Abstract
This study identifies a behavioral and nonpharmacologic means of preventing and reducing newborn pain. Our objective was to determine whether warmth is analgesic in newborn infants undergoing vaccination—a routine painful hospital procedure. We used a prospective randomized controlled trial of 47 healthy full-term newborn infants.
Infants were randomized into one of three conditions prior to vaccination: warmth exposure, pacifier suckling, or sucrose taste. Crying, grimacing, and heart rate differences were analyzed between groups before, during, and after vaccination as outcome measures. Warmer infants cried significantly less than sucrose taste or pacifier suckling after vaccination. Heart rate patterns reflected this analgesia. Core temperature did not differ between study groups. Providing natural warmth to newborn infants during a painful procedure decreases the crying and grimacing on par with the “gold” standard treatments of sucrose or pacifier.
Keywords: Infant, newborn, pain, analgesia, vaccination, crying, grimacing, heart rate, autonomic, sucrose, pacifier
INTRODUCTION
Healthy normal newborns experience pain as part of routine newborn care. This pain comes in the form of invasive procedures such as blood sampling, vaccinations, vitamin K injections, or circumcision[4,7,39]. Sick or preterm neonates require increased medical care, which results in a greater exposure to painful procedures[16,57,59]. There is an increased awareness of this pain and the need to address the burden of early newborn pain[4,57].
Prevention of pain in the newborn is increasingly viewed as a professional imperative and an ethical expectation as untreated pain has detrimental consequences [3,8] including greater pain sensitivity in later childhood[30,33,37,44,55,61]. Newborn experiences with pain may be related to permanent neuroanatomical and behavioral abnormalities as demonstrated in animal models[5,55]. Moreover, newborn pain is a source of concern and distress for new parents and may disturb mother-infant breastfeeding and early bonding[24]. Yet, pain-reduction therapies are often underused for the numerous minor procedures that are part of routine medical and nursing care of neonates[16,57].
Fears about adverse pharmacological drug effects on the newborn’s developing nervous system may be a reason for the under use of effective analgesic techniques[2]. Many anesthetics and pharmacological agents have not yet been tested or proven safe or effective with newborn infants[42]. In this setting, a number of natural nonpharmacologic analgesic techniques have been shown to individually and in combination provide pain relief for newborns undergoing painful procedures. These natural, low-cost, and easy to implement pain relieving measures include: sucrose taste[1,13,33,59], suckling a pacifier[15,19,25,58], direct skin-to-skin contact between mother and the infant[29,36,41], and breastfeeding[17,26,28,45] during the procedure.
While sucrose taste has become the clinically most used analgesic for minor procedures with newborns[16], there are increasing concerns about repeated use[35,59]. First, repeated sucrose tastes in very small preterm infants may place the infant at risk for less optimal neurobehavioral development[18,35]. Second, the effectiveness of sucrose analgesia is being questioned because of its inability to prevent later exaggerated pain responses[21,60]. The rationale for this study arose from the desire to identify an effective and efficient analgesic technique without using sucrose.
In planning this study, we were mindful of our previous clinical experiences that required a necessary physical skin-to-skin connection between the mother and infant during the painful procedures in order to achieve effective analgesia[28,29]. This closeness afforded a thermal transfer between mother and infant that may have reduce the energy or metabolic expenditure of the infant and provided a direct or indirect benefit of pain reduction[40,52]. The purpose of this study, therefore, was to explore the analgesic properties of applied warmth compared to the currently used analgesic techniques of sucrose taste and suckling (pacifier). We hypothesized that the external warmth would protect the infant from minor procedural pain. We compared infants receiving radiant warmth (experimental group) during a painful Hepatitis B vaccination to those receiving either sucrose taste or pacifier suckling. The efficacy of these analgesic interventions were determined by evaluating video recordings of infant crying and facial expressions and by assessing the variations of heart rate that normally accompany painful procedures.
Methods
Participants
Full-term newborns delivered at the University of Chicago Medical Center and whose mothers had already consented for the Hepatitis B vaccination were screened for the following inclusion criteria: full term, appropriate for gestational age weight, Apgar scores at 1 and 5 minutes greater than 7, and negative maternal Hepatitis B panel. Infants excluded from the study were pre-term, low-birth weight, had Apgar score less than 7 at one or five minutes, had mothers’ with unknown or positive Hepatitis B status, or were greater than 48 hours old.
During June and July 2007, 47 parents consented for both the Hepatitis B vaccination for their newborn infants and this pain study protocol. Of these 47 infants, 3 infants were subsequently excluded from data analysis due to technical problems with heart rate recording (1 in the sucrose group and 2 in the warmer). Infants were randomly assigned the morning of the study using a sealed envelope system into one of three groups: warmer (n = 14), sucrose (n = 15), or pacifier (n = 15). (Table. 1)
Table 1.
Clinical Characteristics of Study Population
| Sucrose | Pacifier | Warmer | |
|---|---|---|---|
| Gestation [weeks] Median (range) |
39 (38–40) | 39 (37.6–41) | 39 (37–41.4) |
| Birth Weight [g] Mean (SD) |
3248 (±242) | 3196 (±313) | 3222 (±364) |
| Male Gender | 6 | 12 | 7 |
| Maternal Age [yrs] Mean (SD) |
26 (± 3.8) | 25 (± 7.8) | 27 (± 6.3) |
The comparison groups of sucrose and pacifier, as alternative analgesic interventions, came from the Consensus Statement for the Prevention and Management of Pain in the Newborn, which suggested pain management for all intramuscular injections[4]. Our previous studies revealed that to achieve a significant difference in grimacing and crying (Power 80%, p value < .05) between similarly constructed groups, a sample size of 15 infants in each group was needed.
Protocol
The Hepatitis B vaccination was the first vaccination for all participants, although all infants received a Vitamin K injection at birth. In order to ensure that infants were not experiencing an amplified pain response from a repeat injection to the same site, the vaccine was administered into the opposite leg as the Vitamin K injection. A single physician (LG) performed all vaccinations in a standardized fashion to minimize variability in the protocol and guarantee opposite leg utilization.
At the beginning of the study, all infants were unswaddled in their bassinets. Infants in the warmer group had their clothing removed except for the diaper and were placed under an Ohmeda-Ohio® 3000 Infant Warmer System (GE Healthcare). Infants in the other two study groups (sucrose and pacifier), remained in their bassinets clothed in a shirt, diaper, and a hat.
We controlled for behavioral state by initiating the protocol only after each infant spontaneously reached 1 of 3 quiet behavioral states as defined by Prechtl (State 1: eyes closed, regular respiration, no movements; State 2: eyes closed, irrregular respiration, small movements, or State 3: eyes open, no movements) [51]. The protocol consisted of a baseline period (five minutes), intervention (2-minutes), followed by the vaccination (10 seconds), and a recovery period (5 minutes). The restful state was disturbed for infants in the sucrose group who had to be administered the sweet taste and infants in the pacifier group who had to be given the pacifier. The Ohmeda warmer was placed on servo control during baseline to prevent infant cooling. During the 2-minute intervention period the infants were randomly assigned to receive either: (1) 100% radiant warmth from the Ohmeda warmer on the manual setting (warmer group); (2) 1.0 ml of a 25% sucrose solution (Sweet-Ease®, Philips Children’s Medical Ventures, Monroeville, PA) administered via a syringe (sucrose group); or (3) had a hospital issue pacifier (Soothie® Pacifier, Philips Children’s Medical Ventures, Monroeville, PA) lightly held in their mouths (pacifier group). After the 2-minute intervention period, the physician swabbed the infants’ lateral thigh with an alcohol pad, administered the Hepatitis B vaccine (Recombivax HB®, Merck & Co., Inc., Whitehouse Station, NJ) via a 1mL Kendall Syringe with Safely Needle, and applied a bandage. The infant was then observed for 5 minutes after the vaccination. During the recovery period infants in the warmer group remained under the warmer, which was returned to the servo control setting. Infants in the sucrose and pacifier group rested quietly in their bassinettes, and pacifier infants had continued access to the pacifier.
The University of Chicago IRB Committee approved this study and all mothers signed informed consent forms for both the Hepatitis B vaccination and participation in this study.
Data Collection and Management
All infants had three 3M Red Dot™ Neonatal Monitoring Electrodes (3M Healthcare, St. Paul, MN) placed on their thoracic region for heart rate monitoring. The ECG signal from a Hewlett-Packard© Neonatal heart rate monitor was processed through an interface to an EZ-IBI-3 module (UFI, Morro Bay, CA), which automatically computed cardiac sequential beat-to-beat measures of R-R intervals (i.e., the time in msec betweens successive heart beats). The R-R intervals were visually inspected and edited off-line using CardioEdit software (Brain-Body Center, University of Illinois, Chicago, IL). Editing consisted of identifying the rare occasions of missed or spurious detections of R-waves by superimposing the time-synchronized ECG waveform on the timeline of R-R intervals to estimate the occurrence of the missed R-wave.
Respiratory sinus arrhythmia (RSA) was derived from the edited R-R intervals using CardioBatch software (Brain-Body Center, University of Illinois, Chicago, IL). CardioBatch applies a moving polynomial filter [46–48] and quantifies the amplitude of RSA with age-specific parameters, sensitive to the maturational shifts in the frequency of spontaneous breathing (.3–1.3 Hz for the neonate) [48,54]. Average heart rate and the amplitude of RSA were calculated in sequential 30-second epochs within each of the conditions (i.e., baseline, intervention, recovery). Sequential epochs were used as a repeated measure in the analyses of heart rate and RSA described below.
All infants had continuous temperature monitoring. All infants had Smith ER-400 temperature probe placed 2.5 cm in their rectum and Smith STS-400 probe placed on their abdomen. Abdominal temperature probes were covered with a Kentec® AccuTemp Plus Insulated Hydrogel Thermal Reflective cover. Rectal temperature probes were connected to a DeBusk temperature system Dual Temperature Monitor (Powell, TN). As a precaution against excessive heating or cooling, the warmer infants’ abdominal probe was connected to the Ohmeda-Ohio® 3000 Infant Warmer System at all times. Temperatures were continuously sampled throughout the study and mean temperatures were calculated for each minute interval within each condition.
The infant’s face was videotaped for offline coding of grimace and cry. Research assistants were trained (by L.G.) to record grimace as brow bulge, eye squeeze, and nasolabial furrowing. Facial grimacing was scored continuously from the video portion of the tape, and crying was scored independently from the audio portion with video blank. Crying was scored continuously as the presence of an audible crying sound independent of quality. Facial grimacing was scored when brow bulging, eye squeezing and nasolabial furrowing occurred simultaneously. These facial actions have been reported in 99% of neonates within 6 seconds of heel stick and are believed to be very sensitive indices of infant pain[31,32]. Because sleep state was controlled for at the onset of the study, this allowed us to focus on facial action as the most sensitive behavioral indicator of infant pain[34]. Scorers were uninformed as to experimental condition when scoring cry from the auditory portion of the tape. For grimacing, of course, knowledge of group assignment was unavoidable. The total amount of time each infant spent grimacing or crying throughout the course of the study was quantified and inter- and intrarater reliability was calculated for both cry and grimace times. Finally, inter- and intrarater reliability was > .95 for both measures.
Statistical Analysis
Data were analyzed using SPSS 16.0 (SPSS, Inc., Chicago, IL). Pillai’s Trace multivariate tests were used for univariate repeated measures analyses of variances to evaluate heart rate, RSA, and temperature during the three conditions of the experiment (baseline, intervention, and recovery). The multivariate analyses provide a robust estimate of effects when the repeated measures are autocorrelated, as is the case with heart rate, RSA, and temperature. Since cry and grimace durations were not normal, the durations were transformed into quartile rankings. Group differences in the quartile rankings of cry and grimace times were evaluated with the non-parametric Kruskal-Wallis test.
Results
Cry/Grimace
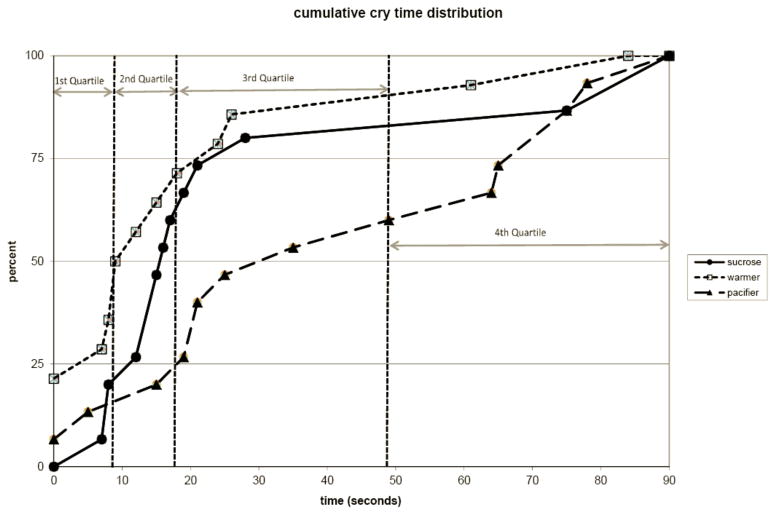
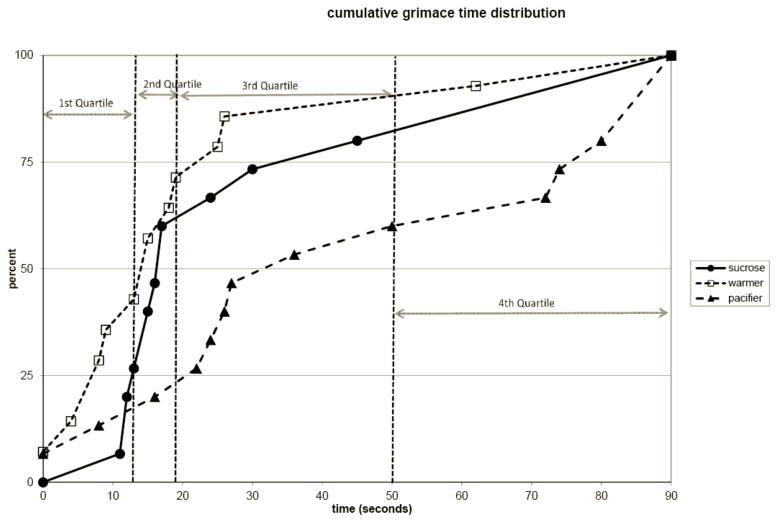
Figures 1 and 2 illustrate the cumulative distribution of time crying and grimacing for all infants. The y-axis of the figure represents the percent of subjects within each treatment group and the x-axis represents that the total amount of time spent crying (Figure 1) or grimacing (Figure 2) after the painful vaccination. For both crying and grimacing, the “warmer” group achieves cessation of crying and grimacing earlier than the other two groups (i.e. has higher percentile track at any given time). Approximately 25% of warmer infants did not cry at any point in the recover and approximately 50 % cried less than 10 seconds. This contrasts with the other two treatment groups with 0% of the sucrose group and about 5% of the pacifier group did not cry at any point during recovery and only 20% of the sucrose group and 18% of the pacifier group cried for less than 10 seconds during the recovery. The warmer group exhibited a similar advantage in total grimace time (Figure 2).
Figure 1. Cumulative Distribution Graph of Cry Times.
Graph represents the cumulative distribution of infants for the amount of time spent crying after vaccination. The quartile distribution cutoffs are indicated with vertical dotted lines. At each crying time there is a consistently higher percentage of warmer infants after vaccination compared to the other groups (p<0.05).
Figure 2. Cumulative Distribution of Grimace Time.
Graph represents the cumulative distribution of infants for the amount of time spent grimacing after vaccination. The quartile distribution cutoffs are indicated with vertical dotted lines. At each grimace time there is a consistently higher percentage of warmer infants after vaccination compared to the other groups (p<0.05).
Across groups the cumulative distributions of cry and grimace were calculated and the subjects were distributed into quartiles. The vertical lines in Figures 1 & 2 represent these quartile distributions cutoffs. The group representation within quartiles for both cry and grimace illustrates the effectiveness of the warmer treatment on newborn behavior (Figure 1). Kruskal-Wallis nonparametric analyses of variance were conducted on the quartile rankings showed significant group effects for both Cry (p<.05) and Grimace (p<.05). Half of the warmer infants had cry times in the first quartile compared to only 20% receiving sucrose and 13% with a pacifier. In addition, 43% of infants in the warmer group had grimace times in the first quartile compared to only 27% of infants receiving sucrose and 13% of infants receiving a pacifier (Figure 2). As illustrated in Figures 1 and 2, the warmer infants consistently demonstrated analgesia in fewer seconds than the sucrose and pacifier groups.
Heart Rate/RSA
During the baseline, heart rate and RSA did not vary across the sequential epochs or between groups and there were no interactions (not shown). Mean heart rate during the baseline was: 134 beats-per-minute (bpm) for the warmer group, 130 bpm for the sucrose group, and 134 bpm for the pacifier group.
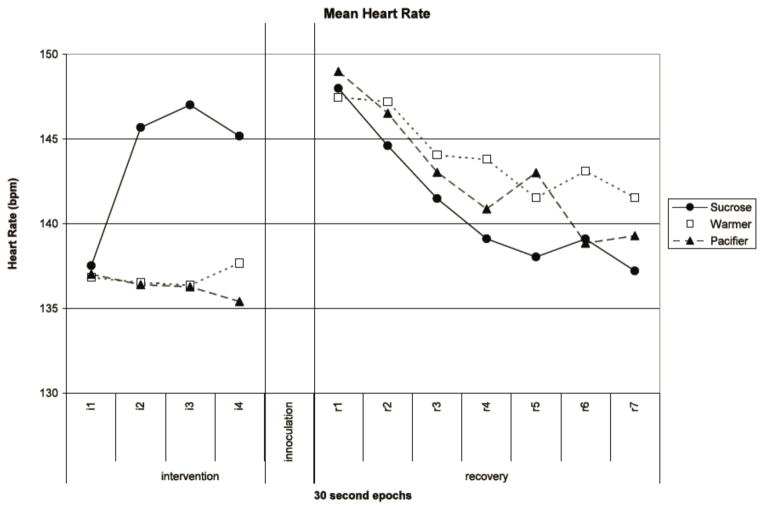
During the intervention heart rate significantly increased (F(3, 39)=4.719, p=0.007). However, the pattern differed among the groups, as documented in the significant group X sequential epoch interaction (F(6, 80)=5.229, p<0.001). As illustrated in Figure 3, heart rate for the sucrose group increased nearly 10 bpm (from 137 bpm to 147 bpm) across the intervention period, while the warmer and pacifier groups remained steady. This increase in heart rate was observed with the increased infant activity required for sucrose ingestion, but not for pacifier suckling or warmer infants.
Figure 3. Mean Heart Rate.
Mean heart rate (bpm) for each intervention group (sucrose, warmer, and pacifier) displayed at 30 second time intervals during intervention and recovery.
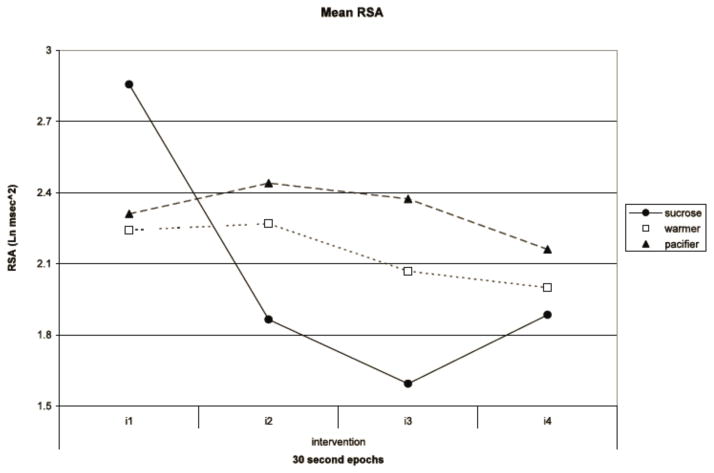
During the intervention, RSA exhibited a trend towards decreasing amplitude (F(3, 39)=2.596, p=0.066). However, there was a significant group by sequential epoch interaction (F(6, 80)=2.499, p=0.032). The interaction was characterized by the sharp decrease in the sucrose group from 2.86 Ln msec2 to 1.59 Ln msec2, while the warmer and pacifier groups varied only between 2.44 Ln msec2 and 1.74 Ln msec2 (See Figure 4).
Figure 4. Mean Respiratory Sinus Arrythmia (RSA).
Mean RSA for each intervention group (sucrose, warmer, and pacifier) displayed at 30 second time intervals during the intervention.
During the recovery period all groups had a similar significant decrease in heart rate across the sequential epochs (F(6, 36)=3.829, p=0.005). Across the groups, heart rate decreased from 148 to 139 bpm during recovery (Figure 3). There were no significant effects or interactions for RSA during the recovery period.
Temperature
Rectal temperatures did not differ among the groups throughout the entire study period. Mean rectal temperature ± standard mean of error for each group was: warmer 36.6±0.1 °C, pacifier 36.6±0.1 °C, and sucrose 36.5±0.1 °C.
Discussion
Providing natural warmth to newborn infants during a painful procedure decreases the crying and grimacing that normally accompanies a painful vaccination on par with the clinically popular treatments of sucrose or pacifier suckling. The brief and limited exposure to warmth had a significant analgesic effect on the infants when receiving the vaccination. More infants in the warmth exposure group rapidly returned to a quiet rest after the standardized 10-second injection procedure was completed. This finding is reflected in the warmer infants uniformly occupied the majority of first quartile positions in the cumulative distributions of crying or grimacing. In fact, they were twice as likely to be represented in the lowest grouping of behavioral indices of pain compared to infants who received sucrose taste or pacifier suckling.
Physiologically, independent of group assignment, the infants demonstrated similar recovery from the large heart rate increases that accompany vaccination. After the expected rapid heart rate acceleration that accompanied the painful insertion of the vaccination needle, infants in all groups had similar and rapid heart rate deceleration during the first 3 minutes of recovery.
Sucrose infants, interestingly, had a heart rate increase prior to the painful vaccination that was not observed in the other two groups. Potentially, the study design might have contributed to this observation, since sucrose infants had to be disturbed from a quiet rest in order for the sucrose taste to be delivered. Infants in the warmer group, in contrast, remained undisturbed as their ambient temperature increased. Interestingly, heart rate did not increase in the pacifier infants, who also were disturbed from a quiet rest when offered the pacifier. Perhaps the stimulating quality of the high concentration sweet taste was responsible for the heart rate rise. Alternatively, the rise in heart rate may reflect sucrose’s effect on the infant’s oral gustatory behavior. Porges and Lipsitt previously described a similar pattern. In their study, as infants increased their sucking frequency to increasingly sweet fluids, heart rate increased and RSA amplitude decreased [49,50].
In the current study, the subsequent painful Hepatitis B vaccination drove up heart rate in all infants regardless of the treatment condition. Moreover, sucrose infants displayed similar heart rate recovery compared to other infants after the vaccination. The differing heart rate and RSA patterns during the intervention period underscores the possibly different oral and gustatory calming mechanisms at work.
From the perspective of understanding how the individual components of breastfeeding provide the infant analgesia, nonpharmacologic infant analgesia likely occurs by activation descending pain modulatory pathways [6,53]. While the analgesic action of sucrose taste analgesia may operate via an endogenous opioid mechanism in the animal model [9,11], parallel data in human infants remain elusive [27,62]. The mechanisms of thermal analgesia appear to be more closely related to the non-opioid mechanisms of maternal contact or skin-to-skin contact [10,12]. No doubt, multiple neural pathways transmit pain signals to the brain and several neural pathways and neurotransmitters play a role in the descending inhibition of this nociceptive transmission [20,43,63]. Alternative mechanisms may better explain this observed thermal analgesia [35] and these untested mechanisms may also be related to the altered pain thresholds that occur during sleep [14,22,56] or ingestion [11,23].
We have shown that exposure to natural external warmth is as effective, if not more effective, as the analgesic and calming properties of sucrose taste and pacifier suckling. Indeed, infants exposed to the warmth of a radiant infant warmer cried and grimaced less after a newborn Hepatitis B vaccination than those who tasted a sweet solution or suckled a pacifier. Physiologically, these infants had identical heart rate recovery from the painful exposure as the infants who received sucrose analgesia or pacifier sucking. We observed no adverse events or side effects from the brief exposure to a heat source or change in the infants’ ambient temperature. In fact, the brief ambient temperature increase as defined here did not vary the mean rectal temps by more than 0.1 degree C.
One limitation of this study, albeit an ethical mandate, is the absence of a no treatment control group. Perhaps more detailed comparisons would be possible between the groups or differences in physiological responses would shed light on differences in the underlying mechanisms. We, however, firmly believe that emerging literature in the basic sciences will continue to elucidate the underlying mechanisms that confer this analgesia [38] and that the ethical guidelines that protect control infants from unbuffered pain are justified.
Conclusions
Providing natural warmth to newborn infants during a painful procedure decreases the crying and grimacing that accompanies the pain of a vaccination. In addition, the rapid heart rate increase associated with a painful stimulus is effectively blunted on par with other analgesic treatments currently available to newborn infants. With infant warmers universal in hospitals today, this warming treatment is natural, easy, and performed better than the currently used analgesic treatments of sucrose taste or pacifier suckling. We encourage further exploration of this and other natural, nonpharmacologic analgesic modalities as we better understand pain control in this vulnerable population.
Summary.
Providing natural warmth to newborn infants during a painful procedure decreases the crying and grimacing on par with standard treatments of sucrose or pacifier.
Acknowledgments
Funding: The research described in this manuscript was supported, in part, by NIH Grants K23 HD049452 (to LG) and R01 HD053570 (to SP) from the National Institute of Child Health and Human Development.
We thank the Nursing Staff at the University of Chicago Medical Center for their cooperation and help in conducting this research as well as the parents who allowed us to study their infants. We thank Reshma Shah, MD, a fellow in Developmental and Behavioral Pediatrics, and Tanvi Patel, Danielle Zageris, and Elizabeth Garza, students at the University of Chicago, for their assistance.
Footnotes
Financial Disclosures: The authors have no financial disclosures to report.
Conflict of Interest: The authors have no conflict of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Larry Gray, Email: larrygray@uchicago.edu, Developmental & Behavioral Pediatrics, Department of Pediatrics, University of Chicago Comer Children’s Hospital, 950 E. 61st Street, Suite 207, Chicago, IL 60637.
Colleen W. Lang, Email: langco@wusmmail.wustl.edu, Developmental & Behavioral Pediatrics, Department of Pediatrics, University of Chicago Comer Children’s Hospital, 950 E. 61st Street, Suite 207, Chicago, IL 60637.
Stephen W. Porges, Email: sporges@psych.uic.edu, Director, Brain-Body Center, Department of Psychiatry, University of Illinois at Chicago, The Psychiatric Institute (MC 912), 1601 W. Taylor Street, Chicago, IL 60612, Office 312 355-1557
References
- 1.Allen KD, White DD, Walburn JN. Sucrose as an analgesic agent for infants during immunization injections. Archives of Pediatrics and Adolescent Medicine. 1996;150:270–274. doi: 10.1001/archpedi.1996.02170280040007. [DOI] [PubMed] [Google Scholar]
- 2.Alvares D, Torsney C, Beland B, Reynolds M, Fitzgerald M. Modelling the prolonged effects of neonatal pain. Progress in Brain Research. 2000;129:365–373. doi: 10.1016/S0079-6123(00)29028-6. [DOI] [PubMed] [Google Scholar]
- 3.Anand KJS, Aranda JV, Berde CB, Buckman S, Capparelli EV, Carlo WA, Hummel P, Lantos J, Johnsto CC, Lehr VT, Lynn AM, Maxwell LG, Oberlander TF, Raju TNK, Soriano SG, Taddio A, Walco GA. Analgesia and anesthesia for neonates: Study design and ethical issues. Clin Ther. 2005;27:814–843. doi: 10.1016/j.clinthera.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 4.Anand KJS, Aynsley-Green A, Bancalari E, Benini F, Champion GD, Craig KD, Dangel TS, Fournier-Charrière E, Franck LS, Grunau RE, Hertel SA, Jacqz-Aigrain E, Jorch G, Kopelman BI, Koren G, Larsson B, Marlow N, McIntosh N, Ohlsson A, Olsson G, Porter F, Richter R, Stevens B, Taddio A. Consensus statement for the prevention and management of pain in the newborn. Archives of Pediatrics and Adolescent Medicine. 2001;155:173–180. doi: 10.1001/archpedi.155.2.173. [DOI] [PubMed] [Google Scholar]
- 5.Anand KJS, Coskun V, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiology and Behavior. 1999;66:627–637. doi: 10.1016/s0031-9384(98)00338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anseloni VCZ, Ren K, Dubner R, Ennis M. A brainstem substrate for analgesia elicited by intraoral sucrose. Neuroscience. 2005;133:231–243. doi: 10.1016/j.neuroscience.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 7.Batton DG, Barrington KJ, Wallman C, Finley GA. Prevention and management of pain in the neonate: An update. Pediatrics. 2006;118:2231–2241. doi: 10.1542/peds.2006-2277. [DOI] [PubMed] [Google Scholar]
- 8.Bellieni CV, Buonocore G. Neonatal pain treatment: Ethical to be effective. Journal of Perinatology. 2008;28:87–88. doi: 10.1038/sj.jp.7211899. [DOI] [PubMed] [Google Scholar]
- 9.Blass EM, Ciaramitaro V. A new look at some old mechanisms in human newborns: taste and tactile determinants of state, affect, and action. Monogr Soc Res Child Dev. 1994:59. [PubMed] [Google Scholar]
- 10.Blass EM, Fillion TJ, Weller A, Brunson L. Separation of Opioid From Nonopioid Mediation of Affect in Neonatal Rats: Nonopioid Mechanisms Mediate Maternal Contact Influences. Behav Neurosci. 1990;104:625–636. doi: 10.1037//0735-7044.104.4.625. [DOI] [PubMed] [Google Scholar]
- 11.Blass EM, Fitzgerald E. Milk-induced analgesia and comforting in 10-day-old rats: Opioid mediation. Pharmacology Biochemistry and Behavior. 1988;29:9–13. doi: 10.1016/0091-3057(88)90266-3. [DOI] [PubMed] [Google Scholar]
- 12.Blass EM, Shide DJ, Zaw-Mon C, Sorrentino J. Mother as shield: Differential effects of contact and nursing on pain responsivity in infant rats - Evidence for nonopioid mediation. Behav Neurosci. 1995;109:342–353. doi: 10.1037//0735-7044.109.2.342. [DOI] [PubMed] [Google Scholar]
- 13.Blass EM, Watt LB. Suckling- and sucrose-induced analgesia in human newborns. Pain. 1999;83:611–623. doi: 10.1016/S0304-3959(99)00166-9. [DOI] [PubMed] [Google Scholar]
- 14.Callahan BL, Gil ASC, Levesque A, Mogil JS. Modulation of Mechanical and Thermal Nociceptive Sensitivity in the Laboratory Mouse by Behavioral State. Journal of Pain. 2008;9:174–184. doi: 10.1016/j.jpain.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Carbajal R, Chauvet X, Coudere S, Olivier-Martin M. Randomised trial of analgesic effects of sucrose, glucose, and pacifiers in term neonates. Br Med J. 1999;319:1393–1397. doi: 10.1136/bmj.319.7222.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carbajal R, Rousset A, Danan C, Coquery S, Nolent P, Ducrocq S, Saizou C, Lapillonne A, Granier M, Durand P, Lenclen R, Coursol A, Hubert P, De Saint Blanquat L, Boëlle P, Annequin D, Cimerman P, Anand KJS, Bréart G. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA - Journal of the American Medical Association. 2008;300:60–70. doi: 10.1001/jama.300.1.60. [DOI] [PubMed] [Google Scholar]
- 17.Carbajal R, Veerapen S, Couderc S, Jugie M, Ville Y. Analgesic effect of breast feeding in term neonates: Randomised controlled trial. Br Med J. 2003;326:13–15. doi: 10.1136/bmj.326.7379.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Celeste Johnston C, Filion F, Snider L, Majnemer A, Limperopoulos C, Walker C, Veilleux A, Pelausa E, Cake H, Stone S, Sherrard A, Boyer K. Routine sucrose analgesia during the first week of life in neonates younger than 31 weeks’ postconceptional age. Pediatrics. 2002;110:523–528. doi: 10.1542/peds.110.3.523. [DOI] [PubMed] [Google Scholar]
- 19.Field T, Goldson E. Pacifying effects of nonnutritive sucking on term and preterm neonates during heelstick procedures. Pediatrics. 1984;74:1012–1015. [PubMed] [Google Scholar]
- 20.Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219–245. doi: 10.1146/annurev.ne.14.030191.001251. [DOI] [PubMed] [Google Scholar]
- 21.Fitzgerald M. When is an analgesic not an analgesic? Pain. 2009;144:9. doi: 10.1016/j.pain.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Foo H, Mason P. Brainstem modulation of pain during sleep and waking. Sleep Medicine Reviews. 2003;7:145–154. doi: 10.1053/smrv.2002.0224. [DOI] [PubMed] [Google Scholar]
- 23.Foo H, Mason P. Analgesia accompanying food consumption requires ingestion of hedonic foods. Journal of Neuroscience. 2009;29:13053–13062. doi: 10.1523/JNEUROSCI.3514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franck LS, Cox S, Allen A, Winter I. Parental concern and distress about infant pain. Archives of Disease in Childhood: Fetal and Neonatal Edition. 2004:89. doi: 10.1136/fn.89.1.F71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franck LS, Lawhon G. Environmental and behavioral strategies to prevent and manage neonatal pain. Semin Perinatol. 1998;22:434–443. doi: 10.1016/s0146-0005(98)80059-1. [DOI] [PubMed] [Google Scholar]
- 26.Gradin M, Finnström O, Schollin J. Feeding and oral glucose - Additive effects on pain reduction in newborns. Early Hum Dev. 2004;77:57–65. doi: 10.1016/j.earlhumdev.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Gradin M, Schollin J. The role of endogenous opioids in mediating pain reduction by orally administered glucose among newborns. Pediatrics. 2005;115:1004–1007. doi: 10.1542/peds.2004-1189. [DOI] [PubMed] [Google Scholar]
- 28.Gray L, Miller LW, Philipp BL, Blass EM. Breastfeeding is analgesic in healthy newborns. Pediatrics. 2002;109:590–593. doi: 10.1542/peds.109.4.590. [DOI] [PubMed] [Google Scholar]
- 29.Gray L, Watt L, Blass EM. Skin-to-skin contact is analgesic in healthy newboms. Pediatrics. 2000;105:110–111. doi: 10.1542/peds.105.1.e14. [DOI] [PubMed] [Google Scholar]
- 30.Grunau RE, Oberlander TF, Whitfield MF, Fitzgerald C, Lee SK. Demographic and therapeutic determinants of pain reactivity in very low birth weight neonates at 32 weeks’ postconceptional age. Pediatrics. 2001;107:105–112. doi: 10.1542/peds.107.1.105. [DOI] [PubMed] [Google Scholar]
- 31.Grunau RVE, Craig KD. Pain expression in neonates: Facial action and cry. Pain. 1987;28:395–410. doi: 10.1016/0304-3959(87)90073-X. [DOI] [PubMed] [Google Scholar]
- 32.Grunau RVE, Johnsto CC, Craig KD. Neonatal facial and cry responses to invasive and non-invasive procedures. Pain. 1990;42:295–305. doi: 10.1016/0304-3959(90)91142-6. [DOI] [PubMed] [Google Scholar]
- 33.Haouari N, Wood C, Griffiths G, Levene M. The analgesic effect of sucrose in full term infants: A randomised controlled trial. Br Med J. 1995;310:1498–1500. doi: 10.1136/bmj.310.6993.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holsti L, Grunau RE. Initial validation of the Behavioral Indicators of Infant Pain (BIIP) Pain. 2007;132:264–272. doi: 10.1016/j.pain.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holsti L, Grunau RE. Considerations for using sucrose to reduce procedural pain in preterm infants. Pediatrics. 2010;125:1042–1047. doi: 10.1542/peds.2009-2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnston CC, Stevens B, Pinelli J, Gibbins S, Filion F, Jack A, Steele S, Boyer K, Veilleux A. Kangaroo Care Is Effective in Diminishing Pain Response in Preterm Neonates. Archives of Pediatrics and Adolescent Medicine. 2003;157:1084–1088. doi: 10.1001/archpedi.157.11.1084. [DOI] [PubMed] [Google Scholar]
- 37.Johnston CC, Stevens BJ. Experience in a neonatal intensive care unit affects pain response. Pediatrics. 1996;98:925–930. [PubMed] [Google Scholar]
- 38.Krauchi K, Deboer T. The interrelationship between sleep regulation and thermoregulation. Frontiers in bioscience : a journal and virtual library. 2010;15:604–625. doi: 10.2741/3636. [DOI] [PubMed] [Google Scholar]
- 39.Lemons JA, Blackmon LR, Kanto WPJ, MacDonald HM, Miller CA, Papile L, Rosenfeld W, Shoemaker CT, Speer ME, Greene MF, Johnson P, McMillan DD, Iyasu S, Wright LL, Molteni R, Langer JC, Escobedo M, Fanaroff A, Ward RM, Bates BA, McCarver DG, Notterman DA, Walson PD, Weismann DN, Wilson JT, Bennett DR, Depp R, III, Dvetkovich T, Hagino OR, MacLeod SM, Mulinare J, Yaffe SJ, Cote CJ, Szefler SJ, Means LJ, Ferrari L, Bailey A, Brown REJ, Davidson P, Davis PJ, Deshpande JK, Mancuso TJ, Yaster M, Hall JK, Rockoff MA, Coran A, Andrassy R, Arensman RM, Azizkhan R, Kosloske AM, Weber TR, Coran AG, McMillan DD, Ohlsson A, Davis DJ, Faucher DJ, Van Aerde JEE, Vincer MJ, Walker R, Lemons J, Saigal S, Levitt C, McCourt C, Fraser-Askin D, Leduc L, Sauve R, Taddio A, Stevens B. Prevention and management of pain and stress in the neonate. Pediatrics. 2000;105:454–461. [Google Scholar]
- 40.Lewis M, Ramsay D. Soothing and Stress. Mahwah, NJ: Lawrence Erlbaum Associates; 1999. [Google Scholar]
- 41.Ludington-Hoe SM, Hosseini R, Torowicz DL. Skin-to-skin contact (Kangaroo Care) analgesia for preterm infant heel stick. AACN Clin Issues. 2005;16:373–387. doi: 10.1097/00044067-200507000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marsh DF, Hatch DJ, Fitzgerald M. Opioid systems and the newborn. Br J Anaesth. 1997;79:787–795. doi: 10.1093/bja/79.6.787. [DOI] [PubMed] [Google Scholar]
- 43.Mayer DJ, Price DD. Central nervous system mechanisms of analgesia. Pain. 1976;2:379–404. doi: 10.1016/0304-3959(76)90080-4. [DOI] [PubMed] [Google Scholar]
- 44.Oberlander TF, Grunau RE, Whitfield MF, Fitzgerald C, Pitfield S, Philip Saul J. Biobehavioral pain responses in former extremely low birth weight infants at four months’ corrected age. Pediatrics. 2000;105:107. doi: 10.1542/peds.105.1.e6. [DOI] [PubMed] [Google Scholar]
- 45.Phillips RM, Chantry CJ, Gallagher MP. Analgesic effects of breast-feeding or pacifier use with maternal holding in term infants. Ambulatory Pediatrics. 2005;5:359–364. doi: 10.1367/A04-189R.1. [DOI] [PubMed] [Google Scholar]
- 46.Porges SW. Method and Apparatus for Evaluating Rhythmic Oscillations in Aperiodic Physiological Response Systems. 1985. [Google Scholar]
- 47.Porges S, Bohrer R. Analyses of periodic processes in psychophysiological research. In: Cacioppo J, Tassinary L, editors. Principles of Psychophysiology: Physical, Social, and Inferential Elements. New York: Cambridge University Press; 1990. pp. 708–753. [Google Scholar]
- 48.Porges SW, Byrne EA. Research methods for measurement of heart rate and respiration. Biol Psychol. 1992;34:93–130. doi: 10.1016/0301-0511(92)90012-j. [DOI] [PubMed] [Google Scholar]
- 49.Porges SW, Lipsitt LP. Neonatal responsivity to gustatory stimulation: The gustatory-vagal hypothesis. Infant Behavior and Development. 1993;16:487–494. [Google Scholar]
- 50.Portales AL, Forges SW, Doussard-Roosevelt JA, Abedin M, Lopez R, Young MA, Beeram MR, Baker M. Vagal regulation during bottle feeding in low-birthweight neonates: Support for the gustatory-vagal hypothesis. Dev Psychobiol. 1997;30:225–233. [PubMed] [Google Scholar]
- 51.Prechtl HFR. The behavioural states of the newborn infant (a review) Brain Res. 1974;76:185–212. doi: 10.1016/0006-8993(74)90454-5. [DOI] [PubMed] [Google Scholar]
- 52.Rao M, Blass EM, Brignol MM, Marino L, Glass L. Reduced heat loss following sucrose ingestion in premature and normal human newborns. Early Hum Dev. 1997;48:109–116. doi: 10.1016/s0378-3782(96)01847-6. [DOI] [PubMed] [Google Scholar]
- 53.Ren K, Blass EM, Zhou Q, Dubner R. Suckling and sucrose ingestion suppress persistent hyperalgesia and spinal fos expression after forepaw inflammation in infant rats. Proc Natl Acad Sci U S A. 1997;94:1471–1475. doi: 10.1073/pnas.94.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riniolo T, Porges SW. Inferential and descriptive influences on measures of respiratory sinus arrhythmia: Sampling rate, R-wave trigger accuracy, and variance estimates. Psychophysiology. 1997;34:613–621. doi: 10.1111/j.1469-8986.1997.tb01748.x. [DOI] [PubMed] [Google Scholar]
- 55.Ruda MA, Ling Q, Hohmann AG, Peng YB, Tachibana T. Altered nociceptive neuronal circuits after neonatal peripheral inflammation. Science. 2000;289:628–630. doi: 10.1126/science.289.5479.628. [DOI] [PubMed] [Google Scholar]
- 56.Seelke AMH, Blumberg MS. Thermal and nutritional modulation of sleep in infant rats. Behav Neurosci. 2005;119:603–611. doi: 10.1037/0735-7044.119.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simons SHP, Van Dijk M, Anand KS, Roofthooft D, Van Lingen RA, Tibboel D. Do We Still Hurt Newborn Babies? A Prospective Study of Procedural Pain and Analgesia in Neonates. Archives of Pediatrics and Adolescent Medicine. 2003;157:1058–1064. doi: 10.1001/archpedi.157.11.1058. [DOI] [PubMed] [Google Scholar]
- 58.South MMT, Strauss RA, South AP, Boggess JF, Thorp JM. The use of non-nutritive sucking to decrease the physiologic pain response during neonatal circumcision: A randomized controlled trial. Obstet Gynecol. 2005;193:537–543. doi: 10.1016/j.ajog.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 59.Stevens B, Yamada J, Ohlsson A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane database of systematic reviews (Online) 2004 doi: 10.1002/14651858.CD001069.pub2. [DOI] [PubMed] [Google Scholar]
- 60.Taddio A, Shah V, Atenafu E, Katz J. Influence of repeated painful procedures and sucrose analgesia on the development of hyperalgesia in newborn infants. Pain. 2009;144:43–48. doi: 10.1016/j.pain.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 61.Taddio A, Shah V, Gilbert-MacLeod C, Katz J. Conditioning and hyperalgesia in newborns exposed to repeated heel lances. J Am Med Assoc. 2002;288:857–861. doi: 10.1001/jama.288.7.857. [DOI] [PubMed] [Google Scholar]
- 62.Taddio A, Shah V, Shah P, Katz J. β-Endorphin Concentration after Administration of Sucrose in Preterm Infants. Archives of Pediatrics and Adolescent Medicine. 2003;157:1071–1074. doi: 10.1001/archpedi.157.11.1071. [DOI] [PubMed] [Google Scholar]
- 63.Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. Journal of Clinical Neurophysiology. 1997;14:2–31. doi: 10.1097/00004691-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]