Abstract
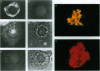
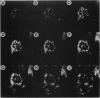
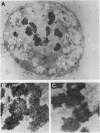
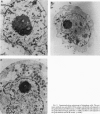
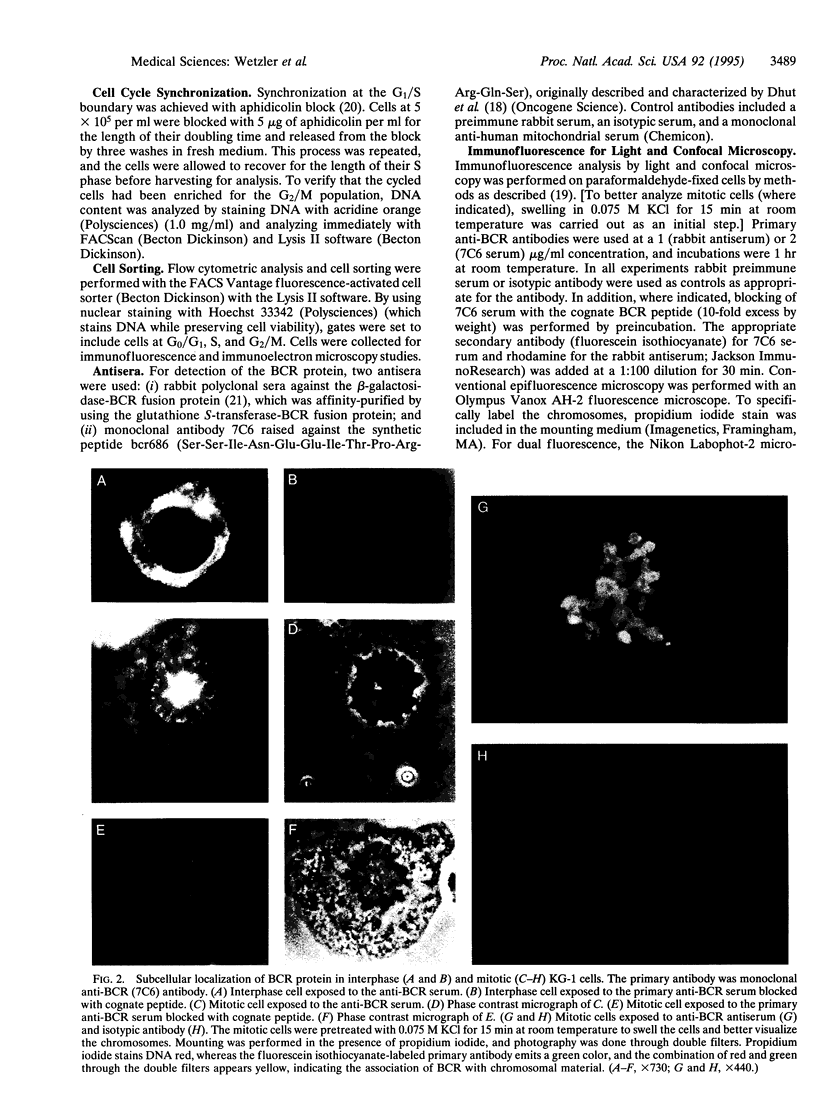
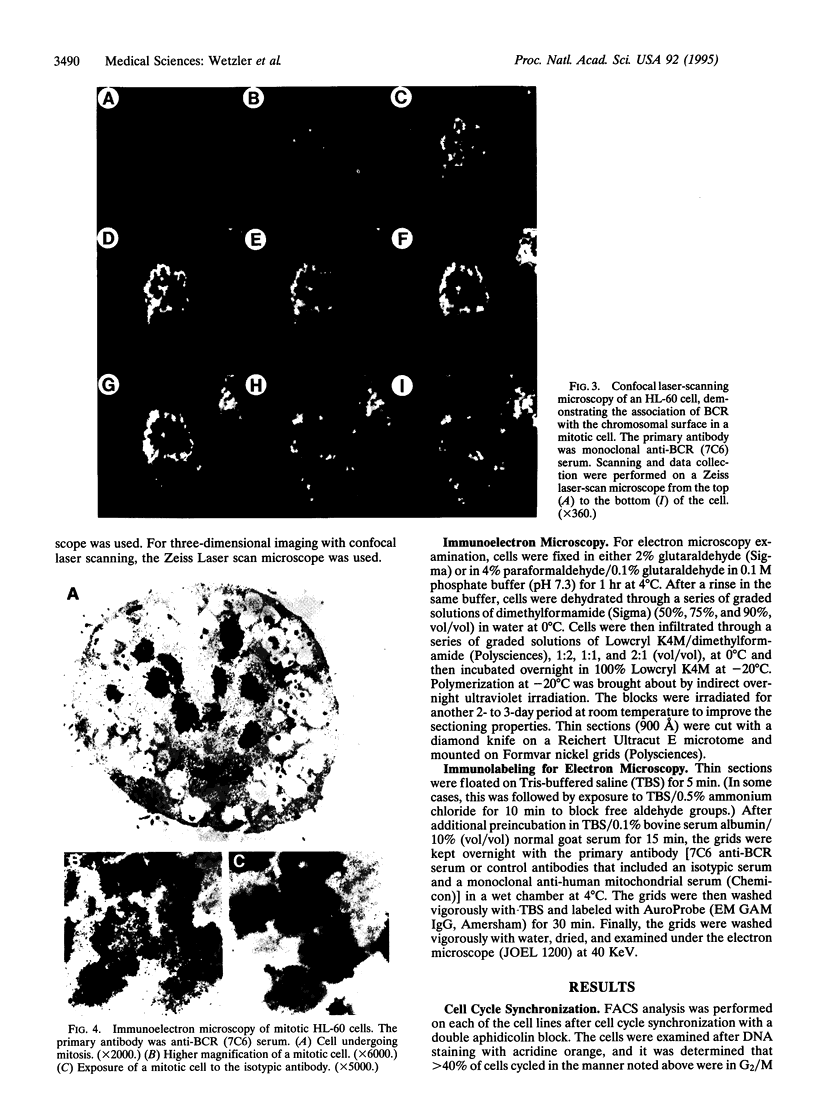
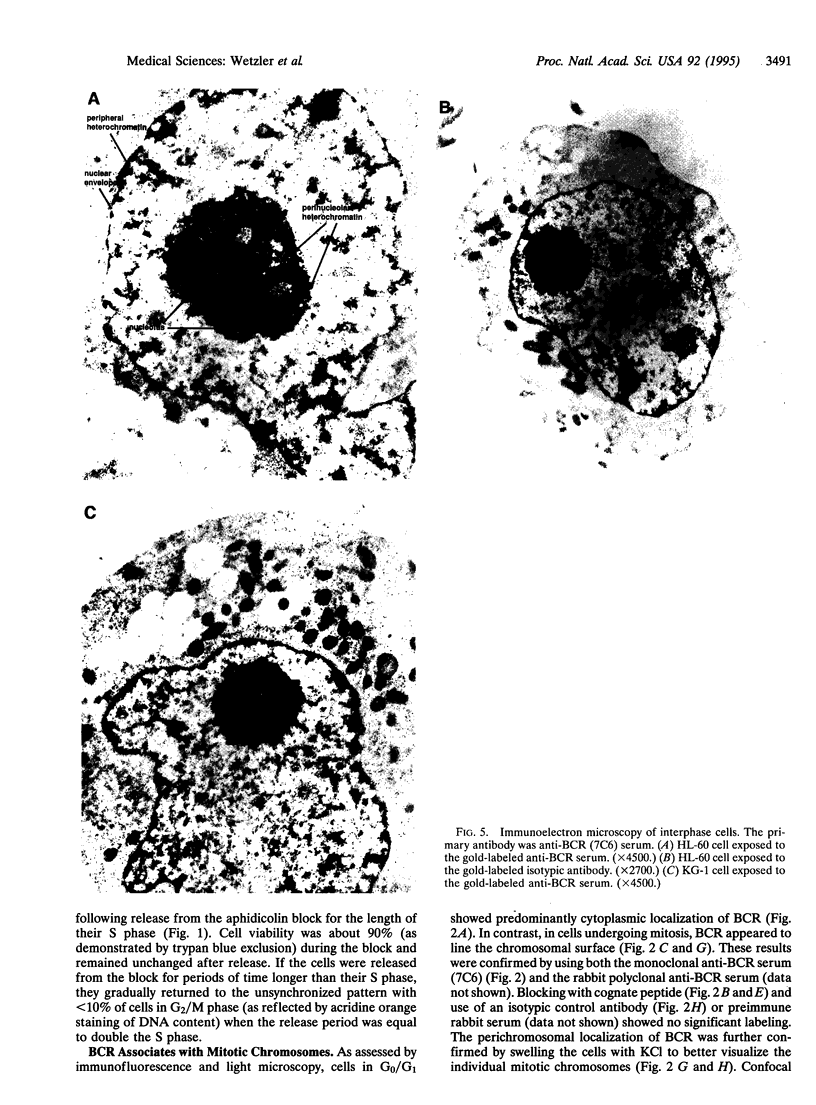
The disruption of the BCR gene and its juxtaposition to and consequent activation of the ABL gene has been implicated as the critical molecular defect in Philadelphia chromosome-positive leukemias. The normal BCR protein is a multifunctional molecule with domains that suggest its participation in phosphokinase and GTP-binding pathways. Taken together with its localization to the cytoplasm of uncycled cells, it is therefore presumed to be involved in cytoplasmic signaling. By performing a double aphidicolin block for cell cycle synchronization, we currently demonstrate that the subcellular localization of BCR shifts from being largely cytoplasmic in interphase cells to being predominantly perichromosomal in mitosis. Furthermore, with the use of immunogold labeling and electron microscopy, association of BCR with DNA, in particular heterochromatin, can be demonstrated even in quiescent cells. Results were similar in cell lines of lymphoid or myeloid origin. These observations suggest a role for BCR in the phosphokinase interactions linked to condensed chromatin, a network previously implicated in cell cycle regulation.
Full text
PDF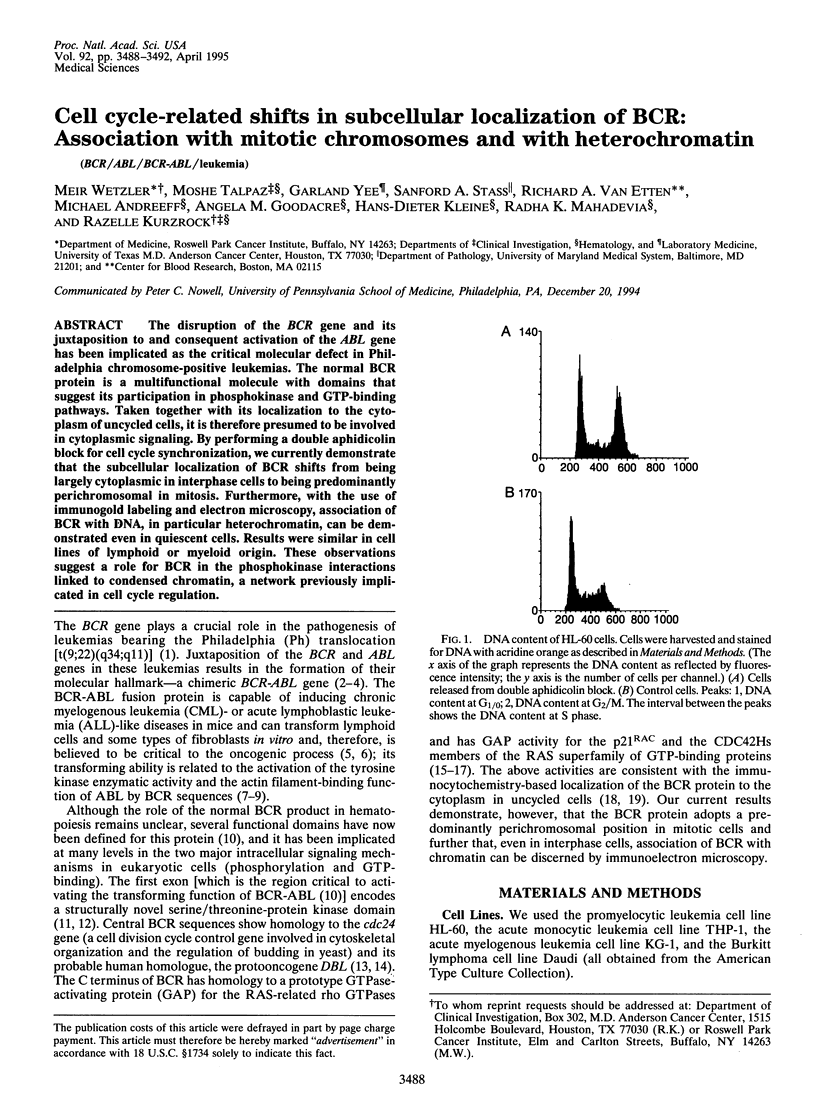

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Houston H., Allen J., Lints T., Harvey R. The hematopoietically expressed vav proto-oncogene shares homology with the dbl GDP-GTP exchange factor, the bcr gene and a yeast gene (CDC24) involved in cytoskeletal organization. Oncogene. 1992 Apr;7(4):611–618. [PubMed] [Google Scholar]
- Arion D., Meijer L., Brizuela L., Beach D. cdc2 is a component of the M phase-specific histone H1 kinase: evidence for identity with MPF. Cell. 1988 Oct 21;55(2):371–378. doi: 10.1016/0092-8674(88)90060-8. [DOI] [PubMed] [Google Scholar]
- Arlinghaus R. B. Multiple BCR-related gene products and their proposed involvement in ligand-induced signal transduction pathways. Mol Carcinog. 1992;5(3):171–173. doi: 10.1002/mc.2940050302. [DOI] [PubMed] [Google Scholar]
- Belenguer P., Caizergues-Ferrer M., Labbé J. C., Dorée M., Amalric F. Mitosis-specific phosphorylation of nucleolin by p34cdc2 protein kinase. Mol Cell Biol. 1990 Jul;10(7):3607–3618. doi: 10.1128/mcb.10.7.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Neriah Y., Daley G. Q., Mes-Masson A. M., Witte O. N., Baltimore D. The chronic myelogenous leukemia-specific P210 protein is the product of the bcr/abl hybrid gene. Science. 1986 Jul 11;233(4760):212–214. doi: 10.1126/science.3460176. [DOI] [PubMed] [Google Scholar]
- Bendayan M., Nanci A., Kan F. W. Effect of tissue processing on colloidal gold cytochemistry. J Histochem Cytochem. 1987 Sep;35(9):983–996. doi: 10.1177/35.9.3302022. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M. Reversible histone modifications and the chromosome cell cycle. Bioessays. 1992 Jan;14(1):9–16. doi: 10.1002/bies.950140103. [DOI] [PubMed] [Google Scholar]
- Brown S. W. Heterochromatin. Science. 1966 Jan 28;151(3709):417–425. doi: 10.1126/science.151.3709.417. [DOI] [PubMed] [Google Scholar]
- Daley G. Q., Van Etten R. A., Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990 Feb 16;247(4944):824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- Dhut S., Dorey E. L., Horton M. A., Ganesan T. S., Young B. D. Identification of two normal bcr gene products in the cytoplasm. Oncogene. 1988 Nov;3(5):561–566. [PubMed] [Google Scholar]
- Diekmann D., Brill S., Garrett M. D., Totty N., Hsuan J., Monfries C., Hall C., Lim L., Hall A. Bcr encodes a GTPase-activating protein for p21rac. Nature. 1991 May 30;351(6325):400–402. doi: 10.1038/351400a0. [DOI] [PubMed] [Google Scholar]
- Fleig U. N., Gould K. L. Regulation of cdc2 activity in Schizosaccharomyces pombe: the role of phosphorylation. Semin Cell Biol. 1991 Aug;2(4):195–204. [PubMed] [Google Scholar]
- Hall A. Signal transduction through small GTPases--a tale of two GAPs. Cell. 1992 May 1;69(3):389–391. doi: 10.1016/0092-8674(92)90441-e. [DOI] [PubMed] [Google Scholar]
- Heintz N., Sive H. L., Roeder R. G. Regulation of human histone gene expression: kinetics of accumulation and changes in the rate of synthesis and in the half-lives of individual histone mRNAs during the HeLa cell cycle. Mol Cell Biol. 1983 Apr;3(4):539–550. doi: 10.1128/mcb.3.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisterkamp N., Jenster G., ten Hoeve J., Zovich D., Pattengale P. K., Groffen J. Acute leukaemia in bcr/abl transgenic mice. Nature. 1990 Mar 15;344(6263):251–253. doi: 10.1038/344251a0. [DOI] [PubMed] [Google Scholar]
- Kipreos E. T., Wang J. Y. Differential phosphorylation of c-Abl in cell cycle determined by cdc2 kinase and phosphatase activity. Science. 1990 Apr 13;248(4952):217–220. doi: 10.1126/science.2183353. [DOI] [PubMed] [Google Scholar]
- Kloetzer W., Kurzrock R., Smith L., Talpaz M., Spiller M., Gutterman J., Arlinghaus R. The human cellular abl gene product in the chronic myelogenous leukemia cell line K562 has an associated tyrosine protein kinase activity. Virology. 1985 Jan 30;140(2):230–238. doi: 10.1016/0042-6822(85)90361-7. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Witte O. N. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984 Jul;37(3):1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Kurzrock R., Gutterman J. U., Talpaz M. The molecular genetics of Philadelphia chromosome-positive leukemias. N Engl J Med. 1988 Oct 13;319(15):990–998. doi: 10.1056/NEJM198810133191506. [DOI] [PubMed] [Google Scholar]
- Kurzrock R., Shtalrid M., Romero P., Kloetzer W. S., Talpas M., Trujillo J. M., Blick M., Beran M., Gutterman J. U. A novel c-abl protein product in Philadelphia-positive acute lymphoblastic leukaemia. Nature. 1987 Feb 12;325(6105):631–635. doi: 10.1038/325631a0. [DOI] [PubMed] [Google Scholar]
- Labbe J. C., Picard A., Peaucellier G., Cavadore J. C., Nurse P., Doree M. Purification of MPF from starfish: identification as the H1 histone kinase p34cdc2 and a possible mechanism for its periodic activation. Cell. 1989 Apr 21;57(2):253–263. doi: 10.1016/0092-8674(89)90963-x. [DOI] [PubMed] [Google Scholar]
- Maru Y., Witte O. N. The BCR gene encodes a novel serine/threonine kinase activity within a single exon. Cell. 1991 Nov 1;67(3):459–468. doi: 10.1016/0092-8674(91)90521-y. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Yasuda H., Mita S., Marunouchi T., Yamada M. Evidence for the involvement of H1 histone phosphorylation in chromosome condensation. Nature. 1980 Mar 13;284(5752):181–183. doi: 10.1038/284181a0. [DOI] [PubMed] [Google Scholar]
- McWhirter J. R., Wang J. Y. Activation of tyrosinase kinase and microfilament-binding functions of c-abl by bcr sequences in bcr/abl fusion proteins. Mol Cell Biol. 1991 Mar;11(3):1553–1565. doi: 10.1128/mcb.11.3.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melan M. A., Sluder G. Redistribution and differential extraction of soluble proteins in permeabilized cultured cells. Implications for immunofluorescence microscopy. J Cell Sci. 1992 Apr;101(Pt 4):731–743. doi: 10.1242/jcs.101.4.731. [DOI] [PubMed] [Google Scholar]
- Nigg E. A. The substrates of the cdc2 kinase. Semin Cell Biol. 1991 Aug;2(4):261–270. [PubMed] [Google Scholar]
- Reeves R., Langan T. A., Nissen M. S. Phosphorylation of the DNA-binding domain of nonhistone high-mobility group I protein by cdc2 kinase: reduction of binding affinity. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1671–1675. doi: 10.1073/pnas.88.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A. J., Self A. J., Kasmi F., Paterson H. F., Hall A., Marshall C. J., Ellis C. rho family GTPase activating proteins p190, bcr and rhoGAP show distinct specificities in vitro and in vivo. EMBO J. 1993 Dec 15;12(13):5151–5160. doi: 10.1002/j.1460-2075.1993.tb06210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D., Zannini M., Lewis M., Wickner R. B., Hunt L. T., Graziani G., Tronick S. R., Aaronson S. A., Eva A. A region of proto-dbl essential for its transforming activity shows sequence similarity to a yeast cell cycle gene, CDC24, and the human breakpoint cluster gene, bcr. New Biol. 1991 Apr;3(4):372–379. [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985 Jun 13;315(6020):550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- Stam K., Heisterkamp N., Reynolds F. H., Jr, Groffen J. Evidence that the phl gene encodes a 160,000-dalton phosphoprotein with associated kinase activity. Mol Cell Biol. 1987 May;7(5):1955–1960. doi: 10.1128/mcb.7.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzler M., Talpaz M., Van Etten R. A., Hirsh-Ginsberg C., Beran M., Kurzrock R. Subcellular localization of Bcr, Abl, and Bcr-Abl proteins in normal and leukemic cells and correlation of expression with myeloid differentiation. J Clin Invest. 1993 Oct;92(4):1925–1939. doi: 10.1172/JCI116786. [DOI] [PMC free article] [PubMed] [Google Scholar]