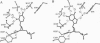Abstract
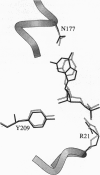
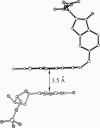
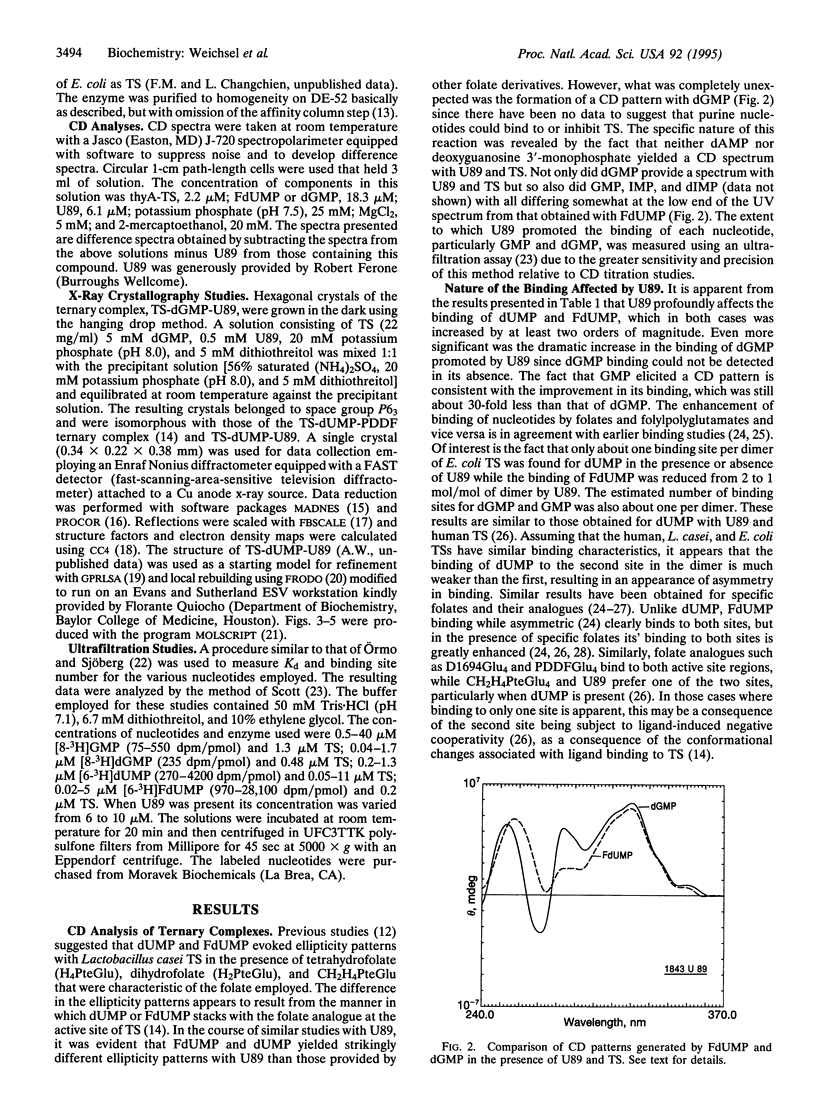
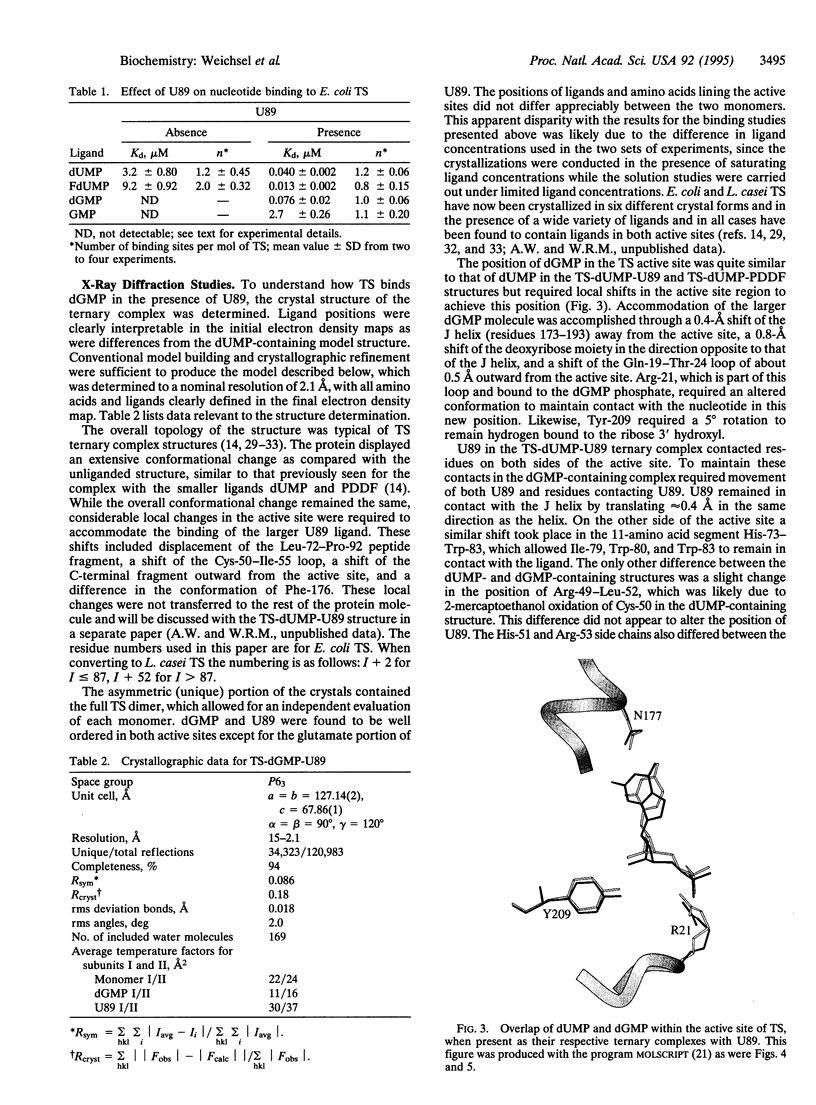
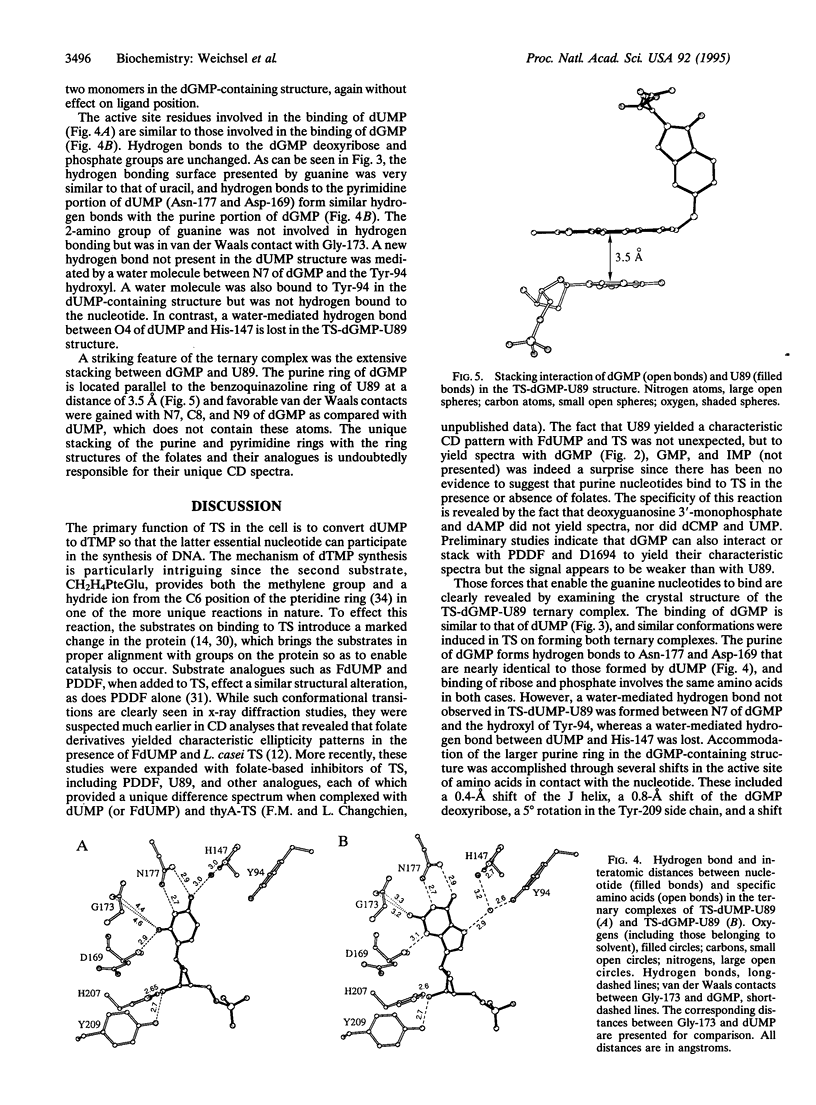
A folate analogue, 1843U89 (U89), with potential as a chemotherapeutic agent due to its potent and specific inhibition of thymidylate synthase (TS; EC 2.1.1.45), greatly enhances not only the binding of 5-fluoro-2'-deoxyuridine 5'-monophosphate (FdUMP) and dUMP to Escherichia coli TS but also that of dGMP, GMP, dIMP, and IMP. Guanine nucleotide binding was first detected by CD analysis, which revealed a unique spectrum for the TS-dGMP-U89 ternary complex. The quantitative binding of dGMP relative to GMP, FdUMP, and dUMP was determined in the presence and absence of U89 by ultrafiltration analysis, which revealed that although the binding of GMP and dGMP could not be detected in the absence of U89 both were bound in its presence. The Kd for dGMP was about the same as that for dUMP and FdUMP, with binding of the latter two nucleotides being increased by two orders of magnitude by U89. An explanation for the binding of dGMP was provided by x-ray diffraction studies that revealed an extensive stacking interaction between the guanine of dGMP and the benzoquinazoline ring of U89 and hydrogen bonds similar to those involved in dUMP binding. In addition, binding energy was provided through a water molecule that formed hydrogen bonds to both N7 of dGMP and the hydroxyl of Tyr-94. Accommodation of the larger dGMP molecule was accomplished through a distortion of the active site and a shift of the deoxyribose moiety to a new position. These rearrangements also enabled the binding of GMP to occur by creating a pocket for the ribose 2' hydroxyl group, overcoming the normal TS discrimination against nucleotides containing the 2' hydroxyl.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alison D. L., Newell D. R., Sessa C., Harland S. J., Hart L. I., Harrap K. R., Calvert A. H. The clinical pharmacokinetics of the novel antifolate N10-propargyl-5,8-dideazafolic acid (CB 3717). Cancer Chemother Pharmacol. 1985;14(3):265–271. doi: 10.1007/BF00258131. [DOI] [PubMed] [Google Scholar]
- Beaudette N. V., Langerman N., Kisliuk R. L. A calorimetric study of the binding of 2'-deoxyuridine-5'-phosphate and its analogs to thymidylate synthetase. Arch Biochem Biophys. 1980 Apr 1;200(2):410–417. doi: 10.1016/0003-9861(80)90371-9. [DOI] [PubMed] [Google Scholar]
- Cohen S. S., Flaks J. G., Barner H. D., Loeb M. R., Lichtenstein J. THE MODE OF ACTION OF 5-FLUOROURACIL AND ITS DERIVATIVES. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1004–1012. doi: 10.1073/pnas.44.10.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danenberg K. D., Danenberg P. V. Evidence for a sequential interaction of the subunits of thymidylate synthetase. J Biol Chem. 1979 Jun 10;254(11):4345–4348. [PubMed] [Google Scholar]
- Dev I. K., Dallas W. S., Ferone R., Hanlon M., McKee D. D., Yates B. B. Mode of binding of folate analogs to thymidylate synthase. Evidence for two asymmetric but interactive substrate binding sites. J Biol Chem. 1994 Jan 21;269(3):1873–1882. [PubMed] [Google Scholar]
- Duch D. S., Banks S., Dev I. K., Dickerson S. H., Ferone R., Heath L. S., Humphreys J., Knick V., Pendergast W., Singer S. Biochemical and cellular pharmacology of 1843U89, a novel benzoquinazoline inhibitor of thymidylate synthase. Cancer Res. 1993 Feb 15;53(4):810–818. [PubMed] [Google Scholar]
- Fauman E. B., Rutenber E. E., Maley G. F., Maley F., Stroud R. M. Water-mediated substrate/product discrimination: the product complex of thymidylate synthase at 1.83 A. Biochemistry. 1994 Feb 15;33(6):1502–1511. doi: 10.1021/bi00172a029. [DOI] [PubMed] [Google Scholar]
- Finer-Moore J., Fauman E. B., Foster P. G., Perry K. M., Santi D. V., Stroud R. M. Refined structures of substrate-bound and phosphate-bound thymidylate synthase from Lactobacillus casei. J Mol Biol. 1993 Aug 20;232(4):1101–1116. doi: 10.1006/jmbi.1993.1463. [DOI] [PubMed] [Google Scholar]
- Galivan J. H., Maley F., Baugh C. M. Demonstration of separate binding sites for the folate coenzymes and deoxynucleotides with inactivated Lactobacillus casei thymidylate synthetase. Biochem Biophys Res Commun. 1976 Jul 26;71(2):527–534. doi: 10.1016/0006-291x(76)90819-6. [DOI] [PubMed] [Google Scholar]
- Galivan J. H., Maley G. F., Maley F. Factors affecting substrate binding in Lactobacillus casei thymidylate synthetase as studied by equilibrium dialysis. Biochemistry. 1976 Jan 27;15(2):356–362. doi: 10.1021/bi00647a018. [DOI] [PubMed] [Google Scholar]
- Galivan J. H., Maley G. F., Maley F. The effect of substrate analogs on the circular dichroic spectra of thymidylate synthetase from Lactobacillus casei. Biochemistry. 1975 Jul 29;14(15):3338–3344. doi: 10.1021/bi00686a008. [DOI] [PubMed] [Google Scholar]
- Galivan J., Maley F., Baugh C. M. Protective effect of the pteroylpolyglutamates and phosphate on the proteolytic inactivation of thymidylate synthetase. Arch Biochem Biophys. 1977 Dec;184(2):346–354. doi: 10.1016/0003-9861(77)90361-7. [DOI] [PubMed] [Google Scholar]
- HARTMANN K. U., HEIDELBERGER C. Studies on fluorinated pyrimidines. XIII. Inhibition of thymidylate synthetase. J Biol Chem. 1961 Nov;236:3006–3013. [PubMed] [Google Scholar]
- Jones T. R., Calvert A. H., Jackman A. L., Brown S. J., Jones M., Harrap K. R. A potent antitumour quinazoline inhibitor of thymidylate synthetase: synthesis, biological properties and therapeutic results in mice. Eur J Cancer. 1981 Jan;17(1):11–19. doi: 10.1016/0014-2964(81)90206-1. [DOI] [PubMed] [Google Scholar]
- Kamb A., Finer-Moore J. S., Stroud R. M. Cofactor triggers the conformational change in thymidylate synthase: implications for an ordered binding mechanism. Biochemistry. 1992 Dec 29;31(51):12876–12884. doi: 10.1021/bi00166a024. [DOI] [PubMed] [Google Scholar]
- Kent R. J., Heidelberger C. Fluorinated pyrimidines. XL. The reduction of 5-fluorouridine 5'-diphosphate by ribonucleotide reductase. Mol Pharmacol. 1972 Jul;8(4):465–475. [PubMed] [Google Scholar]
- Langenbach R. J., Danenberg P. V., Heidelberger C. Thymidylate synthetase: mechanism of inhibition by 5-fluoro-2'-deoxyuridylate. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1565–1571. doi: 10.1016/0006-291x(72)90892-3. [DOI] [PubMed] [Google Scholar]
- Leary R. P., Beaudette N., Kisliuk R. L. Interaction of deoxyuridylate with thymidylate synthetase. J Biol Chem. 1975 Jul 10;250(13):4864–4868. [PubMed] [Google Scholar]
- Lewis C. A., Jr, Ellis P. D., Dunlap R. B. Fluorine-19 nuclear magnetic resonance investigation of the noncovalent and covalent binary complexes of 5-fluorodeoxyuridylate and Lactobacillus casei thymidylate synthetase. Biochemistry. 1980 Jan 8;19(1):116–123. doi: 10.1021/bi00542a018. [DOI] [PubMed] [Google Scholar]
- Lockshin A., Danenberg P. V. Thymidylate synthetase and 2'-deoxyuridylate form a tight complex in the presence of pteroyltriglutamate. J Biol Chem. 1979 Dec 25;254(24):12285–12288. [PubMed] [Google Scholar]
- Lorenson M. Y., Maley G. F., Maley F. The purification and properties of thymidylate synthetase from chick embryo extracts. J Biol Chem. 1967 Jul 25;242(14):3332–3344. [PubMed] [Google Scholar]
- Maley G. F., Maley F., Baugh C. M. Differential inhibition of host and viral thymidylate synthetases by folylpolyglutamates. J Biol Chem. 1979 Aug 25;254(16):7485–7487. [PubMed] [Google Scholar]
- Maley G. F., Maley F. Properties of a defined mutant of Escherichia coli thymidylate synthase. J Biol Chem. 1988 Jun 5;263(16):7620–7627. [PubMed] [Google Scholar]
- Matthews D. A., Appelt K., Oatley S. J., Xuong N. H. Crystal structure of Escherichia coli thymidylate synthase containing bound 5-fluoro-2'-deoxyuridylate and 10-propargyl-5,8-dideazafolate. J Mol Biol. 1990 Aug 20;214(4):923–936. doi: 10.1016/0022-2836(90)90346-N. [DOI] [PubMed] [Google Scholar]
- Matthews D. A., Villafranca J. E., Janson C. A., Smith W. W., Welsh K., Freer S. Stereochemical mechanism of action for thymidylate synthase based on the X-ray structure of the covalent inhibitory ternary complex with 5-fluoro-2'-deoxyuridylate and 5,10-methylenetetrahydrofolate. J Mol Biol. 1990 Aug 20;214(4):937–948. doi: 10.1016/0022-2836(90)90347-O. [DOI] [PubMed] [Google Scholar]
- Montfort W. R., Perry K. M., Fauman E. B., Finer-Moore J. S., Maley G. F., Hardy L., Maley F., Stroud R. M. Structure, multiple site binding, and segmental accommodation in thymidylate synthase on binding dUMP and an anti-folate. Biochemistry. 1990 Jul 31;29(30):6964–6977. doi: 10.1021/bi00482a004. [DOI] [PubMed] [Google Scholar]
- Ormö M., Sjöberg B. M. An ultrafiltration assay for nucleotide binding to ribonucleotide reductase. Anal Biochem. 1990 Aug 15;189(1):138–141. doi: 10.1016/0003-2697(90)90059-i. [DOI] [PubMed] [Google Scholar]
- Santi D. V., McHenry C. S. 5-Fluoro-2'-deoxyuridylate: covalent complex with thymidylate synthetase. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1855–1857. doi: 10.1073/pnas.69.7.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]