton D, Schuman EM. Neuron. 1997;19:653. doi: 10.1016/s0896-6273(00)80378-5. [DOI] [PubMed] [Google Scholar]
- 17.Patterson SL, et al. Neuron. 2001;32:123. doi: 10.1016/s0896-6273(01)00443-3. [DOI] [PubMed] [Google Scholar]
- 18.Mu Y, Poo MM. Neuron. 2006;50:115. doi: 10.1016/j.neuron.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Blum R, Kafitz KW, Konnerth A. Nature. 2002;419:687. doi: 10.1038/nature01085. [DOI] [PubMed] [Google Scholar]
- 20.Li HS, Xu XZ, Montell C. Neuron. 1999;24:261. doi: 10.1016/s0896-6273(00)80838-7. [DOI] [PubMed] [Google Scholar]
- 21.Noguchi J, Matsuzaki M, Ellis-Davies GCR, Kasai H. Neuron. 2005;46:609. doi: 10.1016/j.neuron.2005.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gartner A, Staiger V. Proc. Natl. Acad. Sci. U.S.A. 2002;99:6386. doi: 10.1073/pnas.092129699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aicardi G, et al. Proc. Natl. Acad. Sci. U.S.A. 2004;101:15788. doi: 10.1073/pnas.0406960101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuste R, Denk W. Nature. 1995;375:682. doi: 10.1038/375682a0. [DOI] [PubMed] [Google Scholar]
- 25.Nevian T, Sakmann B, Neurosci J. 2006;26:11001. doi: 10.1523/JNEUROSCI.1749-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santamaria F, Wils S, De SE, Augustine GJ. Neuron. 2006;52:635. doi: 10.1016/j.neuron.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ostroff LE, Fiala JC, Allwardt B, Harris KM. Neuron. 2002;35:535. doi: 10.1016/s0896-6273(02)00785-7. [DOI] [PubMed] [Google Scholar]
- 28.Frey U, Morris RG. Nature. 1997;385:533. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.