Abstract
BACKGROUND:
Hospitalization is an important outcome in pulmonary arterial hypertension (PAH), shown previously to correlate with survival. Using the Registry to Evaluate Early and Long-term PAH Disease Management (REVEAL Registry), we sought to characterize first-time hospitalizations and their effect on subsequent hospitalization and survival in patients with newly diagnosed disease.
METHODS:
Patients with newly diagnosed PAH (n = 862, World Health Organization group 1) were evaluated for first-time hospitalization. The hospitalizations were categorized as PAH related or PAH unrelated based on the case report form. Categories for PAH-related and PAH-unrelated hospitalization were defined before independent review. Patient demographics and disease characteristics are described as well as freedom from hospitalization and survival.
RESULTS:
Of 862 patients, 490 (56.8%) had one or more hospitalizations postenrollment: 257 (52.4%) PAH related, 214 (43.7%) PAH unrelated, and 19 (3.9%) of undetermined causes. The most common causes of PAH-related hospitalization were congestive heart failure and placement/removal of a central venous catheter. Patients with PAH-related hospitalizations were more likely to receive parenteral therapy, be in functional class III/IV, and have higher risk scores before hospitalization at enrollment. Following discharge, 25.4% ± 3.2% and 31.0% ± 4.0% of patients with PAH-related and PAH-unrelated first hospitalization, respectively, remained hospitalization-free for 3 years (P = .11). Survival estimates at 3 years postdischarge were 56.8% ± 3.5% and 67.8% ± 3.6% (P = .037) for patients with PAH-related and PAH-unrelated hospitalization, respectively.
CONCLUSIONS:
In the REVEAL Registry, PAH-related hospitalization was associated with relatively more rehospitalizations and worse survival at 3 years.
TRIAL REGISTRY:
ClinicalTrials.gov; No.: NCT00370214; URL: www.clinicaltrials.gov
A composite end point encompassing hospitalization is considered a “level 1” end point, that is, a true measure of clinical efficacy.1 Clinical studies of pulmonary arterial hypertension (PAH) have incorporated such composite clinical-worsening end points in their study design,2‐6 including a recent long-term event-driven clinical study that used time to PAH-related death or PAH-related hospitalization as a critical secondary end point.7 Clinical worsening, defined as worsening functional class, ≥ 15% reduction in 6-min walk distance, need for parenteral prostacyclin analog therapy, or all-cause hospitalization, has been found to predict mortality at 1 year after any parameter of clinical worsening is met.8,9 Furthermore, hospitalization among patients with PAH appears to be associated with increased subsequent mortality.10‐12 Hospitalization—and rehospitalization—therefore, represent a substantial burden both for patients with PAH and for the health-care system.13
Reports that characterize hospitalizations and/or calculate subsequent survival posthospitalization in patients with PAH have identified factors at hospital admission that correlate with subsequent survival.10‐12,14 However, it is difficult to generalize these reports to routine practice because of their single-center experience and their focus on only certain causes of PAH (World Health Organization [WHO] group 1 pulmonary hypertension). Also, these reports describe patients with disease-related hospitalizations and not patients with disease-unrelated hospitalizations or no hospitalizations, characterizations that could elucidate the relative burdens of the disease, hospitalization for any reason, and hospitalization specifically related to disease.
The Registry to Evaluate Early and Long-term PAH Disease Management (REVEAL Registry) is an observational registry that provides current information about the demographics, disease course, and management of patients with PAH.15 In this retrospective analysis, we characterize first-time hospitalizations after enrollment among patients with newly diagnosed disease in the REVEAL Registry and describe demographics and clinical characteristics of patients at enrollment and/or diagnosis, by category of hospitalization. We also examine the effect of first-time hospitalization on risk of subsequent hospitalization and on survival.
Materials and Methods
The design of the REVEAL Registry has been described previously.15 Briefly, the REVEAL Registry is a multicenter observational, prospective registry involving 55 university-affiliated and community hospital-based pulmonary hypertension centers in the United States. Each of the participating sites received institutional review board approval (e-Table 1 (525.5KB, pdf) ). Patients with PAH (WHO group 1 pulmonary hypertension; Venice 2003 definition),16 confirmed by right-sided heart catheterization, were enrolled consecutively from March 2006 through December 2009 after providing informed consent.
For this analysis we evaluated only patients with newly diagnosed disease with qualifying right-sided heart catheterization within 90 days of enrollment with hemodynamics measured at rest. Only patients with pulmonary capillary wedge pressure ≤ 15 mm Hg were included.
First-time postenrollment hospitalizations were independently reviewed by three investigators (C. D. B., P. K. L., and R. E. S.) and categorized as PAH related or not primarily related to PAH (hereafter referred to as “PAH unrelated”) based on information in case report forms (dates of hospital admission and discharge, primary discharge diagnosis, reason for hospitalization, disease characteristics, and treatments prior to admission). Secondary diagnoses were not available, so the determination of PAH related vs PAH unrelated was made based only on the primary diagnosis. Categories for PAH-related hospitalization were determined before review, and any one of these categories would have constituted a PAH-related hospitalization. Categories for the PAH-unrelated hospitalizations were also determined before review, but some modifications were made afterward to cover all categories with more than one admission—any single discharge diagnosis that did not fit in a category was classified as “other.” More than one category could have been present at any given admission, and in those situations the primary discharge diagnosis was the determinate of hospitalization causality. Data download for these analyses occurred on February 4, 2013.
Group differences in patient demographics and disease characteristics determined at enrollment were tested with Pearson χ2, Mantel-Haenszel χ2, or t tests for binary, ordinal, and continuous variables, respectively. For variables with highly skewed distributions, the Wilcoxon test was used. Kaplan-Meier estimates for freedom from hospitalization and for survival were computed for distinct at-risk periods: (1) for the full cohort (ie, hospitalized and nonhospitalized patients), the risk period began at enrollment; (2) for patients with a first-time hospitalization, the risk period (for freedom from event following hospitalization) began at hospital discharge; (3) for patients event-free through the first year of follow-up, the at-risk period for future follow-up began at 365 days after enrollment.
Results
Causes of PAH-Related and PAH-Unrelated Hospitalizations
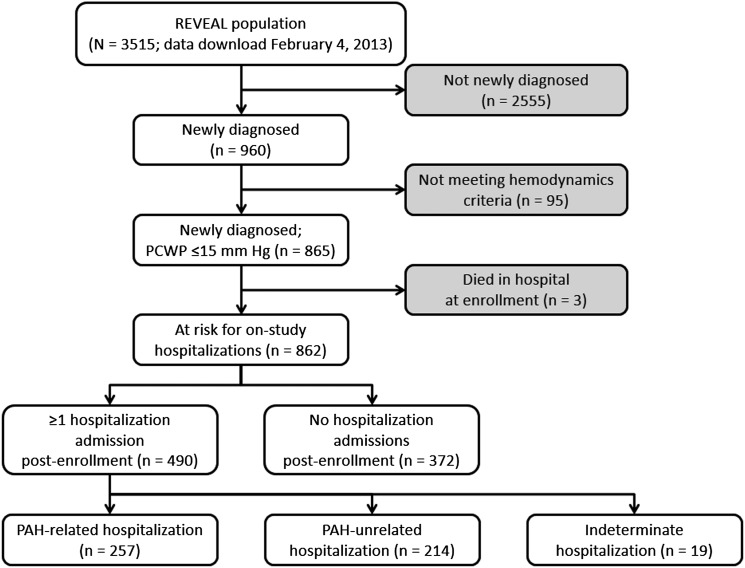
Of 862 patients in the analysis cohort, 490 (56.8%) had at least one hospitalization during follow-up on study, and 372 (43.2%) had none (Fig 1). Of the 490 first-time hospitalizations, 257 (52.4%) were for causes clearly related to PAH, 214 (43.7%) were for PAH-unrelated causes that were considered related to comorbidity, and 19 (3.9%) could not be characterized as PAH related or unrelated because of insufficient data (Fig 1). The median time from right-sided heart catheterization diagnosis to study enrollment was 26 days.
Figure 1 –
Diagram of patients included in the analysis cohort. Patient groups shown in gray boxes were not included in the analysis cohort. PAH = pulmonary arterial hypertension; PCWP = pulmonary capillary wedge pressure; REVEAL = Registry to Evaluate Early and Long-term PAH Disease Management.
Among the 257 patients hospitalized for PAH-related causes, congestive heart failure (n = 81, 31.5%) and placement or removal of a central venous catheter (n = 63, 24.5%) were the two most common causes cited at admission (Table 1). Other common causes for first-time hospitalization were the initial IV line insertion (n = 30, 11.7%), which represents the initiation of IV therapy, and the escalation of therapy for PAH (n = 23, 8.9%). A total of 21 PAH-related hospitalizations (8.2%) were associated with catheter infection: 11 occurring in the first year, three in the second year, and seven in the third year or later.
TABLE 1 ] .
Reasons for First-Time Hospitalization Related and Unrelated to PAH
| Reason | No. (%) |
| Patients with first-time PAH-related hospitalization (n = 257) | |
| Congestive heart failure | 81 (31.5) |
| Placement or removal of central venous catheter | 63 (24.5) |
| Initial IV line insertion | 30 (11.7) |
| Escalation in PAH treatment | 23 (8.9) |
| Catheter infection | 21 (8.2) |
| Syncope | 12 (4.7) |
| Conversion of IV line | 7 (2.7) |
| PH medication-related adverse event | 5 (1.9) |
| Transplant | 5 (1.9) |
| Right-sided heart catheterization | 4 (1.6) |
| Atrial septostomy | 0 (0.0) |
| PAH-related but uncharacterized | 42 (16.3) |
| Patients with first-time PAH-unrelated hospitalization (n = 214) | |
| PAH-unrelated infections (not pneumonia) | 38 (21.1) |
| Pneumonia | 34 (15.9) |
| Surgery/procedure | 24 (11.2) |
| Hemorrhage | 19 (8.9) |
| GI disorder (not hemorrhage or infection) | 12 (5.6) |
| Arrhythmia | 11 (5.1) |
| Respiratory failure | 11 (5.1) |
| Anemia | 10 (4.7) |
| Noncardiac chest pain | 7 (3.3) |
| Respiratory disorder (not pneumonia or respiratory failure) | 7 (3.3) |
| Sepsis | 7 (3.3) |
| Trauma/fracture | 7 (3.3) |
| Electrolyte abnormality | 6 (2.8) |
| Renal failure | 5 (2.3) |
| Hemoptysis | 4 (1.9) |
| Neurologic disorder (not stroke) | 4 (1.9) |
| Acute coronary syndromea | 4 (1.9) |
| Hepatic failure | 2 (0.9) |
| Ischemic stroke | 2 (0.9) |
| Pain (not chest pain) | 2 (0.9) |
| Psychiatric disorder | 2 (0.9) |
| Thromboembolism | 4 (1.9) |
| Other | 15 (7.0) |
Reasons are not mutually exclusive; a patient could have been hospitalized for more than one reason. PAH = pulmonary arterial hypertension; PH = pulmonary hypertension.
Includes angina and myocardial infarction.
Among the 214 patients hospitalized for PAH-unrelated causes, non-line-related infections were cited most commonly: PAH-unrelated infections (n = 38, 21.1%) and pneumonia (n = 34, 15.9%) (Table 1). Other common diagnoses at the time of first hospitalization were surgery/procedures (n = 24, 11.2%) and hemorrhage (n = 19, 8.9%).
Patient Demographics and Disease Characteristics at Enrollment That Predict Hospitalization
Patients hospitalized for any reason were more likely than patients not hospitalized to have comorbidities (diabetes and depression) and to have more severe PAH at enrollment, as measured by functional class, presence of pericardial effusion, higher mean atrial pressure, lower cardiac index, and higher REVEAL Registry risk score. Patients with hospitalizations also had significantly longer follow-up from enrollment than patients without hospitalizations (Table 2).
TABLE 2 ] .
Patient Demographics and Disease Characteristics at Enrollment
| Parameter | Patients With ≥ 1 Hospitalization | Patients With No Hospitalizations (D) | P Value (C vs D) | P Value (A vs B) | ||
| PAH Related (A) | PAH Unrelated (B) | Alla (C) | ||||
| No. of patientsb | 257 | 214 | 471 | 372 | … | … |
| Mean age (SD), y | 51.9 (16.0) | 52.4 (17.0) | 52.1 (16.4) | 52.6 (17.6) | .70c | .73c |
| Age < 18 y, No. (%) | 3 (1.2) | 9 (4.2) | 12 (2.5) | 13 (3.5) | .42d | .037d |
| Age ≥ 65 y, No. (%) | 63 (24.5) | 46 (21.5) | 109 (23.1) | 95 (25.5) | .42d | .44d |
| Female, No. (%) | 201 (78.2) | 159 (74.3) | 360 (76.4) | 283 (76.1) | .90d | .32d |
| PAH cause | ||||||
| Idiopathic PAH | 128 (49.8) | 94 (43.9) | 222 (47.1) | 181 (48.7) | .37d | .38d |
| Familial PAH | 9 (3.5) | 5 (2.3) | 14 (3.0) | 7 (1.9) | … | … |
| Associated PAH | … | … | ||||
| CTD | 68 (26.5) | 74 (34.6) | 142 (30.1) | 108 (29.0) | ||
| CHD | 13 (5.1) | 10 (4.7) | 23 (4.9) | 21 (5.6) | ||
| PoPH | 17 (6.6) | 19 (8.9) | 36 (7.6) | 18 (4.8) | ||
| HIV | 5 (1.9) | 2 (0.9) | 7 (1.5) | 6 (1.6) | ||
| Drugs/toxins | 13 (5.1) | 7 (3.3) | 20 (4.2) | 20 (5.4) | ||
| Other | 2 (0.8) | 3 (1.4) | 5 (1.1) | 4 (1.1) | ||
| PVOD | 2 (0.8) | 0 (0.0) | 2 (0.4) | 7 (1.9) | ||
| Comorbidities, No. (%) | ||||||
| Diabetes | 42 (16.9) | 31 (14.8) | 73 (16.0) | 38 (10.6) | .025d | .54d |
| Hypertension | 105 (42.3) | 104 (49.8) | 209 (45.7) | 151 (41.9) | .28d | .11d |
| COPD | 36 (14.5) | 34 (16.3) | 70 (15.3) | 41 (11.4) | .10d | .60d |
| Depression | 51 (20.6) | 48 (23.0) | 99 (21.7) | 52 (14.4) | .008d | .54d |
| Pre-enrollment parenteral therapy | ||||||
| No. % | 57 (22.2) | 37 (17.3) | 94 (20.0) | 31 (8.3) | < .001 | .19 |
| Median days pre-enrollment | 17 | 33 | 24 | 28 | .75 | .088 |
| NYHA functional class, No. (%) | ||||||
| I | 9 (4.0) | 5 (2.7) | 14 (3.4) | 15 (4.8) | .014e | .034e |
| II | 41 (18.2) | 46 (25.1) | 87 (21.3) | 80 (25.8) | … | … |
| III | 128 (56.9) | 113 (61.7) | 241 (59.1) | 183 (59.0) | … | … |
| IV | 47 (20.9) | 19 (10.4) | 66 (16.2) | 32 (10.3) | … | … |
| 6MWD, m | ||||||
| No. of patients | 157 | 148 | 305 | 226 | .12c | .34c |
| Mean (SD) | 311.5 (124.9) | 298.5 (112.4) | 305.2 (119.0) | 322.5 (137.2) | … | … |
| BNP, pg/mL | ||||||
| No. of patients | 106 | 103 | 209 | 147 | .18f | .36f |
| Mean (SD) | 463.5 (583.4) | 457.0 (742.7) | 460.3 (665.1) | 491.6 (1070.2) | … | … |
| Median (IQR) | 262.5 (87.0-592.0) | 203.0 (81.0-530.0) | 231.0 (86.0-551.0) | 176.0 (64.0-502.0) | … | … |
| Pericardial effusion, No. (%) | 70 (27.2) | 52 (24.3) | 122 (25.9) | 63 (16.9) | .002d | .47d |
| Hemodynamics | ||||||
| Cardiac index, L/min/m2 | ||||||
| No. of patients | 203 | 168 | 371 | 290 | .018c | .026c |
| Mean (SD) | 2.1 (0.8) | 2.3 (0.8) | 2.2 (0.8) | 2.4 (0.9) | … | … |
| PVR, Wood units | ||||||
| No. of patients | 242 | 202 | 444 | 350 | .20c | .15c |
| Mean (SD) | 12.0 (6.0) | 10.5 (6.3) | 11.3 (6.1) | 10.8 (6.2) | … | … |
| mPAP at rest | ||||||
| No. of patients | 245 | 205 | 450 | 354 | .16c | .076c |
| Mean (SD) | 51.1 (12.9) | 48.9 (13.1) | 50.1 (13.0) | 48.8 (13.5) | … | … |
| mRAP, mm Hg | ||||||
| No. of patients | 225 | 186 | 411 | 323 | < .001c | .34c |
| Mean (SD) | 10.6 (6.5) | 10.0 (5.7) | 10.3 (6.2) | 8.8 (5.5) | … | … |
| REVEAL Registry risk score | ||||||
| No. of patients | 257 | 214 | 471 | 372 | < .001c | .84c |
| Mean (SD) | 8.6 (2.1) | 8.6 (2.1) | 8.6 (2.1) | 7.9 (2.2) | … | … |
| Length of follow-up from enrollment, mo | ||||||
| No. of patients | 257 | 214 | 471 | 372 | < .001c | .004c |
| Mean (SD) | 39.6 (22.6) | 45.3 (19.3) | 42.2 (21.3) | 35.6 (22.1) | … | … |
All patients with ≥ 1 hospitalization (C) includes patients with PAH-related (A) and PAH-unrelated (B) hospitalizations. Nineteen patients with undetermined causes of hospitalization were not included in this cohort. 6MWD = 6-min walk distance; BNP = brain natriuretic peptide; CHD = congenital heart disease; CTD = connective tissue disease; IQR = interquartile range; mPAP = mean pulmonary arterial pressure; mRAP = mean right atrial pressure; NYHA = New York Heart Association; PoPH = portopulmonary hypertension; PVOD = pulmonary veno-occlusive disease; PVR = pulmonary vascular resistance. See Table 1 legend for expansion of other abbreviation.
The total number of patients in each cohort with data at enrollment for each parameter is assumed to be 257 (A), 214 (B), 471 (C), and 372 (D), unless otherwise noted.
P value is computed using t test.
P value is computed using χ2 test.
P value is computed using Mantel-Haenszel test.
P value is computed using Wilcoxon signed rank sum test.
Comparison between PAH-related and PAH-unrelated groups revealed few differences at enrollment. In particular, patients with PAH-related hospitalizations were less likely than patients with PAH-unrelated hospitalizations to be < 18 years of age and more likely to have more severe PAH, as measured by functional class and lower cardiac index. Length of follow-up from enrollment was also shorter for the PAH-related group (Table 2).
Characterization of PAH-Related and PAH-Unrelated Hospitalizations
Hospitalization-related characteristics were similar overall between the PAH-related and PAH-unrelated groups, with the notable exception that patients with PAH-related hospitalizations were more likely to be on parenteral therapy, to be in functional class III or IV prior to hospitalization, and to have a higher REVEAL Registry risk score prior to hospitalization (Table 3). Further description of the type and timing of PAH-related hospitalizations, including distribution of specific causes for hospitalization over time, is provided in the online supplement (e-Figs 1, 2 (525.5KB, pdf) , e-Table 2 (525.5KB, pdf) ).
TABLE 3 ] .
Characterization of Hospitalizations, by Type of Hospitalization
| Parameter | PAH Related (A) | PAH Unrelated (B) | Alla (C) | P Value (A vs B) |
| No. of patientsb | 257 | 214 | 471 | … |
| Length of stay, d | ||||
| No. of patients | 248 | 202 | 450 | .13c |
| Mean (SD) | 6.5 (7.9) | 10.3 (38.7) | 8.2 (26.6) | … |
| Median | 4.0 | 4.0 | 4.0 | … |
| Length of stay ≥ 7 d, No. (%) | 73 (29.4) | 54 (26.7) | 127 (28.2) | .53d |
| Total hospital days in the year after first admission | ||||
| No. of patients | 248 | 202 | 450 | .47c |
| Mean (SD) | 14.3 (24.7) | 16.6 (42.6) | 15.3 (33.9) | … |
| Median | 7.5 | 6.5 | 7.0 | … |
| Total days ≥ 14 d, No. (%) | 74 (28.8) | 48 (22.4) | 122 (25.9) | .12d |
| No. of readmissions during study | ||||
| No. of patients | 257 | 214 | 471 | .42c |
| Mean (SD) | 1.8 (2.6) | 2.1 (3.7) | 1.9 (3.1) | … |
| None, No. (%) | 93 (36.2) | 90 (42.1) | 183 (38.9) | … |
| 1 readmission, No. (%) | 71 (27.6) | 47 (22.0) | 118 (25.1) | … |
| ≥ 2 readmissions, No. (%) | 93 (36.2) | 77 (36.0) | 170 (36.1) | … |
| Average length of follow-up from hospital discharge, y | ||||
| No. of patients | 241 | 211 | 452 | .91c |
| Mean (SD) | 2.2 (1.7) | 2.2 (1.5) | 2.2 (1.6) | … |
| Hospitalization rate, No. per year of follow-up | ||||
| No. of patients | 241 | 211 | 452 | .10c |
| Mean (SD) | 2.2 (5.6) | 1.5 (3.0) | 1.8 (4.6) | … |
| No. of PAH medications at admission, No. (%) | ||||
| None | 26 (10.1) | 26 (12.1) | 52 (11.0) | .82d |
| Monotherapy | 133 (51.8) | 106 (49.5) | 239 (50.7) | … |
| Double therapy | 79 (30.7) | 69 (32.2) | 148 (31.4) | … |
| Triple therapy | 19 (7.4) | 13 (6.1) | 32 (6.8) | … |
| Parenteral therapy | 111 (45.5) | 43 (21.6) | 154 (34.8) | < .001d |
| Warfarin use | 76 (29.9) | 48 (22.5) | 124 (26.6) | .072d |
| Diuretic use | 120 (47.2) | 85 (39.9) | 205 (43.9) | .11d |
| Most recent FC assessment prior to hospitalization, No. (%) | ||||
| FC I | 12 (4.9) | 7 (3.5) | 19 (4.3) | .003e |
| FC II | 58 (23.8) | 71 (35.9) | 129 (29.2) | … |
| FC III | 136 (55.7) | 107 (54.0) | 243 (55.0) | … |
| FC IV | 38 (15.6) | 13 (6.6) | 51 (11.5) | … |
| REVEAL Registry risk score prior to hospitalization | ||||
| No. of patients | 257 | 214 | 471 | .018c |
| Mean (SD) | 8.5 (2.3) | 8.0 (2.5) | 8.3 (2.4) | … |
| In-hospital death, No. (%) | 14 (5.4) | 3 (1.4) | 17 (3.6) | .024f |
All patients with ≥ 1 hospitalization (C) includes patients with PAH-related (A) and PAH-unrelated (B) hospitalizations. Nineteen patients with undetermined causes of hospitalization were not included in this cohort. FC = New York Heart Association functional class. See Table 1 legend for expansion of other abbreviation.
The total number of patients in each cohort with data at enrollment for each parameter is assumed to be 257 (A), 214 (B), and 471 (C), unless otherwise noted.
P value is computed using t test.
P value is computed using χ2 test.
P value is computed using Mantel-Haenszel test.
P value is computed using Fisher exact test.
Freedom From Hospitalization
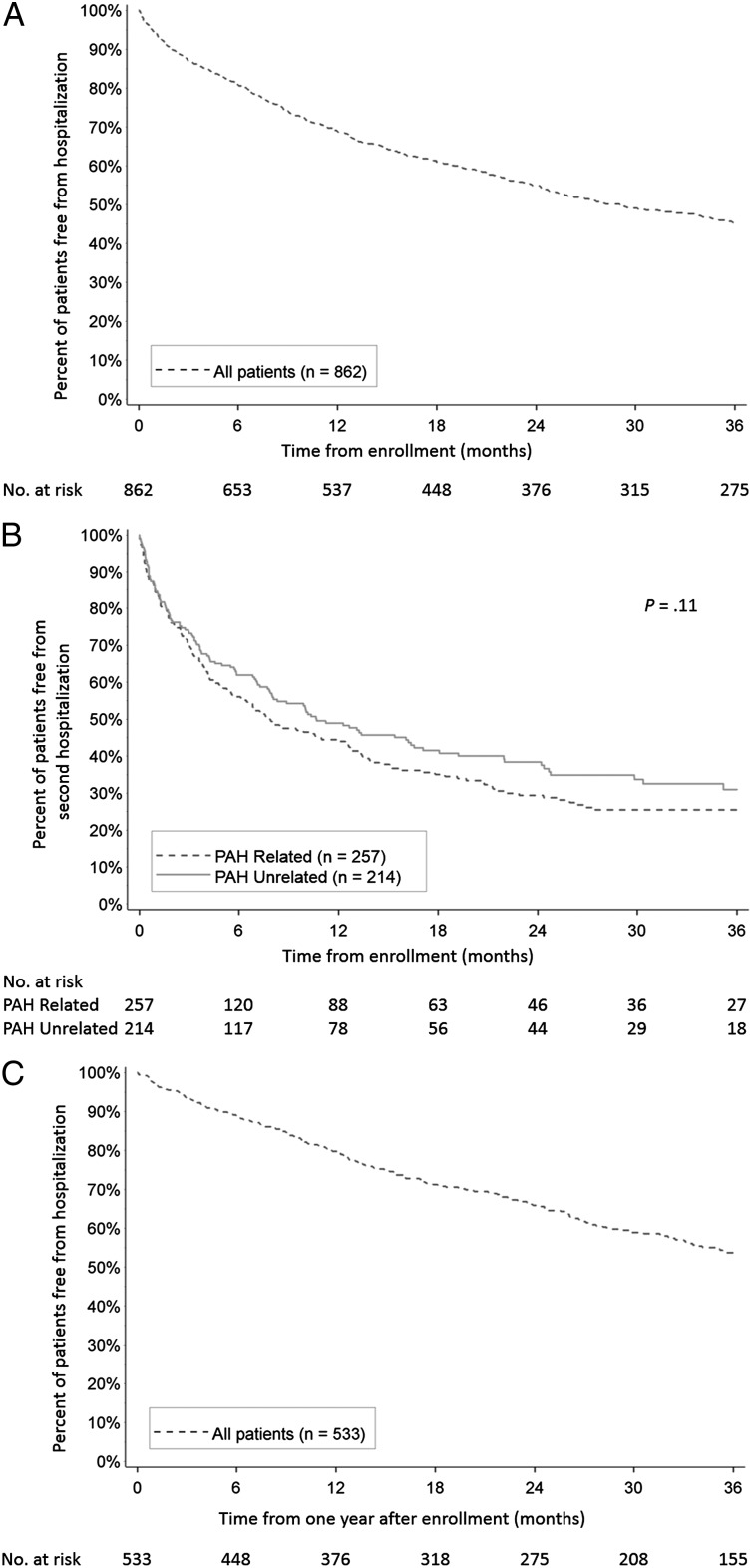
For the entire analysis cohort at 3 years from enrollment, 45.4% ± 1.8% of patients remained free from hospitalization (Fig 2A). Among patients who were hospitalized and discharged alive following the first hospitalization, 25.4% ± 3.2% of patients with PAH-related and 31.0% ± 4.0% of patients with PAH-unrelated first hospitalization remained free from a second hospitalization at 3 years postdischarge (P = .11) (Fig 2B). For patients who remained hospitalization-free for 1 year postenrollment, 53.7% ± 2.5% remained free from admission after an additional 3 years of follow-up (Fig 2C).
Figure 2 –
A-C, Kaplan-Meier estimates of freedom from hospitalization for (A) all patients from time of enrollment; (B) patients with a first-time hospitalization from time of discharge, by type of hospitalization; and (C) patients with no hospitalization in the first year of follow-up (including patients with no hospitalizations and patients with first-time hospitalizations occurring after the first year of follow-up) from 1 y after enrollment. See Figure 1 legend for expansion of abbreviation.
Survival
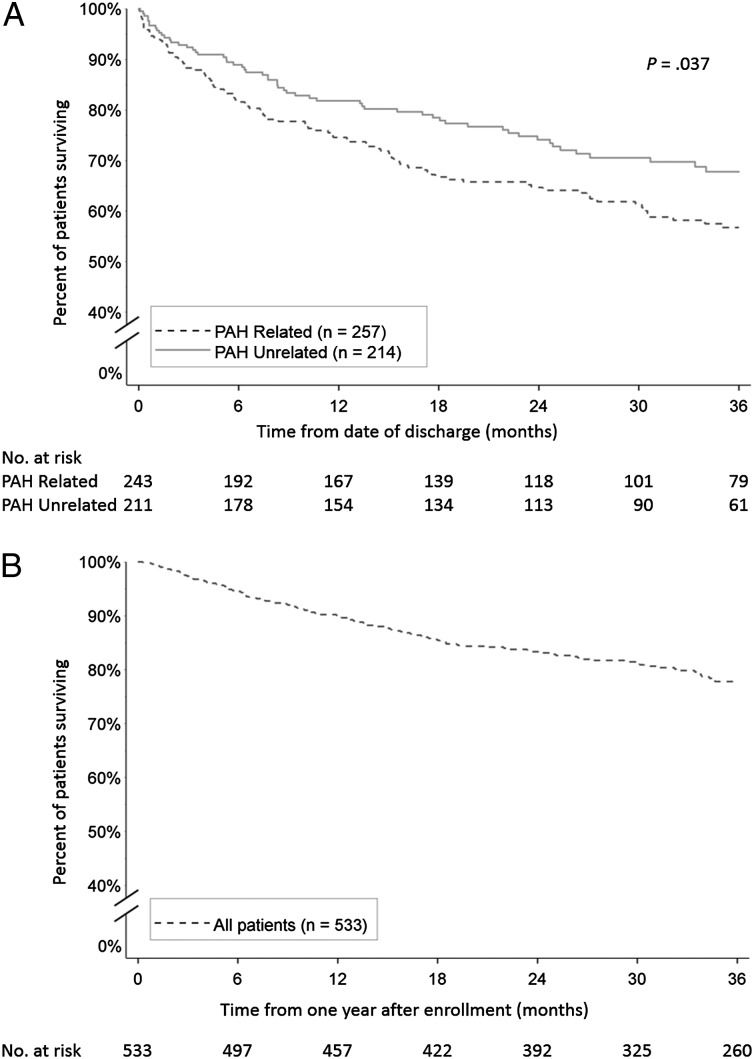
In-hospital mortality was significantly higher for PAH-related hospitalizations compared with PAH-unrelated hospitalizations (5.4% vs 1.4%, P = .024) (Table 3). Among those discharged alive following first-time hospitalization, the survival estimate at 3 years postdischarge was lower for patients with PAH-related hospitalization than for patients with PAH-unrelated hospitalization (56.8% ± 3.5% vs 67.8% ± 3.6%, P = .037) (Fig 3A). Among patients who remained hospitalization-free for 1 year postenrollment, survival after 3 additional years of follow-up was 77.8% ± 1.9% (Fig 3B).
Figure 3 –
A, B, Kaplan-Meier estimates of survival for (A) patients with a first-time hospitalization from time of discharge, by type of hospitalization; and (B) patients with no hospitalization in the first year of follow-up (including patients with no hospitalizations and patients with first-time hospitalizations occurring after the first year of follow-up) from 1 y after enrollment. See Figure 1 legend for expansion of abbreviation.
Discussion
The REVEAL Registry represents the largest database so far of patients newly diagnosed with PAH (WHO group 1) analyzed for characteristics of first-time hospitalization. The burden of disease in patients with PAH is substantial, as evidenced by the high incidence of hospitalization (57%) in this cohort of patients with newly diagnosed disease from the prospective REVEAL Registry. Our findings clearly demonstrate that all-cause hospitalization is very common among patients with newly diagnosed disease. In particular, the rate of PAH-unrelated hospitalizations in this REVEAL Registry cohort is relatively high, suggesting that the term “PAH-unrelated” is a misnomer and that this category of hospitalization actually reflects a degree of risk conferred by the PAH comorbidity. For example, a primary discharge diagnosis of “pneumonia” on the case report form would result in a categorization of that patient’s hospitalization as PAH-unrelated. However, if PAH predisposes the patient to non-line-related infections such as pneumonia or if an infection such as pneumonia were to precipitate congestive heart failure, then one could argue that the hospitalization is truly PAH related. The current study is unable to address that methodologic challenge. Although 53% of patients had first-time hospitalizations that were clearly related to PAH, it is unclear what proportion of so-called “PAH-unrelated” hospitalizations were truly incidental or precipitated by the PAH comorbidity to some extent.
Patients in this analysis who were hospitalized for any reason had a higher prevalence of comorbidities and more severe PAH at enrollment, as measured by functional class, pericardial effusion, mean right atrial pressure, and REVEAL Registry risk score, compared with patients without hospitalizations. Fewer differences at enrollment distinguished between patients with PAH-related and PAH-unrelated hospitalizations, although patients with PAH-related hospitalizations presented with more severe PAH at the time of first admission. The lack of differences between the PAH-related and PAH-unrelated groups at enrollment is an important point, given our finding that both in-hospital and postdischarge survival are significantly worse for patients hospitalized for PAH-related reasons, despite the similar frequencies at which PAH-related and PAH-unrelated hospitalizations occur. The worse outcomes were seen in the PAH-related group despite > 46% use of parenteral prostanoids at the time of the first hospitalization compared with 22% in the PAH-unrelated group.
Hospital readmission is common for patients with PAH, especially those whose first hospitalization was PAH related. Only 25.4% of patients discharged after a PAH-related hospitalization remained free from readmission 3 years later. Furthermore, hospitalization for any cause increases overall risk of death, an association that has been documented previously.8 Even for patients with no hospitalizations in the first year after enrollment, almost one-half experience at least one hospitalization over the subsequent 3 years, despite treatment of PAH, indicating that surviving the first year hospitalization-free does not strongly relate to the likelihood of future hospitalization.
Mean total hospital days in the year after first admission (inclusive of first admission) for all patients with at least one hospitalization was 15.3 days (median, 7.0 days), which represents a significant burden for both the health-care system as a whole and for individual patients. For our analysis, we did not evaluate the burden of cost for PAH-related hospitalization. One retrospective study estimated total per-patient per-month costs (including inpatient and outpatient costs) at $4,021 for patients with PAH.17 Extrapolating from that figure, we calculate that total costs for PAH were about $188 million in 2012 (assuming a prevalence of 12.4 cases per million18 and 313.9 million people in the United States in 201219). Based on the attention currently paid to left-sided heart failure by regulatory and reimbursement third parties,20 for which total costs are estimated at $30 billion in the United States in 2013,21 one might expect similar emphasis to be directed at PAH-related hospitalizations, particularly those for heart failure.
This analysis may have been limited by several factors. First, the analysis presented here was retrospectively determined and may be limited by the biases inherent to any analysis that is not fully prespecified prospectively before the data are collected. This includes the selection of the cohort for the analysis. We chose to focus on patients with newly diagnosed disease to minimize survivor bias, but it must be noted that there was, typically, a delay of about 1 month between diagnosis and enrollment. Perhaps more importantly, at the time of the first hospitalization, patients no longer had newly diagnosed disease, but rather those results should be generalized to patients being discharged after their first postdiagnosis hospitalization.
Second, the categorization of hospitalization as PAH related or unrelated was made based on specific retrospective data available from the case report form, and in 42 cases (16.3%) of hospitalization causality related to PAH could not be determined. Access to full patient charts was not available at the time of investigator review of the data. Third, the hospitalization subcategories overlapped. For example, “placement or removal of a central venous catheter” may overlap with “initial IV line insertion.” Some of the overlap was mitigated by ensuring that the category “PAH-unrelated infection” excluded both “catheter infection” (PAH-related) and “pneumonia” (PAH-unrelated hospitalization), so that these three categories of infection were considered separately. Fourth, the significantly longer period of follow-up for patients with hospitalizations, compared with patients without hospitalizations, may be a source of bias, since the likelihood of a hospitalization event increases with time.
Conclusions
PAH is associated with a considerable burden of disease (as measured by all-cause hospitalization). Patients who are more severely ill (ie, with more severe PAH or multiple comorbidities) are at higher risk for all-cause hospitalization. The degree of freedom from hospitalization is very poor after the first hospitalization, regardless of the cause. Prognosis is relatively better for patients with no hospitalizations in the first year or first hospitalization occurring after 1 year, but it nevertheless remains poor.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: C. D. B. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. D. P. M., C. D. B., and W. W. B., contributed to the design of the statistical analysis plan; C. D. B., P. K. L., R. E. S., and D. P. M. contributed to performing data analysis; M. R. S. and D. P. M. contributed to providing statistical analysis of the data reported in the study; C. D. B., P. K. L., M. R. S., M. D. M., D. P. M., A. J. R., W. W. B., and R. E. S. contributed to reviewing the data analysis for clinical relevance; C. D. B. W. W. B., D. M. P., and R. E. S. contributed to reviewing pertinent literature; and C. D. B., P. K. L., M. R. S., M. D. M., D. P. M., A. J. R., W. W. B., and R. E. S. contributed to discussions of the data and drafting of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Burger has received research grant support as a participant in multicenter pharmaceutical clinical studies from Actelion Pharmaceuticals Ltd; Gilead Sciences, Inc; and United Therapeutics Corporation. He has served on advisory board committees for Actelion Pharmaceuticals US, Inc and Gilead Sciences, Inc. Ms Long has worked as a clinical research coordinator funded in part by research grant support for multicenter pharmaceutical clinical studies from Actelion Pharmaceuticals Ltd; Gilead Sciences, Inc; and United Therapeutics Corporation. Mr Shah is an employee of ICON Clinical Research, a company that receives research support from Actelion Pharmaceuticals US, Inc. Dr McGoon’s institution has received research funding from Medtronic, Inc and Gilead Sciences, Inc. He has served on advisory, steering and/or end point/data and safety monitoring board committees for Actelion Pharmaceuticals US, Inc; Gilead Sciences, Inc; Lung LLC; and GlaxoSmithKline. He has received honoraria for speaking at conferences supported by Actelion Pharmaceuticals US, Inc and Gilead Sciences, Inc. Mr Miller is an employee of ICON Clinical Research, a company that receives funding from Actelion Pharmaceuticals US, Inc and acts as a BioStatistical CRO for the REVEAL Registry. Dr Romero is an employee of Actelion Pharmaceuticals US, Inc. Dr Benton is an employee of Actelion Pharmaceuticals US, Inc. Dr Safford has received research grant support as a participant in multicenter pharmaceutical clinical studies from Actelion Pharmaceuticals Ltd; Gilead Sciences, Inc; and United Therapeutics Corporation.
Role of sponsors: The sponsor, Actelion Pharmaceuticals US Inc, provided the study design, statistical analysis plan, and management of study registry and participated in data analysis, interpretation, and preparation of manuscript.
Other contributions: Assistance in writing the first draft of the manuscript was provided by a professional medical writer, Anna Lau, PhD, of Percolation Communications LLC, and paid by the sponsor. The authors thank Simona Neumann, PhD, of Actelion Pharmaceuticals US, Inc, for final draft writing support.
Additional information: The e-Figures and e-Tables can be found in the Supplemental Materials section of the online article.
ABBREVIATIONS
- PAH
pulmonary arterial hypertension
- REVEAL Registry
Registry to Evaluate Early and Long-term PAH Disease Management
- WHO
World Health Organization
Footnotes
FUNDING/SUPPORT: Actelion Pharmaceuticals US Inc. is the sponsor of the REVEAL Registry and provided funding and support for the analysis presented.
References
- 1.Fleming TR, Powers JH. Biomarkers and surrogate endpoints in clinical trials. Stat Med. 2012;31(25):2973-2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galiè N, Ghofrani HA, Torbicki A, et al. ; Sildenafil Use in Pulmonary Arterial Hypertension (SUPER) Study Group. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353(20):2148-2157 [DOI] [PubMed] [Google Scholar]
- 3.Galiè N, Humbert M, Vachiéry JL, et al. ; Arterial Pulmonary Hypertension and Beraprost European (ALPHABET) Study Group. Effects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol. 2002;39(9):1496-1502 [DOI] [PubMed] [Google Scholar]
- 4.Galiè N, Rubin Lj, Hoeper M, et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet. 2008;371(9630):2093-2100 [DOI] [PubMed] [Google Scholar]
- 5.Jing ZC, Yu ZX, Shen JY, et al. ; Efficacy and Safety of Vardenafil in the Treatment of Pulmonary Arterial Hypertension (EVALUATION) Study Group. Vardenafil in pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med. 2011;183(12):1723-1729 [DOI] [PubMed] [Google Scholar]
- 6.McLaughlin VV, Oudiz RJ, Frost A, et al. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2006;174(11):1257-1263 [DOI] [PubMed] [Google Scholar]
- 7.Pulido T, Adzerikho I, Channick RN, et al. ; SERAPHIN Investigators. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369(9):809-818 [DOI] [PubMed] [Google Scholar]
- 8.Frost AE, Badesch DB, Miller DP, Benza RL, Meltzer LA, McGoon MD. Evaluation of the predictive value of a clinical worsening definition using 2-year outcomes in patients with pulmonary arterial hypertension: a REVEAL Registry analysis. Chest. 2013;144(5):1521-1529 [DOI] [PubMed] [Google Scholar]
- 9.Talavera ML, Favaloro LF, Diez MD, Peradejordis MP, Huerta CH, Favaloro RF. Prognostic value of heart failure hospitalization in pulmonary hypertension [abstract]. Eur J Heart Fail. 2011;10(S1):S158 [Google Scholar]
- 10.Campo A, Mathai SC, Le Pavec J, et al. Outcomes of hospitalisation for right heart failure in pulmonary arterial hypertension. Eur Respir J. 2011;38(2):359-367 [DOI] [PubMed] [Google Scholar]
- 11.Haddad F, Peterson T, Fuh E, et al. Characteristics and outcome after hospitalization for acute right heart failure in patients with pulmonary arterial hypertension. Circ Heart Fail. 2011;4(6):692-699 [DOI] [PubMed] [Google Scholar]
- 12.Sztrymf B, Souza R, Bertoletti L, et al. Prognostic factors of acute heart failure in patients with pulmonary arterial hypertension. Eur Respir J. 2010;35(6):1286-1293 [DOI] [PubMed] [Google Scholar]
- 13.Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart failure–associated hospitalizations in the United States. J Am Coll Cardiol. 2013;61(12):1259-1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domsic RT, Chung L, Gordon JK, Cloonan Y, Steen VD. PHAROS Investigators. Survival, hospitalization or need for combination therapy at one year in patients with scleroderma-associated pulmonary arterial hypertension [abstract]. Arthritis Rheum. 2012;64(S10):S310-S311 [Google Scholar]
- 15.McGoon MD, Krichman A, Farber HW, et al. Design of the REVEAL registry for US patients with pulmonary arterial hypertension. Mayo Clin Proc. 2008;83(8):923-931 [DOI] [PubMed] [Google Scholar]
- 16.Simonneau G, Galiè N, Rubin LJ, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43(12suppl S):5S-12S [DOI] [PubMed] [Google Scholar]
- 17.Said Q, Martin BC, Joish VN, Kreilick C, Mathai SC. The cost to managed care of managing pulmonary hypertension. J Med Econ. 2012;15(3):500-508 [DOI] [PubMed] [Google Scholar]
- 18.Rossano JW, Crystal MA, Morales DL, et al. Increasing prevalence and decreasing mortality in pulmonary hypertension related hospitalizations in the United States: an analysis of over 20,000 hospital admissions [abstract]. Circulation. 2011;124:A9251 [Google Scholar]
- 19.State & county quick facts: USA. US Census Bureau website. http://quickfacts.census.gov/qfd/states/00000.html. Accessed December 16, 2013
- 20.Sieck S. The economics and reimbursement of congestive heart failure. In: Peacock WF, ed. Short Stay Management of Acute Heart Failure. New York, NY: Humana Press; 2012:9-32 [Google Scholar]
- 21.Go AS, Mozaffarian D, Roger VL, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6-e245 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement