Abstract
BACKGROUND:
Hyperventilation has been associated with adverse effects on lung function, symptoms, and well-being in asthma. We examined whether raising end-tidal CO2 levels (ie, Pco2) compared with slow breathing is associated with improvements in asthma control, including peak flow variability.
METHODS:
One hundred twenty patients with asthma were randomly assigned to capnometry-assisted respiratory training (CART) for raising Pco2 or slow breathing and awareness training (SLOW) for slowing respiratory rate. Patients received five weekly sessions and completed bid homework exercises over 4 weeks. Blinded assessments at baseline, posttreatment, 1- and 6-month follow-up of asthma control, Pco2, and diurnal peak flow variability were primary outcome measures. Additionally, we measured pulmonary function (spirometry, forced oscillation, exhaled nitric oxide, and methacholine challenge), symptoms, quality of life, and bronchodilator use. Because the control group received active treatment, we expected improvements in asthma control in both groups but more pronounced benefits from CART.
RESULTS:
Improvements were seen in 17 of 21 clinical indexes (81.0%) in both interventions, including the primary outcome variables asthma control (d = 0.81), peak flow variability (d = 0.54), quality of life, bronchodilator use, lung function, and airway hyperreactivity. Most improvements were sustained across the 6-month follow-up. Compared with slow breathing, CART showed greater increases in Pco2 (d = 1.45 vs 0.64 for CART vs SLOW, respectively) and greater reductions in respiratory impedance during treatment, less distress during methacholine challenge, and greater reduction in asthma symptoms at follow-up (P < .05).
CONCLUSIONS:
Brief interventions aimed at raising Pco2 or slowing respiratory rate provide significant, sustained, and clinically meaningful improvements in asthma control. Raising Pco2 was associated with greater benefits in aspects of lung function and long-term symptoms.
TRIAL REGISTRY:
ClinicalTrials.gov; No.: NCT00975273; URL: www.clinicaltrials.gov
Asthma is one of the most common chronic diseases with high prevalence rates and morbidity but overall unsatisfactory disease management.1 Although medication is undisputedly the first line of treatment in patients with asthma, adverse side effects, cost, and patient desire to reduce or replace medication with alternative treatments have prompted recommendations for broader asthma care.2 Nonpharmacologic methods for asthma management often include breathing retraining.3,4 As early as 1946, Herxheimer5 linked observations of hyperventilation to asthma exacerbations. Relative to other primary care patients, patients with asthma report more symptoms of hyperventilation or overbreathing,6 and such symptoms are associated with lower quality of life.7 Studies have found lower resting Pco2 in asthma,8 acute decreases in Pco2 during severe exacerbations,9 increased airway obstruction,10 and hyperreactivity8 when Pco2 is reduced. In addition, hyperpnea that accompanies hyperventilation exacerbates asthma by airway drying, a mechanism central to exercise-induced asthma.11
Among the best-known hypoventilation trainings is the Buteyko method.2,12 Built on the hypothetical treatment rationale that low Pco2 levels are critical culprits in asthma pathophysiology, patients are trained in breath-holding techniques to reduce ventilation. Controlled studies found reduced rescue medication use and improved quality of life in asthma13 but no improvements in pulmonary function. However, because Pco2 was rarely assessed and never manipulated, the rationale of the training remains untested. The only Buteyko study that measured Pco2 pretreatment and posttreatment showed no effect on this key indicator of hyperventilation.14
Thus, we tested the efficacy of a novel hypoventilation training that affects Pco2 and respiratory rate (RR) directly by using feedback. This training has proven efficacious in controlled trials in patients with panic disorder,15,16 leading to sustained elevations in Pco2 and improvements in panic symptoms. A small proof-of-concept study for asthma showed promising associations of hypoventilation training with better asthma control, improved symptoms, and reduced variability of lung function.17 In the present clinical trial, the effects of hypoventilation training on asthma symptoms and pathophysiology were compared with feedback-guided slowing of RR. Slower breathing alone was not expected to alter Pco2 because of the typical compensatory changes in tidal volume that maintain a stable gas exchange. The two therapeutic procedures were identical on important nonspecific therapy factors, with the exception of capnometry feedback to raise Pco2. Given that both groups received an active treatment, we expected improvements in asthma control in both groups. However, the hypothesis was that superior increases in Pco2, asthma control measured by questionnaire, and peak flow variability would be expected for capnometry feedback at treatment follow-up.
Materials and Methods
Study Design and Participants
One hundred twenty adults (aged 18-65 years) with asthma of all severity grades1 were recruited through physician referrals and advertisements. Diagnosis was verified by either a 20% drop in FEV1 following methacholine challenge or a 12% increase in FEV1 following nebulizer treatment with 0.083% albuterol if initial FEV1 was at least 60% predicted. Patients were randomly assigned to either (1) capnometry-assisted respiratory training (CART) or (2) slow breathing and awareness training (SLOW). A computer-generated allocation and block randomization by initial Pco2 levels was used to ensure that two-thirds of patients in both groups had Pco2 < 35 mm Hg. Assessments were at pretreatment, posttreatment, 1-month follow-up (1MFU), and 6-month follow-up (6MFU). Patients had to remain on their treatment regimen (standard of care) until 1MFU, unless an exacerbation required a change. This was determined by a written asthma action plan1 based on significant worsening in either asthma symptoms, measurements of peak expiratory flow (PEF), or as directed by the treating physician. Patients who experienced stronger exacerbations (PEF < 60% predicted together with development of strong symptoms) during the trial were withdrawn from the study and referred for immediate reevaluation. Short-acting bronchodilators (albuterol) with dose counters embedded in Ventolin HFA metered-dose inhalers (GlaxoSmithKline plc) were provided throughout the study period. Exclusion criteria included present smoking or past smoking (> 10 pack-years), uncontrolled medical or psychiatric comorbidity, oral corticosteroids in the past 3 months, and pregnancy (see full list in e-Appendix 1 (438.4KB, pdf) ). The study was approved by the institutional review boards of the participating sites (#008-180-Baylor, #KS08-051-SMU). All patients provided written informed consent. Treatments were offered in English and Spanish.
Interventions
After a 7-day run-in period, patients received five individual, manualized, therapist-led sessions over 4 weeks. Treatments (duration, dose, homework, patient-therapist contact, and device used) were identical with the exception of feedback of Pco2. Patients received comprehensive handouts for guidance, detailing aspects of pulmonary physiology, the relevance of breathing to asthma symptoms, and instructions for daily exercises during the 4 treatment weeks. The portable capnometer (TIDAL WAVE; Koninklijke Philips NV) used for in-session and at-home training continuously displayed and stored values with time and date stamps for compliance check (e-Fig 1 (438.4KB, pdf) ). All prior-week exercises were downloaded, and printouts depicting continuous Pco2 and RR data were discussed at the weekly treatment sessions.
Capnometry-Assisted Respiratory Training:
The goal of CART is to reach and maintain a Pco2 of around 40 to 42 mm Hg by reducing ventilation primarily through shallow breathing aided by continuous feedback of Pco2 and RR. Slow, regular, nasal, and abdominal breathing is also emphasized. Patients were educated about the mechanisms of asthma symptoms through hyperventilation and hyperpnea. They were prescribed bid, 17-min audio-directed exercises with (1) 2-min quiet sitting, eyes-closed (baseline); (2) 10-min paced breathing following tones at 13, 11, 9, and 6 breaths/min across subsequent weeks; and (3) 5 min without pacing. Additional observation of oxygen saturation as measured by pulse oximetry was used to educate patients about the stability of oxygen saturation despite possible symptoms of dyspnea.
Slow Breathing and Awareness Training:
SLOW followed the same structure and dose as CART but only by using the capnometer display of RR for feedback (Pco2 display was deactivated) to achieve week-by-week reductions in RR while following the same pacing tones. Slow, abdominal, nasal, and regular breathing was also trained, with the rationale focused on irregular and fast breathing as a source of symptoms. Awareness of breathing was also emphasized.
Assessments
Assessments of primary and most secondary outcomes were performed at pretreatment and posttreatment, 1MFU, and 6MFU by independent assessors blinded to participant treatment assignment (e-Fig 2 (438.4KB, pdf) ). Primary outcome measures were asthma control, Pco2 (measured during 2-min quiet sitting in the clinic without feedback), and diurnal PEF variability. Participants completed the Asthma Control Test (ACT),18 a guideline-supported measure of asthma impairment1 with demonstrated reliability, sensitivity to change, and predictive validity for future exacerbations.19,20 Diurnal PEF variability was assessed during the 3-day run-in period, at posttreatment, 1MFU, and 6MFU using an electronic pocket spirometer (AM2; Jaeger Toennies Erich Jaeger GmbH). Participants took measurements at six different times throughout the day (see e-Appendix 1 (438.4KB, pdf) for details).
Secondary outcome measures assessed bronchodilator use, asthma symptoms, mastery of asthma management, quality of life, lung function, airway hyperreactivity, and perception of obstruction. Bronchodilator use was recorded with a dose counter embedded in the shell of the metered-dose inhaler. Patients received as many refills as needed across the study period but were asked to abstain from using their short-acting bronchodilators for 8 h before the assessments. Frequency of symptoms in the past week was assessed with the 10-item Asthma Symptom Questionnaire.21 The 36-item Asthma Symptom Checklist measured participant perceptions of asthma symptom episodes or exacerbations on dimensions of panic-fear, dyspnea, irritability, congestion, fatigue, and hyperventilation.22 Mastery of asthma management was measured by the Knowledge, Attitude, and Self-efficacy Asthma Questionnaire.23 Physical and mental quality of life was measured using the Survey Form-12 (SF-12).24
PEF and FEV1 were based on the best of three blows and expressed as percent predicted values. Additional measures of FEV1 were taken at the beginning of each treatment session preceded by respiratory impedance measurement at 5-Hz oscillation frequency (Zrs5Hz) (impulse oscillometry; Jaeger Toennies Erich Jaeger GmbH/Cardinal Health Inc) as a more-sensitive measure of airway diameter.25 The fraction of exhaled nitric oxide (in parts per billion) at a flow of 50 mL/s was measured with an electrochemical gas analyzer (NIOX MINO; Aerocrine). At pretreatment and 1MFU, airway hyperreactivity to methacholine was assessed according to American Thoracic Society standards26 expressed as the provocation concentration leading to a 20% fall in FEV1 (PC20). Change in perception of obstruction was based on dyspnea and distress differences between maxima during provocation vs saline.
Control variables were prescribed inhaled corticosteroid intake (converted to beclomethasone equivalent),27 leukotriene modifier prescription (0 = none and 1 = any use), reported frequency of corticosteroid and leukotriene intake as an indicator of adherence, and cold and flu symptoms in the week prior to assessment (presence = 1, absence = 0). Adverse events were assessed at each visit. Treatment expectancy and credibility28 were rated after the first treatment session on a scale of 1 (not at all logical), 5 (somewhat logical), and 9 (very logical).
Statistical Analysis
Mixed-model repeated-measures analysis of covariances allowed the inclusion of all participants regardless of missing data, thus improving power and generalizability. It is the recommended method for longitudinal data analysis.29 The 2 × 4 design included two treatment conditions (CART vs SLOW) and the four assessments over time (baseline, posttreatment, 1MFU, and 6MFU). Each model initially included as covariates age, cold symptoms, flu symptoms, beclomethasone-equivalent doses, frequency of inhaled corticoid steroid use, and frequency of leukotriene modifier use. Nonsignificant covariates were dropped for the final analysis of each outcome. We enrolled 120 participants to detect a medium-sized group difference (d = 0.5) at 80% power and P < .05 at 6MFU.
Results
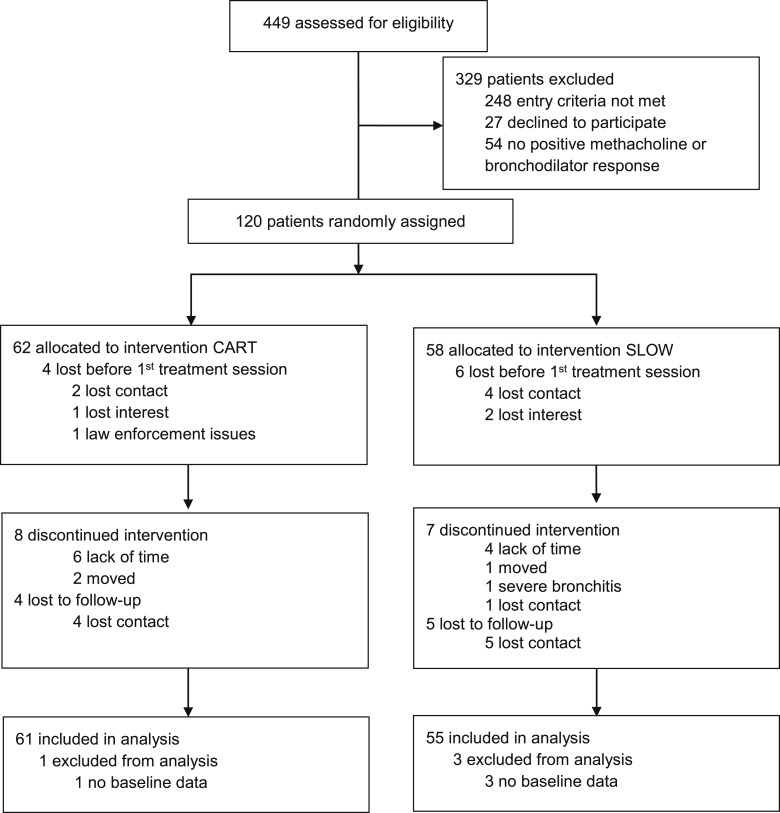
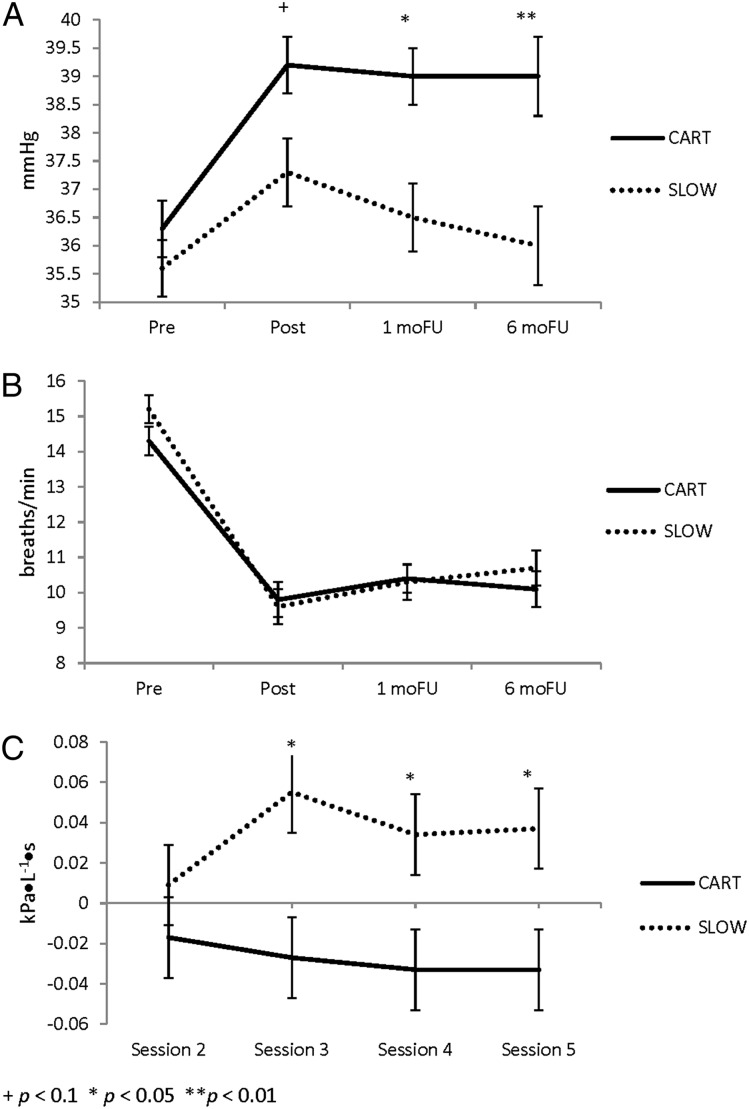
Condition did not differ significantly on dropout rates, completion rates, demographics, anthropometrics, or disease-specific characteristics, except for age (Fig 1, Table 1). The results of the primary outcome measures indicated that asthma control improved equally in both groups from baseline to follow-up (Cohen d = 0.81). In contrast to SLOW, in which Pco2 increased only slightly during treatment but was unchanged at 6MFU (35.6-35.6 mm Hg, d = 0.64), Pco2 levels in CART increased and were sustained through 6MFU (36.3-39.0 mm Hg, d = 1.45) (Fig 2). Diurnal PEF variability decreased equally in both groups (d = 0.54) (Table 2).
Figure 1 –
Study flow diagram. CART = capnometry-assisted respiratory training; SLOW = slow breathing and awareness training.
TABLE 1 ] .
Baseline Demographic and Clinical Characteristics of Study Participants
| Characteristic | CART (n = 61) | SLOW (n = 55) |
| Age, ya | 37.3 ± 12.1 | 42.4 ± 12.9 |
| Women | 35 (57.4) | 36 (65.5) |
| Race | ||
| White | 40 (65.6) | 36 (65.6) |
| Black | 18 (29.5) | 17 (30.9) |
| Other | 3 (4.9) | 2 (3.6) |
| Hispanic | 9 (14.8) | 8 (14.5) |
| BMI, kg/m2 | 28.6 ± 6.0 | 31.3 ± 7.0 |
| Symptoms mostly at night | 14 (23.3) | 11 (20.4) |
| Asthma controlb | ||
| Well controlled | 9 (14.8) | 4 (7.3) |
| Not well controlled | 23 (37.7) | 22 (40.0) |
| Very poorly controlled | 29 (47.5) | 29 (52.7) |
| Asthma severityb,c | ||
| Intermittent | 0 (0.0) | 1 (1.8) |
| Mild persistent | 8 (13.1) | 6 (10.9) |
| Moderate persistent | 12 (19.7) | 11 (20.0) |
| Severe persistent | 2 (3.3) | 5 (9.1) |
| Short-term bronchodilator use only | 22 (36.1) | 23 (41.8) |
| Bronchodilator use, times/d | 1.4 ± 2.4 | 1.3 ± 2.7 |
| Inhaled corticosteroid use | 35 (57.4) | 32 (58.2) |
| Leukotriene inhibitor use | 13 (21.3) | 6 (10.9) |
Data are presented as mean ± SD or No. (%). CART = capnometry-assisted respiratory training; SLOW = slow breathing and awareness training.
P < .05.
Asthma control and asthma severity is based on National Heart, Lung, and Blood Institute criteria.1
Severity was only calculated for participants not taking maintenance medication.
Figure 2 –
A-C, Changes in end-tidal Pco2 (A), respiratory rate (B), and respiratory impedance (C) (from initial assessment at treatment session 1). 1moFU = 1-mo follow-up; 6moFU = 6-mo follow-up. See Figure 1 legend for expansion of other abbreviations.
TABLE 2 ] .
Change in Primary Outcome Parameters Between Baseline and Follow-up
| Outcome | Mean ± SE | Time Effect P Value | Group × Time Effect P Value | Mean Change (95% CI) From Baseline to | |||||
| Baseline | Post | 1MFU | 6MFU | Post | 1MFU | 6MFU | |||
| ACT | < .001 | .157 | |||||||
| CART | 17.6 ± 0.5 | 19.2 ± 0.5 | 18.8 ± 0.5 | 19.6 ± 0.6 | 1.6 (0.6 to 2.6) | 1.2 (−0.0 to 2.5) | 2.1 (0.6 to 3.6) | ||
| SLOW | 16.6 ± 0.5 | 19.1 ± 0.5 | 19.8 ± 0.6 | 19.5 ± 0.6 | 2.5 (1.5 to 3.5) | 3.2 (1.9 to 4.5) | 2.9 (1.3 to 4.4) | ||
| Differential change | … | … | … | … | −0.9 (−2.3 to 0.5) | −2.0 (−3.8 to −0.2) | −0.8 (−3.0 to 1.4) | ||
| P value | … | … | … | … | .217 | .034 | .466 | ||
| Pco2 | < .001 | .049 | |||||||
| CART | 36.3 ± 0.5 | 39.2 ± 0.5 | 39.0 ± 0.5 | 39.0 ± 0.7 | 2.9 (2.0 to 3.9) | 2.7 (1.6 to 3.8) | 2.7 (1.3 to 4.0) | ||
| SLOW | 35.6 ± 0.5 | 37.3 ± 0.6 | 36.5 ± 0.6 | 35.6 ± 0.7 | 1.7 (0.7 to 2.8) | 0.9 (−0.2 to 2.1) | 0.0 (−1.4 to 1.5) | ||
| Differential change | … | … | … | … | 1.2 (−0.2 to 2.7) | 1.8 (0.2 to 3.3) | 2.7 (0.7 to 4.6) | ||
| P value | … | … | … | … | .094 | .031 | .009 | ||
| Diurnal peak flow | .005 | .169 | |||||||
| CART | 10.0 ± 0.8 | 8.7 ± 0.7 | 10.1 ± 0.8 | 9.2 ± 0.7 | −1.3 (−2.5 to −0.1) | 0.1 (−1.5 to 1.6) | −0.8 (−2.4 to 0.7) | ||
| SLOW | 9.8 ± 0.8 | 7.9 ± 0.7 | 8.1 ± 0.8 | 9.1 ± 0.7 | −1.9 (−3.1 to −0.6) | −1.7 (−3.3 to −0.1) | −0.7 (−2.3 to 0.9) | ||
| Differential change | … | … | … | … | 0.6 (−1.2 to 2.3) | 1.8 (−0.4 to 4.0) | −0.2 (−2.4 to 2.1) | ||
| P value | … | … | … | … | .535 | .114 | .891 | ||
Differential change was calculated as the difference from baseline in the CART condition (eg, 1MFU − baseline) minus the difference from baseline for the SLOW condition; higher numbers indicate greater increases in CART vs SLOW. 1MFU = 1-mo follow-up; 6MFU = 6-mo follow-up; ACT = Asthma Control Test. See Table 1 legend for expansion of other abbreviations.
Results for the secondary outcome measures indicated that number of bronchodilator doses per day decreased in both groups from baseline through follow-up. Frequency of weekly asthma symptoms and exacerbation symptoms (Asthma Symptom Checklist) were reduced from baseline to posttreatment and 1MFU in both groups, with significant advantages of CART over SLOW at 6MFU (see e-Table 1 (438.4KB, pdf) for subscale results). Self-efficacy and physical quality of life improved equally in both groups (Table 3).
TABLE 3 ] .
Change in Secondary Outcome Parameters Between Baseline and Follow-up
| Outcome | Mean ± SE | Time Effect P Value | Group × Time Effect P Value | Mean Change (95% CI) From Baseline to | |||||
| Baseline | Post | 1MFU | 6MFU | Post | 1MFU | 6MFU | |||
| ASQ | < .001 | .039 | |||||||
| CART | 2.4 ± 0.1 | 1.9 ± 0.1 | 1.9 ± 0.1 | 1.7 ± 0.1 | −0.4 (−0.6 to −0.2) | −0.4 (−0.7 to −0.2) | −0.7 (−0.9 to −0.4) | ||
| SLOW | 2.3 ± 0.1 | 1.8 ± 0.1 | 1.8 ± 0.1 | 2.0 ± 0.1 | −0.5 (−0.7 to −0.3) | −0.5 (−0.7 to −0.2) | −0.3 (−0.5 to −0.0) | ||
| Differential change | … | … | … | … | 0.1 (−0.2 to 0.4) | 0.0 (−0.3 to 0.4) | −0.4 (−0.7 to −0.0) | ||
| P value | … | … | … | … | .491 | .836 | .026 | ||
| ASC | < .001 | .042 | |||||||
| CART | 52.5 ± 1.3 | 49.2 ± 1.3 | 50.0 ± 1.4 | 46.5 ± 1.3 | −3.2 (−5.8 to −0.6) | −2.5 (−5.3 to 0.2) | −6.0 (−8.4 to −3.6) | ||
| SLOW | 52.4 ± 1.3 | 50.2 ± 1.3 | 48.7 ± 1.4 | 49.8 ± 1.3 | −2.2 (−4.8 to 0.4) | −3.7 (−6.5 to −0.9) | −2.5 (−4.9 to −0.1) | ||
| Differential change | … | … | … | … | −1.1 (−4.7 to 2.6) | 1.2 (−2.7 to 5.1) | −3.5 (−6.9 to −0.1) | ||
| P value | … | … | … | … | .565 | .545 | .044 | ||
| SF-12 physical | < .001 | .260 | |||||||
| CART | 49.7 ± 1.1 | 51.4 ± 1.0 | 51.0 ± 1.0 | 51.2 ± 0.9 | 1.7 (−0.1 to 3.5) | 1.3 (−1.0 to 3.5) | 1.5 (−0.8 to 3.7) | ||
| SLOW | 47.7 ± 1.2 | 51.5 ± 1.0 | 51.0 ± 1.1 | 52.2 ± 1.0 | 3.8 (1.9 to 5.8) | 3.3 (1.0 to 5.7) | 4.5 (2.1 to 6.8) | ||
| Differential change | … | … | … | … | −2.1 (−0.8 to 0.5) | −2.0 (−5.3 to 1.2) | −3.0 (−6.2 to 0.2) | ||
| P value | … | … | … | … | .116 | .214 | .067 | ||
| SF-12 mental | .244 | .922 | |||||||
| CART | 51.1 ± 1.1 | 51.9 ± 1.1 | 52.8 ± 1.2 | 52.5 ± 1.2 | 0.8 (−1.2 to 2.8) | 1.7 (−0.5 to 3.9) | 1.3 (−1.1 to 3.7) | ||
| SLOW | 52.1 ± 1.2 | 52.4 ± 1.1 | 53.7 ± 1.2 | 52.4 ± 1.3 | 0.3 (−1.8 to 2.4) | 1.6 (−0.7 to 3.9) | 0.3 (−2.2 to 2.8) | ||
| Differential change | … | … | … | … | 0.5 (−2.4 to 3.4) | 0.1 (−3.1 to 3.3) | 1.0 (−2.5 to 4.5) | ||
| P value | … | … | … | … | .740 | .959 | .564 | ||
| KASE-AQ | < .001 | .665 | |||||||
| CART | 53.9 ± 1.3 | 60.3 ± 1.3 | 60.6 ± 1.2 | 62.1 ± 1.3 | 6.5 (4.0 to 8.9) | 6.7 (4.3 to 9.0) | 8.3 (5.6 to 10.9) | ||
| SLOW | 52.5 ± 1.3 | 57.0 ± 1.3 | 58.0 ± 1.2 | 58.6 ± 1.3 | 4.5 (2.0 to 7.0) | 5.6 (3.2 to 7.9) | 6.2 (3.4 to 8.9) | ||
| Differential change | … | … | … | … | 2.0 (−1.5 to 5.5) | 1.1 (−2.2 to 4.5) | 2.1 (−1.8 to 6.0) | ||
| P value | … | … | … | … | .258 | .508 | .283 | ||
| Bronchodilator use | < .001 | .922 | |||||||
| CART | 1.4 ± 0.3 | 1.0 ± 0.3 | 0.5 ± 0.1 | 0.4 ± 0.1 | −0.4 (−1.0 to 0.3) | −0.9 (−1.5 to −0.3) | −1.0 (−1.6 to −0.4) | ||
| SLOW | 1.3 ± 0.4 | 0.8 ± 0.3 | 0.4 ± 0.1 | 0.3 ± 0.1 | −0.5 (−1.2 to 0.2) | −1.0 (−1.6 to −0.3) | −1.0 (−1.7 to −0.3) | ||
| Differential change | … | … | … | … | 0.1 (−0.8 to 1.1) | 0.1 (−0.8 to 1.0) | 0.0 (−0.9 to 0.9) | ||
| P value | … | … | … | … | .758 | .836 | .982 | ||
Differential change was calculated as the difference from baseline in the CART condition (eg, 1MFU − baseline) minus the difference from baseline for the SLOW condition; higher numbers indicate greater increases in CART vs SLOW. ASC = Asthma Symptom Checklist; ASQ = Asthma Symptom Questionnaire; KASE-AQ = Knowledge, Attitude, and Self-efficacy Asthma Questionnaire; SF-12 = Survey Form-12. See Table 1 and 2 legends for expansion of other abbreviations.
PEF improved steadily from baseline to 6MFU in both groups, whereas FEV1 and fraction of exhaled nitric oxide remained unchanged. Zrs5Hz systematically decreased in CART but increased in SLOW during treatment (group effect for differences from first session baseline P = .026) (Fig 2). Airway hyperreactivity improved equally in both groups, with increases in PC20 from baseline to 1MFU and maximum dyspnea increasing during the challenge. Maximum distress remained stable in CART but increased in SLOW (Table 4). RR was reduced from baseline to follow-up, attesting to the success in manipulating it in both CART and SLOW (Fig 2).
TABLE 4 ] .
Airway Pathophysiology Measures: Model-Derived Estimates for Baseline, Posttreatment, Follow-up, and Change From Baseline
| Measure | Mean ± SE | Time Effect P Value | Group × Time Effect P Value | Mean Change (95% CI) From Baseline to | |||||
| Baseline | Post | 1MFU | 6MFU | Post | 1MFU | 6MFU | |||
| FEV1 % predicted | .178 | .833 | |||||||
| CART | 77.7 ± 2.1 | 76.6 ± 2.0 | 74.5 ± 2.3 | 78.2 ± 2.2 | −1.1 (−4.5 to 2.3) | −3.2 (−6.3 to −0.2) | 0.4 (−2.8 to 3.7) | ||
| SLOW | 80.2 ± 2.2 | 80.1 ± 2.1 | 79.0 ± 2.4 | 80.7 ± 2.3 | −0.2 (−3.7 to 3.4) | −1.2 (−4.5 to 2.0) | 0.5 (−3.0 to 4.0) | ||
| Differential change | … | … | … | … | −0.9 (−5.8 to 4.0) | −2.0 (−6.5 to 2.5) | −0.0 (−4.8 to 4.8) | ||
| P value | … | … | … | … | .712 | .383 | .984 | ||
| PEF % predicted | < .001 | .176 | |||||||
| CART | 88.1 ± 2.6 | 89.8 ± 2.5 | 90.5 ± 2.4 | 93.0 ± 2.8 | 1.8 (−1.7 to 5.3) | 2.4 (−1.4 to 6.3) | 5.0 (0.9 to 9.0) | ||
| SLOW | 87.0 ± 2.8 | 94.5 ± 2.7 | 93.6 ± 2.5 | 95.8 ± 3.0 | 7.5 (3.8 to 11.3) | 6.6 (2.5 to 10.6) | 8.8 (4.3 to 13.2) | ||
| Differential change | … | … | … | … | −5.8 (−10.9 to −0.6) | −4.1 (−9.7 to 1.4) | −3.8 (−9.8 to 2.2) | ||
| P value | … | … | … | … | .028 | .144 | .214 | ||
| Feno | .375 | .442 | |||||||
| CART | 43.5 ± 5.6 | 47.5 ± 6.1 | 43.7 ± 5.9 | 37.1 ± 5.4 | 4.1 (−4.9 to 13.1) | 0.3 (−8.8 to 9.3) | −6.4 (−17.4 to 4.5) | ||
| SLOW | 50.5 ± 5.6 | 49.9 ± 6.2 | 51.4 ± 6.2 | 50.2 ± 5.7 | −0.5 (−9.5 to 8.4) | 1.0 (−8.3 to 10.3) | −0.3 (−11.5 to 11.0) | ||
| Differential change | … | … | … | … | 4.6 (−8.0 to 17.2) | −0.7 (−13.7 to 12.3) | −6.1 (−21.9 to −9.6) | ||
| P value | … | … | … | … | .469 | .914 | .439 | ||
| PC20 | |||||||||
| CART | 4.4 ± 0.8 | 6.5 ± 2.3 | … | .013 | .227 | … | 2.1 (−2.1 to 6.4) | … | |
| SLOW | 4.0 ± 0.8 | 10.0 ± 2.6 | … | … | … | … | 6.0 (1.3 to 10.7) | … | |
| Differential change | … | … | … | … | … | … | … | −3.9 (−10.2 to 2.5) | … |
| P value | … | … | … | … | … | … | … | .227 | … |
| PC20 distress | .033 | .049 | |||||||
| CART | 3.5 ± 0.3 | 3.5 ± 0.3 | … | … | 0.0 (−0.8 to 0.9) | … | |||
| SLOW | 2.8 ± 0.3 | 4.0 ± 0.3 | … | … | 1.2 (0.4 to 2.0) | … | |||
| Differential change | … | … | … | … | … | −1.2 (−2.3 to −0.0) | … | ||
| P value | … | … | … | … | … | .049 | … | ||
| PC20 dyspnea | .050 | .973 | |||||||
| CART | 4.4 ± 0.3 | 5.1 0.4 | … | … | 0.7 (−0.3 to 1.7) | … | |||
| SLOW | 4.0 ± 0.3 | 4.7 ± 0.4 | … | … | 0.7 (−0.3 to 1.7) | … | |||
| Differential change | … | … | … | … | … | −0.0 (−1.4 to 1.4) | … | ||
| P value | … | … | … | … | … | .973 | … | ||
Differential change was calculated as the difference from baseline in the CART condition (eg, 1MFU − baseline) minus the difference from baseline for the SLOW condition; higher numbers indicate greater increases in CART vs SLOW. Feno = fraction of exhaled nitric oxide; PC20 = provocation concentration of methacholine leading to a 20% fall in FEV1; PEF = peak expiratory flow. See Table 1 and 2 legends for expansion of other abbreviations.
Treatment completion and homework adherence was high for both groups (92.7% and 70.1%, respectively). Treatment credibility (mean ± SE, 8.1 ± 1.0 and 8.1 ± 1.1, respectively) and expectancy (mean ± SE, 7.9 ± 1.1 and 7.9 ± 1.1, respectively) was also high, with no substantial moderating effect on outcome. No significant changes were observed in beclomethasone equivalency and frequency of maintenance medication use (e-Appendix 1 (438.4KB, pdf) ). Neither treatment was associated with adverse or serious adverse events (no study-related adverse events [e-Appendix 1 (438.4KB, pdf) ]).
Discussion
We have shown that brief, five-session biofeedback-guided respiratory treatments aimed at either reversing hyperventilation or slowing RR lead to significant clinical improvements across a range of asthma-specific symptoms and pathophysiology. Improvements were seen in 17 of 21 clinical indices (81.0%), including asthma control and symptoms, quality of life, bronchodilator use, lung function, and PC20. Most improvements were sustained at 6MFU. No study-related adverse events were recorded. The present hypothesis about primary outcome variables was only partially supported in that Pco2 was elevated selectively by CART, whereas improvements in the ACT and PEF variability were seen in both interventions. However, patients trained in raising Pco2 showed superior gains at 6MFU on the majority of secondary asthma symptom outcome measures. Treatments also differed in Pco2 and Zrs5Hz. Resting Pco2 increased in CART but was unchanged in SLOW at 6MFU. Session-by-session Zrs5Hz significantly decreased in CART but increased in SLOW. The improvements in PEF in both groups but lack thereof in FEV1 may be due to improved effort or motivation of patients across the trial. Lung volume increases, which reduce impedance measured by forced oscillations,25 were not a likely cause of Zrs5Hz improvements in CART because treatment instructions emphasized shallower breathing. However, deep breaths may have increased Zrs5Hz in SLOW because moderate to severe, persistent asthma has been linked to lack of bronchodilator or even bronchoconstrictor response to deep inspiration.30 Others also found improvements in Zrs5Hz but not in spirometry when training patients with asthma to increase heart rate variability.31 Overall, the 5.6% decrease in Zrs5Hz associated with the Pco2 increase of 2.7 mm Hg in CART by treatment week 4 was mild and only slightly more than reported from earlier research on laboratory-induced hypercapnia.10
The Controlling Asthma by Training of Capnometry-Assisted Hypoventilation (CATCH) trial is the first, to our knowledge, to examine whether Pco2 levels can be increased in asthma and whether this is associated with therapeutic benefits. It is also the first, to our knowledge, to systematically assess the impact of biofeedback-supported slow breathing on Pco2, showing little change in resting Pco2 and lesser long-term improvements in symptoms. The reported effects are clinically important and show promise in addressing an unmet need in a population where overall level of control remains unsatisfactory. Hypoventilation training for asthma has been advocated for some time,2,12,32 but despite the centrality of elevating Pco2 in its therapeutic rationale, no prior study has targeted Pco2 levels directly, except for our pilot study.17 In the present study, we instituted the most rigorous test possible for Pco2 as the active ingredient in that the comparison group followed the same prescriptions of slow breathing and feedback of RR as CART but was not provided with shallow breathing instructions or feedback of Pco2. Thus, SLOW comprised an active treatment group in itself, as interventions using slow abdominal breathing have been shown to improve the experience of asthma symptoms and quality of life.3,4 Although the aims of SLOW matched those of traditional breathing training, they may also have been enhanced by the use of biofeedback of RR. It is therefore not surprising that a number of variables showed robust time effects. In fact, ACT was improved in both groups equally, suggesting that the Pco2 elevation in CART was not needed to improve overall asthma control. Improvements in both groups were also seen in PEF and PC20, whereas prior clinical trials of breathing and meditation techniques only found effects limited to self-report.3,4,33 Increases in PC20 may indicate more general benefits of any breathing training in this cornerstone of asthma pathophysiology. One prior study showed improvements in PC20 through slow breathing and reduced inspiratory and expiratory duration, although observed changes were smaller than those in the present study and were not replicated.34,35 A potential mechanism may involve a reduction in airway drying by reductions in ventilation. Interestingly, maximum dyspnea during the challenge also increased in both groups. In addition to the higher dose of methacholine, this may be due to an improvement in the perception of airway obstruction, which has been observed as one of the outcomes of successful antiasthma medication treatment.36,37 Distress over methacholine challenge increased in SLOW but not in CART, suggesting that training of Pco2 elevations increased patients’ tolerance to adverse respiratory symptoms, particularly dyspnea, which would be compatible with the demonstrated benefits of CART for panic disorder.15,16
The choice of an active treatment as control instead of treatment as usual could be perceived as a limitation of the present study. SLOW was chosen to account for nonspecific effects of biofeedback and to test whether slow breathing combats hyperventilation, as is often speculated. We did not include treatment as usual because it had not resulted in notable improvements in our CART pilot study,17 although the sample was small. We also elected to exclude patients with recent oral corticosteroid use to avoid the massive effects of corticosteroids on markers of asthma pathophysiology and symptoms.
Having demonstrated benefits in a sample of patients with less severe symptoms paves the way for future studies in more severely affected patients. Although reducing hyperventilation did not appear to be critical for a majority of primary and secondary outcome measures, health benefits of hypercapnic breathing beyond asthma have been proposed or demonstrated,38,39 and potent effects of CART have been shown for the treatment of panic disorder.15,16 Thus, patients with asthma with comorbid anxiety disorders40 could benefit specifically from CART.41 Adaptation of CART or SLOW for children and adolescents, for whom behavioral asthma management techniques are less developed, should be a priority of future intervention studies. Differential efficacy based on various sociodemographic characteristics should also be explored in larger trials. Future basic research needs to elucidate pathways that mediate airway impedance reductions and benefits on symptom distress achieved by CART training, which could involve local inflammatory42 and central limbic (insular) pathways.43
In conclusion, both CART and SLOW show potential as future adjunct treatment options, offering sustained benefits for asthma control. Feedback to train slower breathing confers benefits comparable to CART on two primary outcome variables—asthma control and PEF variability—but does not achieve enduring elevations in Pco2 and is associated with fewer benefits for respiratory impedance during training, more distress during methacholine challenge, and less symptom reduction at follow-up.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: T. R. and A. E. M. served as the principal investigators, had full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. T. R., D. R., A. M. S., M. W. M., and A. E. M. contributed to the study design and implementation, data analysis and interpretation, results review and interpretation, manuscript preparation, and approval of the final manuscript; T. R. and A. E. M. wrote the manuscript; D. R. contributed to the statistical analysis; D. R. and M. W. M. were coinvestigators; D. R. and A. M. S. controlled the database; and A. M. S. was the study coordinator.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Millard has participated in speaking activities for GlaxoSmithKline plc, Boehringer Ingelheim GmbH, AstraZeneca, and Novartis AG. Dr Meuret served on the technical expert panel for the comparative effectiveness review on breathing training by the US Department of Health and Human Services, Agency for Healthcare Research and Quality. Drs Ritz and Rosenfield and Ms Steele have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Other contributions: The authors thank the patients for willingly giving their time to this study; the local research teams for their hard work, especially Noelle Bassi Smith, PhD, Hwatcha C. Kim, PhD, Erica Simon, PhD, Anne Morris, PhD, and Ana F. Trueba, PhD; and Michelle Bill, BA, and William R. Lumry, MD, for assistance in patient physical evaluations.
Additional information: The e-Appendix, e-Figures, and e-Table can be found in the Supplemental Materials section of the online article.
ABBREVIATIONS
- 1MFU
1-month follow-up
- 6MFU
6-month follow-up
- ACT
Asthma Control Test
- CART
capnometry-assisted respiratory training
- PC20
provocation concentration leading to a 20% fall in FEV1
- PEF
peak expiratory flow
- RR
respiratory rate
- SF-12
Survey Form-12
- SLOW
slow breathing and awareness training
- Zrs5Hz
respiratory impedance at 5-Hz oscillation frequency
Footnotes
FUNDING/SUPPORT: This study was supported by the National Heart, Lung, and Blood Institute [Grant R01-HL089761 to Drs Ritz and Meuret].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma.Bethesda, MD: National Heart, Lung, and Blood Institute; 2007 [Google Scholar]
- 2.British Thoracic Society Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. Thorax. 2008;63(suppl 4):iv1-iv121 [DOI] [PubMed] [Google Scholar]
- 3.Holloway EA, West RJ. Integrated breathing and relaxation training (the Papworth method) for adults with asthma in primary care: a randomised controlled trial. Thorax. 2007;62(12):1039-1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas M, McKinley RK, Mellor S, et al. Breathing exercises for asthma: a randomised controlled trial. Thorax. 2009;64(1):55-61 [DOI] [PubMed] [Google Scholar]
- 5.Herxheimer H. Hyperventilation asthma. Lancet. 1946;247(6386):83-87 [DOI] [PubMed] [Google Scholar]
- 6.Thomas M, McKinley RK, Freeman E, Foy C, Price D. The prevalence of dysfunctional breathing in adults in the community with and without asthma. Prim Care Respir J. 2005;14(2):78-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ritz T, Rosenfield D, Meuret AE, Bobb C, Steptoe A. Hyperventilation symptoms are linked to a lower perceived health in asthma patients. Ann Behav Med. 2008;35(1):97-104 [DOI] [PubMed] [Google Scholar]
- 8.Osborne CA, O’Connor BJ, Lewis A, Kanabar V, Gardner WN. Hyperventilation and asymptomatic chronic asthma. Thorax. 2000;55(12):1016-1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McFadden ER, Jr, Lyons HA. Arterial-blood gas tension in asthma. N Engl J Med. 1968;278(19):1027-1032 [DOI] [PubMed] [Google Scholar]
- 10.van den Elshout FJJ, van Herwaarden CLA, Folgering HTM. Effects of hypercapnia and hypocapnia on respiratory resistance in normal and asthmatic subjects. Thorax. 1991;46(1):28-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiler JM, Anderson SD, Randolph C, et al. ; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Pathogenesis, prevalence, diagnosis, and management of exercise-induced bronchoconstriction: a practice parameter. Ann Allergy Asthma Immunol. 2010;105(6 suppl):S1-S47 [DOI] [PubMed] [Google Scholar]
- 12.Bruton A, Lewith GT. The Buteyko breathing technique for asthma: a review. Complement Ther Med. 2005;13(1):41-46 [DOI] [PubMed] [Google Scholar]
- 13.O’Connor E, Patnode CD, Burda BU, et al. Breathing Exercises and/or Retraining Techniques in the Treatment of Asthma: Comparative Effectiveness. Comparative Effectiveness Review No. 71 (Prepared by the Oregon Evidence-based Practice Center under Contract No. 290-2007-10057-I). Rockville, MD: Agency for Healthcare Research and Quality; 2012. AHRQ Publication No. 12-EHC092-EF
- 14.Bowler SD, Green A, Mitchell CA. Buteyko breathing techniques in asthma: a blinded randomised controlled trial. Med J Aust. 1998;169(11-12):575-578 [DOI] [PubMed] [Google Scholar]
- 15.Meuret AE, Wilhelm FH, Ritz T, Roth WT. Feedback of end-tidal pCO2 as a therapeutic approach for panic disorder. J Psychiatr Res. 2008;42(7):560-568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meuret AE, Rosenfield D, Seidel A, Bhaskara L, Hofmann SG. Respiratory and cognitive mediators of treatment change in panic disorder: evidence for intervention specificity. J Consult Clin Psychol. 2010;78(5):691-704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meuret AE, Ritz T, Wilhelm FH, Roth WT. Targeting pCO(2) in asthma: pilot evaluation of a capnometry-assisted breathing training. Appl Psychophysiol Biofeedback. 2007;32(2):99-109 [DOI] [PubMed] [Google Scholar]
- 18.Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59-65 [DOI] [PubMed] [Google Scholar]
- 19.Cloutier MM, Schatz M, Castro M, et al. Asthma outcomes: composite scores of asthma control. J Allergy Clin Immunol. 2012;129(3 suppl):S24-S33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schatz M, Zeiger RS, Yang SJ, et al. The relationship of asthma impairment determined by psychometric tools to future asthma exacerbations. Chest. 2012;141(1):66-72 [DOI] [PubMed] [Google Scholar]
- 21.Steen N, Hutchinson A, McColl E, et al. Development of a symptom based outcome measure for asthma. BMJ. 1994;309(6961):1065-1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ritz T, Bobb C, Edwards M, Steptoe A. The structure of symptom report in asthma: a reevaluation. J Psychosom Res. 2001;51(5):639-645 [DOI] [PubMed] [Google Scholar]
- 23.Wigal JK, Stout C, Brandon M, et al. The knowledge, attitude, and self-efficacy asthma questionnaire. Chest. 1993;104(4):1144-1148 [DOI] [PubMed] [Google Scholar]
- 24.Ware JE, Jr. SF-36 health survey update. Spine. 2000;25(24):3130-3139 [DOI] [PubMed] [Google Scholar]
- 25.Ritz T, Dahme B, Dubois AB, et al. Guidelines for mechanical lung function measurements in psychophysiology. Psychophysiology. 2002;39(5):546-567 [DOI] [PubMed] [Google Scholar]
- 26.American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152(3):1107-1136 [DOI] [PubMed] [Google Scholar]
- 27.Blais R, Grégoire JP, Rouleau R, Cartier A, Bouchard J, Boulet LP; Comité de revue de l’utilisation des médicaments. Ambulatory use of inhaled beta(2)-agonists for the treatment of asthma in Quebec: a population-based utilization review. Chest. 2001;119(5):1316-1321 [DOI] [PubMed] [Google Scholar]
- 28.Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. 2000;31(2):73-86 [DOI] [PubMed] [Google Scholar]
- 29.Hamer RM, Simpson PM. Last observation carried forward versus mixed models in the analysis of psychiatric clinical trials. Am J Psychiatry. 2009;166(6):639-641 [DOI] [PubMed] [Google Scholar]
- 30.Scichilone N, Marchese R, Soresi S, Interrante A, Togias A, Bellia V. Deep inspiration-induced changes in lung volume decrease with severity of asthma. Respir Med. 2007;101(5):951-956 [DOI] [PubMed] [Google Scholar]
- 31.Lehrer PM, Vaschillo E, Vaschillo B, et al. Biofeedback treatment for asthma. Chest. 2004;126(2):352-361 [DOI] [PubMed] [Google Scholar]
- 32.Bruton A, Holgate ST. Hypocapnia and asthma: a mechanism for breathing retraining? Chest. 2005;127(5):1808-1811 [DOI] [PubMed] [Google Scholar]
- 33.Pbert L, Madison JM, Druker S, et al. Effect of mindfulness training on asthma quality of life and lung function: a randomised controlled trial. Thorax. 2012;67(9):769-776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh V, Wisniewski A, Britton J, Tattersfield A. Effect of yoga breathing exercises (pranayama) on airway reactivity in subjects with asthma. Lancet. 1990;335(8702):1381-1383 [DOI] [PubMed] [Google Scholar]
- 35.Cooper S, Oborne J, Newton S, et al. Effect of two breathing exercises (Buteyko and pranayama) in asthma: a randomised controlled trial. Thorax. 2003;58(8):674-679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi IS, Chung SW, Han ER, et al. Effects of anti-asthma therapy on dyspnea perception in acute asthma patients. Respir Med. 2006;100(5):855-861 [DOI] [PubMed] [Google Scholar]
- 37.Salome CM, Reddel HK, Ware SI, et al. Effect of budesonide on the perception of induced airway narrowing in subjects with asthma. Am J Respir Crit Care Med. 2002;165(1):15-21 [DOI] [PubMed] [Google Scholar]
- 38.Laffey JG, Kavanagh BP. Carbon dioxide and the critically ill—too little of a good thing? Lancet. 1999;354(9186):1283-1286 [DOI] [PubMed] [Google Scholar]
- 39.Laffey JG, Kavanagh BP. Hypocapnia. N Engl J Med. 2002;347(1):43-53 [DOI] [PubMed] [Google Scholar]
- 40.Feldman JM, Siddique MI, Morales E, Kaminski B, Lu SE, Lehrer PM. Psychiatric disorders and asthma outcomes among high-risk inner-city patients. Psychosom Med. 2005;67(6):989-996 [DOI] [PubMed] [Google Scholar]
- 41.Meuret AE, Ritz T. Hyperventilation in panic disorder and asthma: empirical evidence and clinical strategies. Int J Psychophysiol. 2010;78(1):68-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis MS, Freed AN. Repeated hyperventilation causes peripheral airways inflammation, hyperreactivity, and impaired bronchodilation in dogs. Am J Respir Crit Care Med. 2001;164(5):785-789 [DOI] [PubMed] [Google Scholar]
- 43.von Leupoldt A, Sommer T, Kegat S, et al. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. Am J Respir Crit Care Med. 2008;177(9):1026-1032 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement