Abstract
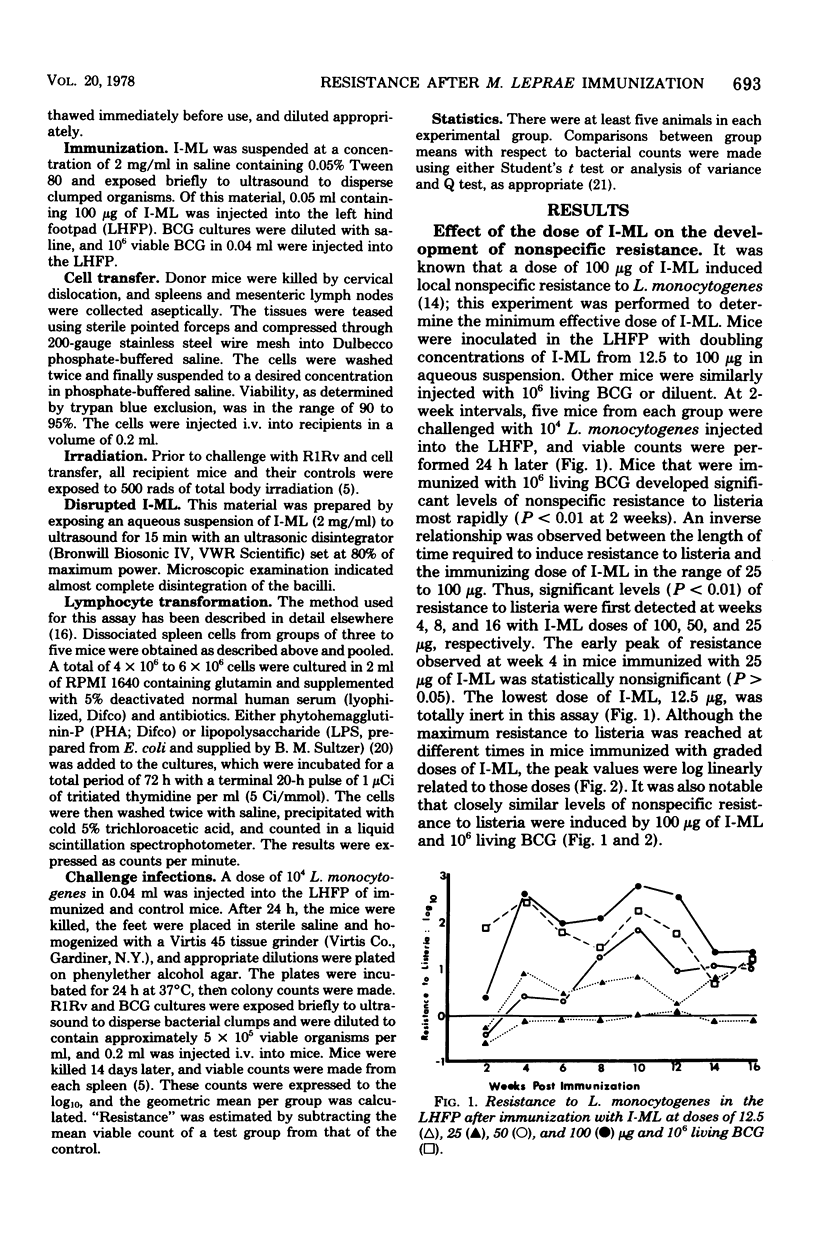
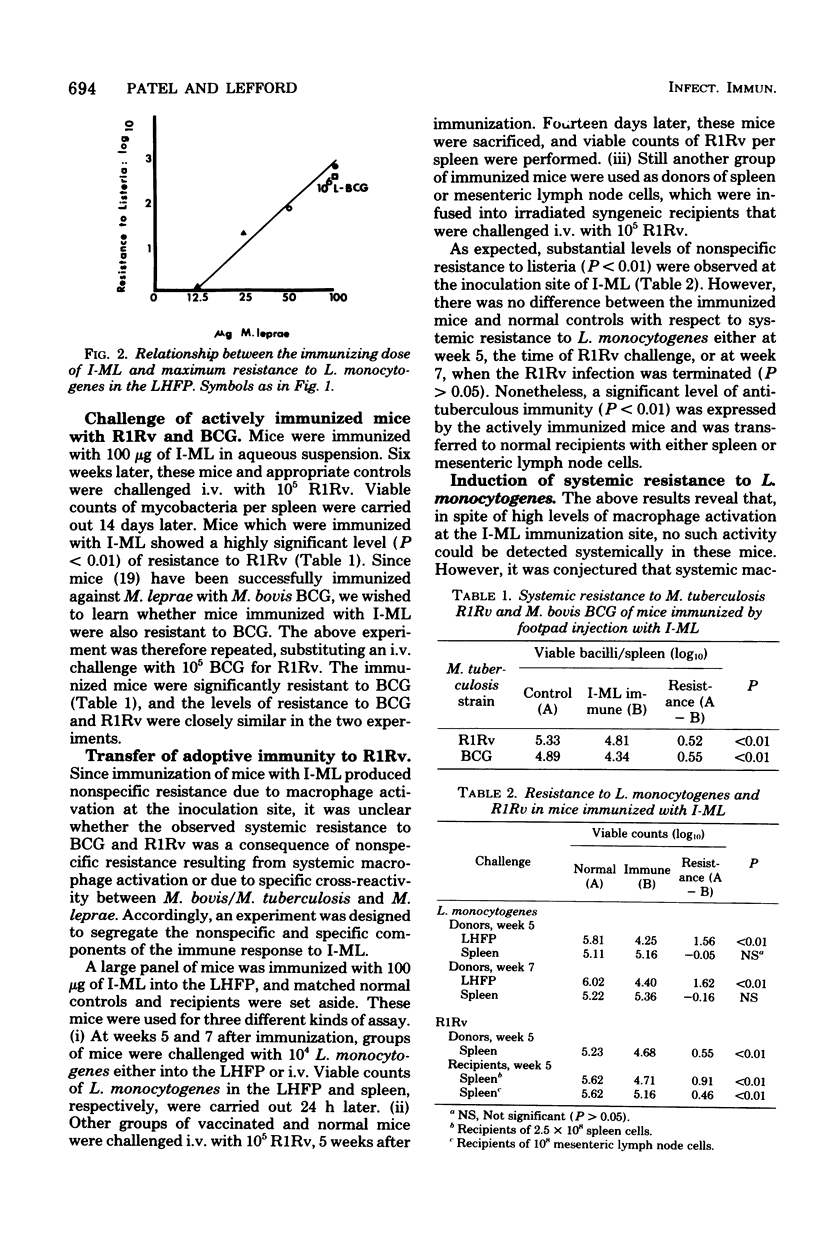
Following subcutaneous inoculation of irradiated Mycobacterium leprae (I-ML) into the left hind footpad of mice, there was increased resistance to Listeria monocytogenes, indicative of macrophage activation, at the immunization site. In spite of the high level of localized macrophage activation which was proportioned to the immunizing dose of I-ML, no such activity could be demonstrated systemically in these mice, as evidenced by the absence of increased resistance to an intravenous challenge with L. monocytogenes. Under these conditions, I-ML-immunized mice were nonetheless resistant to intravenous infection with either M. tuberculosis or M. bovis BCG, and this immunity was transferred to normal recipients using spleen or lymph node cells. Neonatal thymectomy completely abolished the development of antimycobacterial immunity after vaccination with I-ML, but immunity was restored by an intraperitoneal infusion of syngeneic thymocytes. Systemic nonspecific resistance could be generated in I-ML-immunized mice by an intravenous injection of disrupted I-ML. This study reveals that, after subcutaneous vaccination with I-ML, there is local accumulation of activated macrophages at the inoculation site and a widespread distribution of lymphocytes which are sensitized to mycobacterial antigens. Nonspecific resistance is mediated by the former cells and specific antimycobacterial immunity by the latter.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanden R. V., Lefford M. J., Mackaness G. B. The host response to Calmette-Guérin bacillus infection in mice. J Exp Med. 1969 May 1;129(5):1079–1107. doi: 10.1084/jem.129.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr, Meyer O. T., Esterly J. R., Kambara T. The local nature of immunity in tuberculosis, illustrated histochemically in dermal BCG lesions. J Immunol. 1968 May;100(5):931–941. [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J., McGregor D. D., Mackaness G. B. Properties of lymphocytes which confer adoptive immunity to tuberculosis in rats. Immunology. 1973 Oct;25(4):703–715. [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J. The effect of inoculum size on the immune response to BCG infection in mice. Immunology. 1971 Aug;21(2):369–381. [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J. Transfer of adoptive immunity to tuberculosis in mice. Infect Immun. 1975 Jun;11(6):1174–1181. doi: 10.1128/iai.11.6.1174-1181.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B., Blanden R. V. Cellular immunity. Prog Allergy. 1967;11:89–140. [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrvang B., Feek C. M., Godal T. Antimycobacterial antibodies in sera from patients throughout the clinico-pathological disease spectrum of leprosy. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Oct;82B(5):701–706. doi: 10.1111/j.1699-0463.1974.tb00238.x. [DOI] [PubMed] [Google Scholar]
- Myrvang B., Godal T., Ridley D. S., Fröland S. S., Song Y. K. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and histopathological spectrum of leprosy. Clin Exp Immunol. 1973 Aug;14(4):541–553. [PMC free article] [PubMed] [Google Scholar]
- North R. J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973 Apr;7(1):166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- North R. J., Kirstein D. P. T-cell-mediated concomitant immunity to syngeneic tumors. I. Activated macrophages as the expressors of nonspecific immunity to unrelated tumors and bacterial parasites. J Exp Med. 1977 Feb 1;145(2):275–292. doi: 10.1084/jem.145.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P. J., Lefford M. J. Induction of cell-mediated immunity to Mycobacterium leprae in mice. Infect Immun. 1978 Jan;19(1):87–93. doi: 10.1128/iai.19.1.87-93.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Demonstration in tissue culture of lymphocyte-mediated immunity to tuberculosis. Infect Immun. 1970 Jun;1(6):600–603. doi: 10.1128/iai.1.6.600-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPARD C. C. VACCINATION AGAINST EXPERIMENTAL INFECTION WITH MYCOBACTERIUM LEPRAE. Am J Epidemiol. 1965 Mar;81:150–163. doi: 10.1093/oxfordjournals.aje.a120504. [DOI] [PubMed] [Google Scholar]
- Shepard C. C. Vaccination of mice against M. leprae infection. Int J Lepr Other Mycobact Dis. 1976 Jan-Jun;44(1-2):222–226. [PubMed] [Google Scholar]
- Shepard C. C., Van Landingham R., Walker L. L. Immunity to Mycobacterium leprae infections in mice stimulated by M. leprae, BCG, and graft-versus-host reactions. Infect Immun. 1976 Oct;14(4):919–928. doi: 10.1128/iai.14.4.919-928.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Migration inhibitory factor and macrophage bactericidal function. Infect Immun. 1972 Aug;6(2):101–103. doi: 10.1128/iai.6.2.101-103.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M., Nilsson B. S., Kirschenbaum D. Nonspecific stimulation of lymphocytes by tuberculin. Infect Immun. 1977 Mar;15(3):799–806. doi: 10.1128/iai.15.3.799-806.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


