Abstract
Physical and sexual assault during adolescence is a potent risk factor for mental health and psychosocial problems, as well as revictimization, especially among female victims. To better understand this conferred risk, we conducted an exploratory study comparing assaulted and non-assaulted girls’ behavioral and brain responses during a trust learning task. Adolescent girls (14 assaulted, 16 non-assaulted) performed a functional magnetic resonance imaging task that manipulated the percentages of which three different faces delivered positive and negative outcomes. Analyses focused on comparing unexpected to expected outcomes. We found that assaulted adolescent girls demonstrated less behavioral slowing in response to unexpected negative social outcomes, or trust violations (i.e., when a presumably trustworthy face delivered a negative outcome), relative to control girls. Trust violations were also associated with less activation in anterior insular and anterior cingulate regions - regions implicated in reinforcement learning - among the assaulted group compared to the control group. Furthermore, we found that the severity of participants’ exposure to assaultive events scaled negatively with recruitment of these regions. These preliminary results suggest that assault victims may engage aberrant learning processes (e.g., diminishment of prediction error signals) upon unexpected negative social outcomes. These findings have implications for understanding impaired trust learning and social functioning among assault victims.
Keywords: Adolescence, Functional magnetic resonance imaging (fMRI), Trauma, Learning, Trust
1. Introduction
Assaultive violence exposure is a significant problem among adolescents due to both its prevalence and its conferred risk for negative outcomes. The National Survey of Adolescents found a national prevalence rate of 47% for exposure to physical assault, sexual assault, or witnessed violence among adolescents aged 12–17 (Kilpatrick et al., 2000; Kilpatrick et al., 2003). Exposure to assaultive violence among adolescents is strongly associated with (1) an increased risk for mental health disorders, including posttraumatic stress disorder (PTSD), depression, and substance use (Kilpatrick et al., 2000; Kilpatrick et al., 2003; Wolitzky-Taylor et al., 2008; Danielson et al., 2009; Cisler et al., 2011b, 2011a; Cisler et al., 2012) and (2) an increased risk for subsequent exposures to assaultive violence in later adolescence and adulthood (Cougle et al., 2009; Cisler et al., 2011b).
Betrayal trauma events, which are characterized by an assault that violates or betrays an extant interpersonal trust relationship between the perpetrator and victim, seem to pose a particular psychosocial risk. When they occur in childhood and adolescence, these events are associated with a greater incidence of high-betrayal assaultive events in later life (Gobin and Freyd, 2009), suggesting a pre- or post-morbid deficit in correctly evaluating (i.e., learning) trustworthiness of conspecifics and social situations and/or adaptively responding to violations of trust. In support of this, prior studies have found that young adult women with prior exposure to interpersonal violence demonstrated higher thresholds for judging social situations as risky and reported less response effectiveness in risky social situations (Wilson et al., 1999; Gidycz et al., 2006; Yeater et al., 2010; Yeater and Viken, 2010; Yeater et al., 2011). It has also been found that adults who retrospectively reported high betrayal trauma demonstrated greater errors in detecting violations to rules involving social exchange and/or safety precaution (DePrince, 2005). Collectively, these behavioral findings suggest that exposure to interpersonal or assaultive violence is associated with an impairment of cognitive processes that underlie social decision-making and that this impairment is a possible cognitive mechanism by which assault victims become predisposed to revictimization. The present study sought to further investigate this association between exposure to interpersonal violence and impaired social decision-making by using a functional magnetic resonance imaging (fMRI) social contingency “trust” learning paradigm. This novel task manipulated the differential trustworthiness of same-sex conspecific faces in order to observe and compare assaulted and non-assaulted girls according to their behavioral and neural responses to violations of social expectations.
Based on existing literature concerning social-cognitive differences among assault victims, we hypothesized that interpersonal assaultive violence exposure among adolescent girls would be associated with diminished behavioral and brain responses to social expectancy violations – that is, when a conspecific behaves contrary to what prior behavior would predict. Reduced reactivity to unexpected behavior could provide one possible mechanism through which prior assault exposure alters social decision-making processes to confer risk for future victimization. For this initial exploratory study, we focused here on adolescent girls, a potentially vulnerable population, given that (1) adolescence marks a time of increased social, cognitive, and neural development (Casey et al., 2005; Guroglu et al., 2009; Blakemore, 2012; Blakemore and Robbins, 2012; Crone and Dahl, 2012), making adolescence a critical time period during which assault exposure could impact these developmental processes, and (2) girls are at greater risk for mental health problems following trauma relative to boys (Danielson et al., 2009). Furthermore, based on an accumulation of cognitive and neurobiological research on reinforcement learning and prediction error processing (Behrens et al., 2007; Rushworth and Behrens, 2008; d’Acremont et al., 2009; Bossaerts, 2010; Harris and Fiske, 2010; Jones et al., 2011), we expected a network of coactivated brain regions including striatal, insular, anterior cingulate, and ventromedial prefrontal cortical regions to be activated for expectancy violations for controls and less active (relative to controls) among the assaulted sample, given our hypothesis of diminished reactivity to social expectancy violations.
2. Methods
2.1. Participant recruitment and assessment
Participants were recruited from the general community and from trauma specialty outpatient clinics. Exclusionary criteria for the study included major medical conditions, psychotic disorders, and internal ferromagnetic objects precluding MR scans. Thirty-six adolescent girls, aged 12–16, consented, fulfilled all inclusionary criteria for the study, and completed all study procedures. Six participants, however, were excluded from analyses due to excessive head movement causing intractable residual signal artifact in their functional imaging data. This reduced our effective sample size to 30 adolescent girls. See Table 1 for a complete description of demographic and clinical characteristics of the sample. Direct assaultive violence exposure was assessed via the trauma assessment section of the National Survey of Adolescents (Kilpatrick et al., 2000; Kilpatrick et al., 2003; Cisler et al., 2012), designed to assess both the incidence and characteristics of discrete types of physical assault, physical abuse, and sexual abuse. When the presence of a form of assault was affirmed (i.e., an event had occurred meeting the criteria of physical or sexual abuse/assault), a series of more detailed follow-up questions were asked to further characterize the instance(s) of assault (e.g., the frequency, location, and identity of perpetrator). Participants’ past and current mental health statuses were assessed using the K-SADS inventory (Kaufman et al., 1997) and their clinical and social functioning was measured through both self-rated and caregiver-rated assessments. Caregivers completed the Child-Behavior Checklist (CBCL) (Achenbach, 1991), which provided measures of social problems, depression symptoms, and aggressive behavior. Participants completed the UCLA PTSD Index–Adolescent (Steinberg et al., 2004), which provided measures of posttraumatic stress disorder (PTSD) symptom severity. All participants’ Verbal IQs were assessed via the Receptive One-word Vocabulary Test (Brownell, 2000).
Table 1.
Group differences across all collected demographic and clinical variables.
Demographic and clinical characteristics of the sample according to both dichotomous and ordinal assault characterizations.
| Measure | Girls with History of Assault Exposure (n=14) | Girls with High Assault Exposure Severity (n=8) | Girls with Low Assault Exposure Severity (n=6) | Girls with No Assault Exposure (n=16) |
|---|---|---|---|---|
| Age (y) | 15.1(1.1) | 15.4 (0.74) | 14.7 (1.5) | 14.6 (1.1) |
| Ethnicity | 64% Caucasian 21% African-American 7% Biracial 7% Hispanic |
88% Caucasian 0% African-American 13% biracial 0% Hispanic |
33% Caucasian 50% African-American 0% biracial 17% Hispanic |
69% Caucasian 31% African-American 0% biracial 0% Hispanic |
| Direct Assaults % Sexually Assaulted % Physically Assaulted Age at First Assault Exposure Age at Most Recent Assault Exposure (y) Time Since Most Recent Assault Exposure (y) % Assault(s) Perpetrated by Acquaintance % Assault(s) Perpetrated by Stranger |
3.8 (2.7) 57%a 100%a 7.4 (3.4) 12.3 (3.0) 2.8 (3.1) 100% 0% |
5.6 (2.0) 100%bc 100%b 6.0 (2.6) 13.6 (2.3) 1.8 (2.7) 100% 0% |
1.3 (0.5) 0%c 100%c 9.3 (3.7) 10.5 (3.1) 4.2 (3.4) 100% 0% |
0 (0) 0%ab 0%abc n/a n/a n/a n/a n/a |
| Current PTSD | 21% | 38%b | 0% | 0%b |
| Past PTSD | 36%a | 50%b | 17% | 0%ab |
| Current GAD | 21% | 25% | 17% | 7% |
| Past GAD | 7% | 13% | 0% | 0% |
| Current MDD | 15% | 25% | 0% | 0% |
| Past MDD | 31% | 50% | 0% | 14% |
| Current Alcohol Abuse | 14% | 25%b | 0% | 0%b |
| Past Alcohol Abuse | 29% | 50%bc | 0%c | 0%b |
| Current Substance Abuse | 0% | 0% | 0% | 0% |
| Past Substance Abuse Substances Tried |
14%a 2.3 (1.8)a |
13% 2.8 (2.2)b |
17% 1.7 (1.0)c |
0%a 0.2 (0.5)abc |
| Previous Psychological or Psychiatric Treatment | 57% | 75%a | 33% | 25%a |
| UCLA PTSD Symptoms | 25.3 (20.3)a | 37.3 (12.2)bc | 9.3 (18.0)c | 2.1 (4.4)ab |
| CBCL anxious-depressed | 5.9 (5.3)a | 8.4 (5.6)bc | 2.7 (2.4)c | 2.3 (2.4)ab |
| CBCL aggressive behavior | 11.4 (8.7)a | 13.0 (6.4)b | 9.2 (11.4) | 3.1 (3.4)ab |
| CBCL social problems | 3.4 (3.0) | 4.3 (2.7)b | 2.3 (3.1) | 1.8 (1.8)b |
| Verbal IQ | 100.4(11.3) | 100.8 (9.3) | 99.8 (14.4) | 104.6 (19.3) |
Note. Matching superscripts between columns for each row indicate significant between-group differences (p <0.05). Values in parentheses indicate SD.
We characterized assault exposure using two methods. (1) We used a dichotomous assault exposure variable (i.e., assaulted vs. non-assaulted) to assess an effect of assault exposure per se. (2) To test for an effect of assault severity, we used a variable representing the severity of each participant’s assault exposure history. While past epidemiological studies have used a scalar assault severity variable consisting of the sum of the different types of assaultive events to which the individual was exposed (Neuner et al., 2004; Kolassa et al., 2010b; Cisler et al., 2011b; Cisler et al., 2012), in our sample the distribution of this scalar assault variable was right skewed. To improve the normality of this distribution, we instead calculated an ordinal assault exposure severity variable to test for a linear relationship between assault exposure severity and dependent measures of interest. This variable was defined as 0 = no assault exposures (n=16), 1 = one or two exposures (n = 6), and 2 = three or more exposures (n=8).
2.2. fMRI task
All participants engaged in an identical social learning task in which they were given a hypothetical cache of money (initialized at $50). In each trial, $10 of their money was invested by the computer pseudorandomly to one of three neutral female faces (from the published NimStim facial stimuli set (Tottenham et al., 2009)). The face either kept the money (− $10) or returned twice as much (+ $20) back to the participant. After each of the 96 trials, participants rated which face was the most trustworthy. Participants were instructed to track which face over the course of the entire experiment was the most trustworthy. Fig. 1 indicates two potential trial sequences. To manipulate the differential trustworthiness of the faces, each of the three faces, for the first 48 trials, gave the money back to participants a predetermined percentage of trials, 80, 50, and 20%, respectively (e.g., face 1 gave participants’ money back 80% of the time across the first 48 trials). After the initial 48 trials, face 1 and face 3’s percentages switched such that face 1 henceforth gave back only 20% of the time and face 3 80% of the time, thereby violating previously established behavioral expectancies and creating a high conflict epoch. The last 24 trials were excluded from analyses to focus on conflict and prediction error processing rather than reversal learning (see Supplementary Fig. 1 for more detailed behavioral analyses and further explanation for excluding of the last epoch). The trials in which face 1 (the overall most trustworthy face) took participants’ money were modeled as unexpected negative outcomes, and the trials in which face 3 (the overall least trustworthy face) gave participants money back were modeled as unexpected positive outcomes. Conversely, trials in which face 1 gave participants money were modeled as expected positive outcomes, and trials in which face 3 took participants’ money were modeled as expected negative outcomes. However, because the first six trials at the beginning of the experiment occurred before expectations could have reasonably been formed and therefore represented a learning acquisition phase rather than an expectation phase, they were excluded from expectation analyses and modeled as a separate regressor. We excluded the first six trials (as opposed to the first five or seven, etc.) because they were counterbalanced across faces such that the first six trials comprised the first two trials of each face—an effort to conserve experimental power without compromising the validity of the experimental manipulations.
Fig. 1.
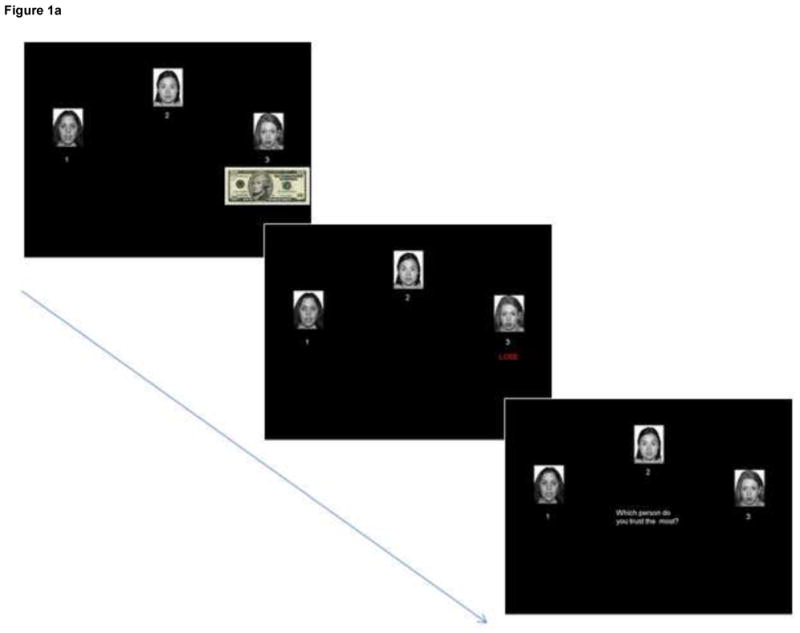
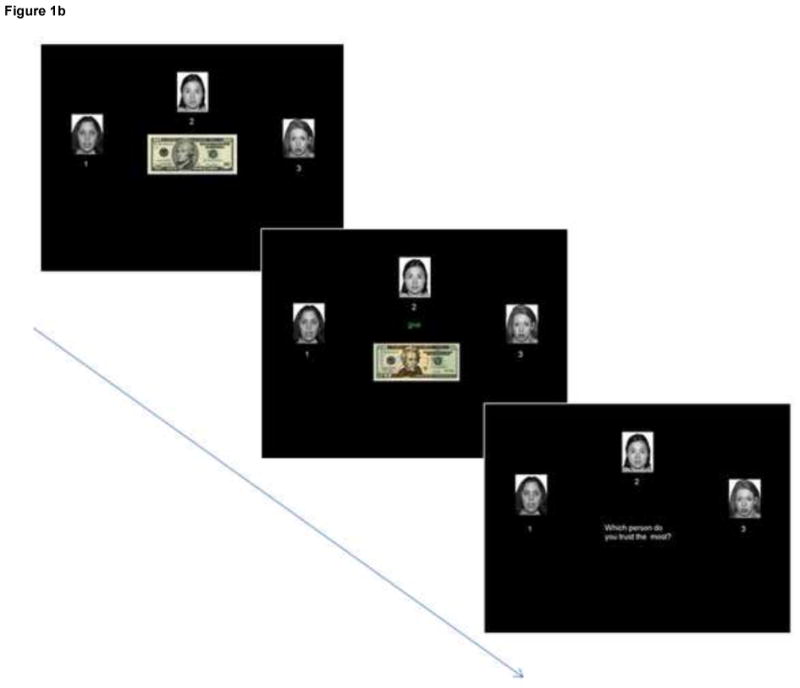
Fig. 1a. An example trial sequence resulting in a negative outcome in which Face 3 takes the participant’s investment of money.
Fig. 1b. An example trial sequence resulting in a positive outcome in which Face 2 doubles the participant’s investment of money.
2.3. Image acquisition and preprocessing
Imaging data were acquired using a Philips 3T Achieva X-series MRI system with an eight-channel head coil (Philips Healthcare, USA), with functional images acquired at a final resolution of 3×3×3 mm3 with a repetition time of 2000 ms. See Supplementary Materials for a detailed description of acquisition parameters and preprocessing steps.
2.4. fMRI analyses
Explicit deconvolution was conducted to estimate the hemodynamic response function (HRF) for each individual participant at each voxel for each task condition, using regression with 7 cubic splines over the 12-s interval following the onset of each stimulus. The first and last time point estimates were constrained to zero in order to ensure neurophysiologically plausible shapes of the HRF. This approach was used to account for the wide range of inter- and intra-individual variability of the HRF. The areas under the curve (AUC) of each individual’s estimated HRFs were calculated using a numerical integration function and transformed into percent signal change to compare across voxels, task conditions, and participants (Urry et al., 2006; Greene et al., 2007; Borst et al., 2010).
Contrasts between different conditions were computed to compare relative percent signal change as a function of task manipulation. To test our social expectancy violation hypothesis, the contrasts of interest were those comparing unexpected and expected outcomes. Specifically, we tested for effects of unexpected relative to expected takes (i.e., trust violations), unexpected relative to expected gives, and unexpected relative to expected outcomes collapsed across all valences. We tested for relationships between assault exposure status and each contrast of interest using a series of whole-brain robust regression analyses (Wager et al., 2005), which provided t-tests of the beta coefficient values for the control group (i.e., the intercept) and for the difference between the control and assaulted groups. Separate regression analyses were conducted for the dichotomous assault exposure variable and the ordinal assault severity variable. Using AFNI software (Cox, 1996), we set a corrected alpha level of p < 0.05 with cluster-thresholding (Forman et al., 1995). In this procedure, we first estimated the amount of spatial smoothing (from 3dFWHMx) in the residuals of the data (from 3dREMLfit). This estimate is then used in Monte Carlo simulations implemented in 3dClustSim (with 10,000 iterations), which demonstrated that a cluster size of 38 contiguous voxels surviving an uncorrected p-value of 0.005 would yield a corrected p < 0.05.
3. Results
3.1. Demographic results
There were no differences between assaulted and control groups on dimensions of age, IQ, and ethnicity. The assaulted group demonstrated higher rates of PTSD, alcohol abuse, substance use, and caregiver-rated aggressive, anxious-depressed, and socially problematic behavior (see Table 1).
3.2. Behavioral results
Participants’ trial-by-trial trustworthy response choices (Supplementary Fig. 2) indicate, among assaulted and control subjects, a clear preference toward face 1 in the first half of the experiment. In the epoch occurring directly after the reversal, subjects’ responses are more volatile—lending support to our categorization of the third epoch as high conflict. Response times (RTs) during the response trials were also recorded and used as a measure of cognitive conflict (i.e., greater RT indicates greater conflict between prior expectancies and observed outcome on the current trial). RT bias scores were created to indicate conflict on trials with unexpected versus expected outcomes, with separate bias scores for positive versus negative expectancy violations. RT bias scores were significantly different (t(28) = −2.14; p = 0.041) between the dichotomized groups (assaulted vs. non-assaulted girls) during trust violations such that assaulted girls demonstrated significantly less RT bias during unexpected take versus expected take trials (Supplementary Fig. 1).
3.3. fMRI contrast results
3.3.1. Testing an effect of trust violations among controls
Given the novelty of the current study’s task and the uniqueness of our sample, it is relevant to define normative brain activity among control adolescent girls during task contrasts in order to facilitate the interpretation of group comparisons. For the trust violation contrast (unexpected takes-expected takes), controls demonstrated activity in bilateral anterior insula (AI), perigenual anterior cingulate cortex (pgACC), dorsomedial prefrontal cortex (dmPFC), and left visual cortex (Supplementary Fig. 2; see Supplementary Table 1 for more detailed descriptions of clusters). To test the effects of trust violations controlling for the effects of expectancy violations per se (i.e., in order to test the specificity of trust violation effects), we created a negative versus positive expectancy violation contrast ([unexpected takes-expected takes]-[unexpected gives-expected gives]). For this contrast (that is, for trust violations specifically), controls demonstrated activity in dmPFC (XYZ=−2, 41, 18, peak t(28)=3.67, cluster size=50) (Supplementary Fig. 3).
3.3.2. Testing for between-group differences during trust violations
When testing for differences in brain activation as a function of assault exposure per se (i.e., using the dichotomous assault variable to test for between-group differences) during unexpected-expected takes, we found clusters of lesser activity among the assaulted group in the perigenual ACC, right superior temporal gyrus (STG), and bilateral insulae (see Supplementary Table 1 for more detailed descriptions of clusters).
We next tested for a linear effect of assault exposure using the ordinal assault variable (coded as 0=no assaults, 1=one or two assaults, 2=greater than two assaults) using the same trust violation contrast. This yielded significant clusters in pgACC and mPFC, bilateral AI, and left dorsal striatum that all negatively scaled with assault severity (Fig. 2; Supplementary Table 1; see Supplementary Fig. 5 for % signal change of clusters, stratified across ordinal labels, for which there was a significant linear relationship). Performing the same analysis with a contrast collapsed across the valence of unexpected outcomes (i.e., unexpected takes combined with unexpected gives), we observed a significant cluster in the right hippocampus that scaled negatively with assault exposure severity (XYZ= 23, −20, −13, peak t(28)= −4.90, cluster size=55) (Supplementary Fig. 6). Comparing the effects of negative versus positive expectancy violations to test for specificity of the unexpected outcomes (i.e., (unexpected-expected takes)-(unexpected-expected gives), we found a cluster in the dmPFC (XYZ=8, 53, 18, peak t(28)= −4.49, cluster size=38) that negatively scaled with assault severity (Supplementary Fig. 3). We found no significant findings of a priori theoretical interest for the unexpected-expected gives contrast. Refer to Supplementary Table 1 for a complete description of findings across all analyses.
Fig. 2.
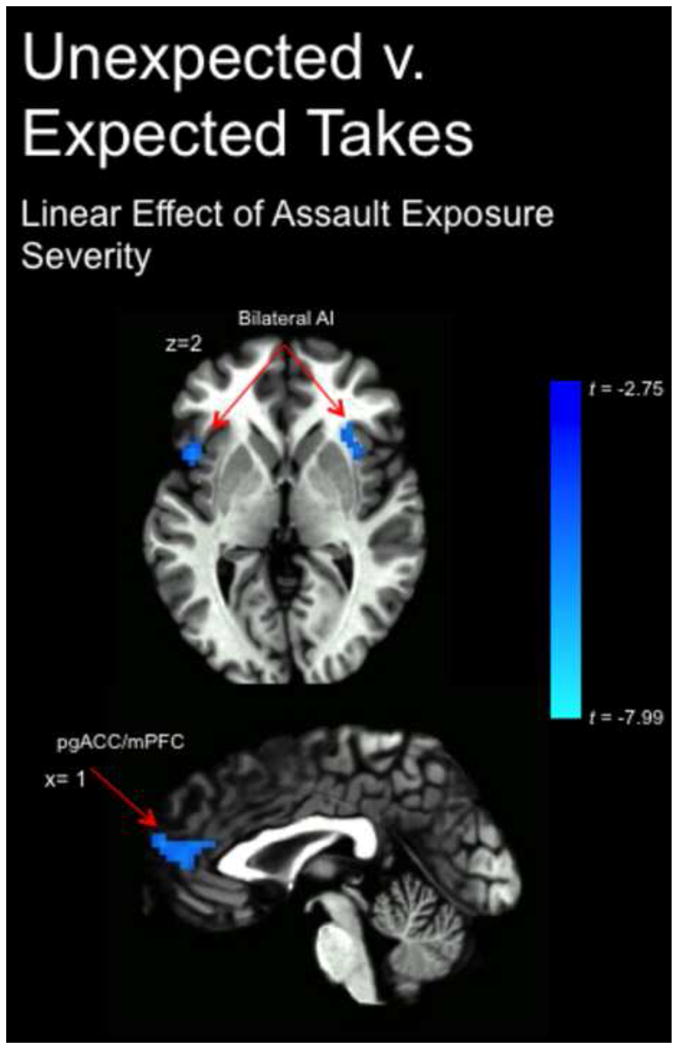
Significant clusters identified in whole-brain group-level regression analysis using the ordinal assault severity variable (coded as 0=no assaults, 1=one or two assaults, 2=more than two assaults) during trust violations. X, Y, and Z coordinates and t-values for the peak voxels within each cluster listed in Supplementary Table 1. Statistical parametric map is thresholded at 2.75 to correct for multiple comparisons and overlaid onto a high-spatial-resolution anatomic image (i.e., N27 Colin template).
3.3.3. Regression with RT bias scores
RT bias scores (RT on unexpected trials minus RT on expected trials) as a continuous variable was also used as a regressor for the unexpected take versus expected take contrast, and we found a cluster in the left AI (XYZ=−47, 11, 6, peak t(28)=3.84, cluster size=48) that scaled positively with greater RT bias scores (Supplementary Fig. 7a). To determine the voxels that significantly scaled with both assault exposure severity and RT bias scores, we performed a conjunction analysis on the two statistical spatial maps revealing a common cluster in the left AI (XYZ= −47, 17, −4, cluster size=17).
3.4. Mediation analyses
Given the observations of (1) a relationship between assault severity and RT bias, (2) a relationship between assault severity and activity in the anterior insula, and (3) a scalar relationship between RT bias and activity in left AI during unexpected takes, we performed a mediation analysis for left insular activity using the clusters identified in the conjunction analysis above. This model tested whether altered recruitment of left AI operated as a mediating mechanism between assault and attenuated RT bias. Following the standard mediation model (Baron and Kenny, 1986), these analyses included paths A (the effect of the independent variable on the mediator), B (the effect of the mediator on the outcome controlling for the independent variable), and C (the effect of the independent variable on the outcome). To quantify these paths, assault exposure severity was regressed onto % signal change AUC estimates of clusters in the AI, and activity of the AI was regressed on RT bias scores after statistically partitioning out the effect of paths A onto paths B. The indirect effect of assault exposure severity on RT bias through left AI was calculated as the sum of the products of the A and B paths. To test the significance of the indirect path, we implemented a bootstrapping method with 10,000 iterations and calculated the lower and upper bounds of the 95% CI of the indirect effect term (MacKinnon et al., 2007). This demonstrated a significant indirect mediation effect of assault exposure on RT bias through altered left AI activity (indirect effect = −184.69, 95% CI = −332.72 to −36.67, Supplementary Fig. 7b).
3.5. Relationship with clinical variables among the assaulted group
Given that the assault group also differed from the control group in clinical symptoms (see Table 1), we conducted a subsequent exploratory analysis to test whether the altered brain responses identified among the assaulted group during the unexpected versus expected take contrast were related to clinical symptom severity (e.g., substance use, aggressive behavior) within the assault group only. We defined regions of interest (ROIs) in the pgACC, right and left AI, and left IFG from clusters identified in the whole-brain regression analysis results using the ordinal assault variable. To correct for the number of hypotheses we tested, we used a False Discovery Rate (FDR) control (Genovese et al., 2002). Among the assaulted girls only, assault severity scores (i.e., the total number of assaultive events to which the individual was exposed (Neuner et al., 2004; Kolassa et al., 2010a; Kolassa et al., 2010b) were found to be negatively related to activity in both left and right AI (t(12)= −3.38, pFDR-corrected < 0.05; t(12)= −3.38, pFDR-corrected < 0.05, respectively). Additionally, CBCL-rated aggressive behavior was negatively related to activity in the left IFG (t(12)= −3.915, pFDR-corrected < 0.05). Of the 14 assaulted participants, eight had either received psychological/psychiatric treatment in the past or currently; thus, we also used robust regression to examine the effect of treatment history on brain function within these ROIs. There was no difference between groups (treatment history vs. no treatment history) on activation within any of the ROIs (pFDR-corrected > 0.40).
4. Discussion
To the investigators’ knowledge, this is the first neuroimaging study of social learning among adolescent victims of assaultive violence. The results of this study demonstrate that (1) assault victims exhibit both reduced behavioral and brain responses to trust violations, (2) the greater the severity of assault exposure, the lesser the magnitude of these brain responses, and (3) this reduced brain activity mediated the reduced behavioral responses among the assaulted group. Additionally, that assaulted adolescent girls’ reduced AI activity during trust violations was predictive of greater presence of mental health-related symptoms (e.g., aggressive behavior) suggests the clinical relevance of the current findings; specifically, these observations suggest that reduced brain activity during trust violations might be indicative of poorer clinical functioning. Improved mental health outcomes might be potentiated by cognitive behavioral interventions that specifically target social decision-making by, for instance, incorporating risk assessment and risk reduction techniques into therapies (Danielson et al., 2010; Danielson et al., 2012).
In the context of biological psychiatry and clinical neuroimaging research, we believe the current study’s findings emphasize the context dependence of clinically meaningful behavioral and neural differences among people who are at risk for or are diagnosed with psychopathology. For instance, anxiety disorders — and in particular PTSD — have been broadly associated with hyperactivity in cingulate and insular regions (Frewen et al., 2008; Pitman et al., 2012). Indeed, we found that in an overlapping sample as that of the current study assault exposure among adolescent girls was associated with greater activity, during an emotion-processing task, in a frontocingulate ‘salience’ network composed primarily of loadings in the anterior insular cortex and dorsal and perigenual anterior cingulate cortex (Cisler et al., 2013). This suggests that the vulnerable population of assaulted adolescents do not demonstrate broad, uniform changes in brain activity but rather exhibit dynamic, context-sensitive or domain-specific changes. Furthermore, corresponding to the aforementioned differences in hyper- versus hypo-activity, there has been relatively widespread evidence that anxiety disorders, including PTSD (Cisler et al., 2011), are generally associated with greater cognitive conflict and emotional reactivity, particularly to negatively valenced events and stimuli (Bar-Haim et al., 2007). The current evidence of reduced conflict in response to trust violations among assault victims again demonstrates the importance of context in understanding function within core cognitive and neural mechanisms. Important to appreciate in examining these apparent discrepancies are the perhaps unique characteristics of the sample composition of the current study (as our sample was not strictly clinical but rather an at-risk sample that demonstrated specific characteristics in terms of age, sex, and trauma type) and experimental paradigm (as our study employed a cognitively complex task, the performance of which requires the coordinated recruitment of various cognitive processes). With these differences in mind, however, it seems the current results advocate for a more nuanced view of the neurobiological and cognitive substrates of assault exposure specifically and perhaps of psychopathology more generally.
In the reinforcement learning literature, studies have found that the magnitude of value prediction errors (i.e., discrepancies between predicted and observed outcomes) and risk prediction errors (i.e., discrepancies between predicted and observed outcome variance, or risk) predict activity in anterior cingulate regions (Behrens et al., 2007; Rushworth and Behrens, 2008) and the AI (Preuschoff et al., 2008; d’Acremont et al., 2009; Bossaerts, 2010), respectively. During our task, activity in these particular regions was negatively associated with both assault severity and negative RT bias scores and, critically, this relationship was observed during trials in which there would be both high value prediction errors (expectancy violations) and high risk prediction errors (volatility of outcomes). Insofar as activation of AI and ACC during trust violation trials would be consistent with prior literature regarding the neural correlates of domain-general as well as domain-specific social prediction errors (Harris and Fiske, 2010; Jones et al., 2011), the reduced recruitment of these regions among assaulted girls supports the hypothesis that assaulted adolescent girls demonstrate attenuated prediction errors.
Although we cannot claim that the current study’s task, which notably used simulated faces instead of actual conspecifics, can clearly generalize to the more social cognitively complex concept of trust, we believe that at the very least the task engages similar cognitive processes as would underlie real-world social decision-making and therefore could serve as a reasonable proxy or laboratory analogue. Additionally, to the extent we are uncertain whether the task measures social-specific learning versus domain-general learning, we also cannot make firm inferences regarding whether the observed differences represent altered social-specific learning mechanisms. Nonetheless, these observed differences among assaulted girls may have clinically meaningful implications regardless of whether they are social-specific or domain-general.
There are several potential explanations for the observed findings. One explanation is that assaulted girls have a preexisting negative expectancy bias such that they are biased toward expecting negative events and, in the context of the experiment, preemptively expect a negative outcome from the otherwise presumably trustworthy face 1. Accordingly, they would generate no prediction error when face 1 takes their money because this negative outcome was, either implicitly or explicitly, expected. This expectancy bias could be an adaption of the assaulted girls in response to seemingly unpredictable, severely negative social outcomes (e.g., assaultive events). This general negative expectancy bias would still be compatible with the increased risk for revictimization observed among interpersonal violence victims. For example, it could be the case that chronic general negative evaluations of other conspecifics leads to difficulty in navigating complex social environments, such as discriminating between ‘safe’ and ‘unsafe’ conspecifics depending on situational contexts or dispositional characteristics. An alternative explanation is that assaulted girls could fail to assign salience, informational value, and/or significance to unexpected negative behavior. This desensitization could also function as an adaptation to volatile social (familial, community) environments and a history of interpersonal trust violations (e.g., assaultive events perpetrated by family members and acquaintances, as were the cases for the current study’s entire sample). Future research should seek to more specifically characterize and localize the cognitive etiologies of these group differences. Despite the etiology, however, these data might provide a potential mechanism explaining the high rates of revictimization among assault victims. That is, the failure to generate a prediction error signal when a presumably trustworthy individual behaves in a socially negative manner (whether due to alterations in forming expectations or in prediction error processing) could potentially explain how a victim may fail to detect and avoid risky social situations. Indeed, past research has found that young adult women with victimization histories have higher thresholds for judging social situations as risky (Gidycz et al., 2006; Yeater et al., 2010; Yeater and Viken, 2010). However, because the current study’s findings among assaulted girls were demonstrated to be related to assault severity and the more severely assaulted girls by definition had been revictimized more frequently, it is not possible to infer causality between these two related findings. That is, the observed differences among assaulted girls could be a neurocognitive consequence of revictimization, a neurocognitive mechanism of revictimization, or a non-causally-related neurocognitive correlate of assault. Any of the three interpretations, however, is clinically relevant and might indicate a need to incorporate procedures to improve judgments of socially risky situations into treatments for trauma-related psychiatric conditions in order to reduce the likelihood of revictimization and improve clinical outcomes (Danielson et al., 2010; Danielson et al., 2012).
4.1. Study limitations
While novel and promising in terms of recommending future research among victims of interpersonal violence, the current study is not without limitations. That all of the severely assaulted girls (ordinal label of 2) and none of the mildly to moderately assaulted girls (ordinal label of 1) were exposed to sexually assaultive events introduces an ambiguity as to whether effects observed as a function of assault severity were in part also due to sexual assault exposure. Although this possibility reduces the discriminative validity of the current study’s findings, it highlights a need to examine both main and interaction effects of different types of assault. Another potential limitation of the current study concerns the psychiatric composition of our sample (i.e., the uneven distribution of psychiatric diagnoses among the sample). What might be a limitation of the study’s internal validity, however, might also be—to the extent the sample represented a real-world population of assaulted adolescents who rarely demonstrate complex assault histories without also demonstrating complex psychological profiles including pre-, co-, and post-morbid psychiatric disorders—a strength of the study’s external validity and clinical relevance. Furthermore, the current exploratory study’s sample of assaulted adolescents was relatively small, highlighting the need for replication and extension in future studies.
The cross-sectional design of the study precludes inferences as to whether the observed differences associated with assault were caused by assault exposure or due to pre-existing characteristics among the assaulted group. Regardless of this inability to infer causality, prior research indicates that assaulted adolescents are a significantly at-risk sample for mental health disorders (Kilpatrick et al., 2000; Kilpatrick et al., 2003; Wolitzky-Taylor et al., 2008; Danielson et al., 2009; Cisler et al., 2011a; Cisler et al., 2012) and revictimization (Cougle et al., 2009; Cisler et al., 2011b), and while this study cannot determine whether the observed differences were due to assault specifically, our results do suggest significant behavioral and brain differences in this vulnerable population which can potentially help explain the increased risk observed in this population.
Notwithstanding the study’s limitations, the results support the hypothesis of diminished behavioral and brain responding to trust violations among adolescent female victims of assaultive violence.
Supplementary Material
Acknowledgments
We thank Cindy Mosley, Andi Ham, Shanti Tripathi, and George Andrew James for help with recruitment, analysis, and administration.
Funding sources
Portions of this work were supported through grants 1R21MH097784-01, T32 DA022981-02, and UL1RR029884. No additional external funding was received for this study.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM. Integrative Guide to the 1991 CBCL/4-18, YSR, and TRF Profiles. Department of Psychology, University of Vermont; Burlington, VT: 1991. [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator mediator variable distinction in social psychological-research - conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Walton ME, Rushworth MF. Learning the value of information in an uncertain world. Nature Neuroscience. 2007;10:1214–1221. doi: 10.1038/nn1954. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. Development of the social brain in adolescence. Journal of the Royal Society of Medicine. 2012;105:111–116. doi: 10.1258/jrsm.2011.110221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Robbins TW. Decision-making in the adolescent brain. Nature Neuroscience. 2012;15:1184–1191. doi: 10.1038/nn.3177. [DOI] [PubMed] [Google Scholar]
- Borst JP, Taatgen NA, Stocco A, van Rijn H. The neural correlates of problem states: testing fMRI predictions of a computational model of multitasking. PLoS ONE. 2010:5. doi: 10.1371/journal.pone.0012966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossaerts P. Risk and risk prediction error signals in anterior insula. Brain Structure and Function. 2010;214:645–653. doi: 10.1007/s00429-010-0253-1. [DOI] [PubMed] [Google Scholar]
- Brownell R. Receptive One-word Picture Vocabulary Test. Academic Therapy Publications; Novato, CA: 2000. [Google Scholar]
- Cisler JM, Wolitzky-Taylor KB, Adams TG, Jr, Babson KA, Badour CL, Willems JL. The emotional Stroop task and posttraumatic stress disorder: a meta-analysis. Clinical Psychology Review. 2011;31:817–828. doi: 10.1016/j.cpr.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Amstadter AB, Begle AM, Resnick HS, Danielson CK, Saunders BE, Kilpatrick DG. A prospective examination of the relationships between PTSD, exposure to assaultive violence, and cigarette smoking among a national sample of adolescents. Addictive Behaviors. 2011a;36:994–1000. doi: 10.1016/j.addbeh.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Amstadter AB, Begle AM, Resnick HS, Danielson CK, Saunders BE, Kilpatrick DG. PTSD symptoms, potentially traumatic event exposure, and binge drinking: a prospective study with a national sample of adolescents. Journal of Anxiety Disorders. 2011b;25:978–987. doi: 10.1016/j.janxdis.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Begle AM, Amstadter AB, Resnick HS, Danielson CK, Saunders BE, Kilpatrick DG. Exposure to interpersonal violence and risk for PTSD, depression, delinquency, and binge drinking among adolescents: data from the NSA-R. Journal of Traumatic Stress. 2012;25:33–40. doi: 10.1002/jts.21672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Steele JS, Smitherman S, Lenow JK, Kilts CD. Neural processing correlates of assaultive violence exposure and PTSD symptoms during implicit threat processing: A network-level analysis among adolescent girls. Psychiatry Research: Neuroimaging. 2013;214:238–246. doi: 10.1016/j.pscychresns.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cougle JR, Resnick H, Kilpatrick DG. A prospective examination of PTSD symptoms as risk factors for subsequent exposure to potentially traumatic events among women. Journal of Abnormal Psychology. 2009;118:405–411. doi: 10.1037/a0015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- d’Acremont M, Lu ZL, Li X, Van der Linden M, Bechara A. Neural correlates of risk prediction error during reinforcement learning in humans. Neuroimage. 2009;47:1929–1939. doi: 10.1016/j.neuroimage.2009.04.096. [DOI] [PubMed] [Google Scholar]
- Danielson CK, Amstadter AB, Dangelmaier RE, Resnick HS, Saunders BE, Kilpatrick DG. Trauma-related risk factors for substance abuse among male versus female young adults. Addictive Behaviors. 2009;34:395–399. doi: 10.1016/j.addbeh.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson CK, McCart MR, de Arellano MA, Macdonald A, Doherty LS, Resnick HS. Risk reduction for substance use and trauma-related psychopathology in adolescent sexual assault victims: findings from an open trial. Child Maltreatment. 2010;15:261–268. doi: 10.1177/1077559510367939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson CK, McCart MR, Walsh K, de Arellano MA, White D, Resnick HS. Reducing substance use risk and mental health problems among sexually assaulted adolescents: a pilot randomized controlled trial. Journal of Family Psychology. 2012;26 (4):628–635. doi: 10.1037/a0028862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Frewen PA, Dozois DJA, Lanius RA. Neuroimaging studies of psychological interventions for mood and anxiety disorders: empirical and methodological review. Clinical Psychology Review. 2008;28:228–246. doi: 10.1016/j.cpr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gobin RL, Freyd JJ. Betrayal and revictimization: preliminary findings. Psychological Trauma-Theory Research Practice and Policy. 2009;1:242–257. [Google Scholar]
- Greene AJ, Gross WL, Elsinger CL, Rao SM. Hippocampal differentiation without recognition: an fMRI analysis of the contextual cueing task. Learning and Memory. 2007;14:548–553. doi: 10.1101/lm.609807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guroglu B, van den Bos W, Crone EA. Neural correlates of social decision making and relationships: a developmental perspective. Annals of the New York Academy of Sciences. 2009;1167:197–206. doi: 10.1111/j.1749-6632.2009.04502.x. [DOI] [PubMed] [Google Scholar]
- Harris LT, Fiske ST. Neural regions that underlie reinforcement learning are also active for social expectancy violations. Social Neuroscience. 2010;5:76–91. doi: 10.1080/17470910903135825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RM, Somerville LH, Li J, Ruberry EJ, Libby V, Glover G, Voss HU, Ballon DJ, Casey BJ. Behavioral and neural properties of social reinforcement learning. Journal of Neuroscience. 2011;31:13039–13045. doi: 10.1523/JNEUROSCI.2972-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Acierno R, Saunders B, Resnick HS, Best CL, Schnurr PP. Risk factors for adolescent substance abuse and dependence: data from a national sample. Journal of Consulting and Clinical Psychology. 2000;68:19–30. doi: 10.1037//0022-006x.68.1.19. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Ruggiero KJ, Acierno R, Saunders BE, Resnick HS, Best CL. Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: results from the National Survey of Adolescents. Journal of Consulting and Clinical Psychology. 2003;71:692–700. doi: 10.1037/0022-006x.71.4.692. [DOI] [PubMed] [Google Scholar]
- Kolassa IT, Ertl V, Eckart C, Glockner F, Kolassa S, Papassotiropoulos A, de Quervain DJ, Elbert T. Association study of trauma load and SLC6A4 promoter polymorphism in posttraumatic stress disorder: evidence from survivors of the Rwandan genocide. Journal of Clinical Psychiatry. 2010a;71:543–547. doi: 10.4088/JCP.08m04787blu. [DOI] [PubMed] [Google Scholar]
- Kolassa IT, Kolassa S, Ertl V, Papassotiropoulos A, De Quervain DJ. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-o-methyltransferase Val(158)Met polymorphism. Biological Psychiatry. 2010b;67:304–308. doi: 10.1016/j.biopsych.2009.10.009. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annual Review of Psychology. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuner F, Schauer M, Karunakara U, Klaschik C, Robert C, Elbert T. Psychological trauma and evidence for enhanced vulnerability for posttraumatic stress disorder through previous trauma among West Nile refugees. BMC Psychiatry. 2004;4:34. doi: 10.1186/1471-244X-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MS, Milad MR, Liberzon I. Biological studies of post-traumatic stress disorder. Nature Reviews Neuroscience. 2012;13:769–778. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. Journal of Neuroscience. 2008;28:2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Annals of the New York Academy of Sciences. 2006;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nature Neuroscience. 2008;11:389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- Steinberg AM, Brymer MJ, Decker KB, Pynoos RS. The University of California at Los Angeles Post-traumatic Stress Disorder Reaction Index. Current Psychiatry Reports. 2004;6:96–100. doi: 10.1007/s11920-004-0048-2. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Keller MC, Lacey SC, Jonides J. Increased sensitivity in neuroimaging analyses using robust regression. Neuroimage. 2005;26:99–113. doi: 10.1016/j.neuroimage.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Wolitzky-Taylor KB, Ruggiero KJ, Danielson CK, Resnick HS, Hanson RF, Smith DW, Saunders BE, Kilpatrick DG. Prevalence and correlates of dating violence in a national sample of adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:755–762. doi: 10.1097/CHI.0b013e318172ef5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeater EA, McFall RM, Viken RJ. The relationship between women’s response effectiveness and a history of sexual victimization. J Interpers Violence. 2011;26:462–478. doi: 10.1177/0886260510363425. [DOI] [PubMed] [Google Scholar]
- Yeater EA, Treat TA, Viken RJ, McFall RM. Cognitive processes underlying women’s risk judgments: associations with sexual victimization history and rape myth acceptance. Journal of Consulting and Clinical Psychology. 2010;78:375–386. doi: 10.1037/a0019297. [DOI] [PubMed] [Google Scholar]
- Yeater EA, Viken RJ. Factors affecting women’s response choices to dating and social situations. Journal of Interpersonal Violence. 2010;25:1411–1428. doi: 10.1177/0886260509354588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


