SUMMARY
The nervous system promotes adaptive responding to myriad environmental stimuli by ascribing emotion to specific stimulus domains. This affects the salience of different stimuli, facilitates learning, and likely involves the amygdala. Recent studies suggest a strong homology between adaptive responses that result from learning and those that emerge during development. As in motivated learning, developmental studies have found the salience of different classes of stimuli (eg peers) undergoes marked fluctuation across maturation and may involve differential amygdala engagement. We suggest that variability in amygdala response to different stimulus domains may play an active and functional role in shaping emerging cortical circuits across development by highlighting the importance of particular stimulus categories during sensitive periods of development.
Keywords: Sensitive periods, amygdala, cortex, social
INTRODUCTION
A primary function of the central nervous system is to optimally match behavior with environmental conditions. An important modulator of this process is emotion. Emotion can be construed as the synergistic response of multiple independent body systems in response to a stimulus1. Emotion induction enables both an orchestrated response and flexible attribution of salience to environmental stimuli Importantly differential emotional responses can be tailored to match the internal state of the organism with the stimulus or context in which it is encountered1–3. Thus, an emotional response is an intrinsic signal ascribed to a stimulus that flexibly signals its importance. Another important function of emotion is that it facilitates learning by enhancing sensory experience, focusing attention, and promoting long-term consolidation of sensory experience4–6. Emotional responses also promote expression of optimal responses to emotion eliciting stimuli7 which can be rapidly executed during subsequent encounters.
Such emotional mediation effects are typically studied in a learning context where relatively rapid shifts in both emotion and behavior take place. Another process in which response tendencies are crafted by environmental conditions to optimize the match between individual and environment is development8 (Box 1). Indeed the similarities between learning and development are such that they are often considered analogous processes9 (Box 2). Many studies have demonstrated that in development, as in learning, emotional experiences induced by particular stimuli can act as a key modulator of stimulus impact and profoundly influence maturational outcome10–13. Thus, just as in learning, emotional responses to stimuli in development play an important role in highlighting the significance of different stimuli and promote an ideal match between organism, behavior, and environment.
BOX 1- DEVELOPMENT: A DANCE BETWEEN NATURE AND NURTURE.
Although the central nervous system is one of the earliest organ systems to emerge during gestation, maturation is a protracted process. Indeed, in humans, neural development is now thought to extend well beyond puberty, into early adulthood30. Over the first several years of life, brain development is marked by neurogenesis and establishment of new synaptic contacts. This expansion is then followed by a gradual refinement process which consists of synaptic pruning and myelination27, 30. This refinement process follows a developmental sequence, from phylogenetically conserved regions involved in sensory and motor processes to regions involved in more complex functions like multisensory integration and socio-perceptual functions27, 30, 32.
The extent to which developmental refinement is driven by intrinsic maturational processes or constructed primarily as a consequence of experiential inputs has been a source of considerable debate92. Over the last several decades, a pendulum has swung from nativist intrinsic explanations, to constructivist empirical explanations, which emphasize environmental contingencies. More recently, models have begun to characterize development as a culmination of the inseparable interaction between biological and environmental factors8, 11. These models suggest that while there is a loosely defined sequence, timeline, and process associated with maturation of the nervous system, the output of this maturation is largely influenced by the environmental context in which development occurs. Some models also suggest a differential susceptibility to environmental influence8.
There are many examples that illustrate how developmental outcomes are shaped by interactions between biology and environment. A classic example is language. While language acquisition is a milestone of normative development, the specific language acquired is completely dependent on the context in which maturation occurs44, 93. Similar examples of this principle are found across many domains and include “programming” the dynamics of the hypothalamo-pituitary-adrenal axis, where early stress can alter the homeostatic balance of cortisol detection and release11, 85; tuning of sensory and perceptual sensitivity, where discrimination of subtle elements becomes more or less sensitive with early life exposure12, 41, 45; acquisition of parental behavior patterns, which are influenced by patterns of one’s own parental behavior63; and even adoption of preferred characteristics for subsequent mate preferences43. Therefore for most neural circuits the final maturational outcome of depends completely on an interaction between genes and experience.
BOX 2: NEUROBIOLOGICAL MECHANISMS OF LEARNING AND DEVELOPMENT: STRENGTHENING CONNECTIONS.
Over the last several decades great strides have been made in delineating the molecular mechanisms that mediate learning94. Much of this research is guided by models of simple associative conditioning which demonstrate that synaptic strength between two weakly connected cells increases when they fire simultaneously94. A key molecular component of this strengthening is activation of the glutamate NMDA receptor. This receptor acts as a “coincidence detector” that strengthens connectivity of co-active cells by opening calcium ion channels, which ultimately induces protein-based cellular changes responsible for long term stabilization of circuits82, 95. Inhibitory GABAergic connections may also play an important modulatory role in this strengthening process. Because GABA exerts tonic inhibitory control on some NMDA circuits, modulation of GABA input is thought to be an important precursor for NMDA- mediated learning under some circumstances67, 82, 96
Likewise, in development, circuit strength is refined through patterns of synaptic activity that are affected by environmental context27. The early stages of this refinement are characterized by a highly dynamic phase of formation and retraction of synaptic contacts on newly formed dendritic spines95. In a manner very similar to synaptic strengthening implicated in traditional hebbian learning, activity within these nascent synaptic contacts are stabilized and maintained via activation of NMDA receptors, influx of intracellular calcium ions, induction of transcription factors, and neurotrophin release95. Moreover, recent evidence indicates that inhibitory GABA inputs may also help to tune receptive fields and gate neuronal sensitivity to environmental input across development96. Thus, in both maturation and traditional learning, synaptic and circuit stabilization is a process of amplifying and strengthening connections that are weakly co-active. Mechanistically this depends on glutamate NMDA receptors, a cascade of events triggered by intracellular calcium influx, and GABA mediated inhibition of competing influences.
During critical developmental phases, rapid synaptic refinement occurs in specific circuits. Thus, environmental contexts which induce synapse-specific neuronal activity may have a particularly strong influence on specific circuit function. At a systems level, sensory processing has been used to model the effects of environment on brain organization across development. This work demonstrates the experience of visual, auditory, or somatosensory stimuli during specific developmental windows are critical for the organization of cortical circuitry, parcelation of cortical space and functional responsivity36, 37, 97. These models of sensory development may serve as more general models of experiential influence during sensitive periods.
A key aspect of developmental adaptations is that the timing of stimulus encounters is of utmost importance. Development progresses through a series of sensitive periods or time windows during which particular classes of stimuli (or stimulus domains) are highly influential in affecting the course and trajectory of maturation14, 15. This time-sensitive and domain specific property of development highlights the importance of maximizing the impact of relevant experiences during developmentally appropriate periods. While there are a number of factors that influence the potency of discrete classes of stimuli across development, here we suggest that differences in emotional response may be an important, and often overlooked, means of heightening the salience of environmental features at specific sensitive periods.
Based on a similar role in learning paradigms we suggest that an important neuronal hub in this process is the amygdala. The amygdala is thought to be a key region for the orchestration of emotional responding, and for the flexible attribution of salience to different stimuli in the environment. In addition the amygdala is a critical site for emotional facilitation of memory3, 16, 17. A number of recent studies have revealed systematic differences in patterns of amygdala activity across development18–23 and differential consequences of amygdala damage incurred at discrete periods of development have also been observed24, 25. These findings suggest that shifting amygdala-mediated affective processes are a key component of development. We argue that differential emotional reactivity and amygdala response in particular are likely to play an important role in highlighting developmentally relevant features of the environment. Such a fluctuation in amygdala sensitivity may serve to orient the organism to important features of the environment during sensitive maturational periods.
PERIODS OF SENSITIVITY AND INSENSITIVITY IN DEVELOPMENT: TIMING IS EVERYTHING
In broad terms, there are two components involved in the development of the nervous system. The first is an organizational framework which is primarily mediated by intrinsic factors such as gene expression, which spur the generation and elaboration of neurons and determine the general spatial organization of neural tissue26, 27. In the second phase, the organizational framework is fine-tuned. This fine-tuning is based on use-dependent preservation or elimination of neural circuits, which ultimately results in an extensive network of differentially weighted neural networks28. This second, fine tuning, process is affected to a much greater extent by the environmental context than is establishment of the initial organizational framework27, 28
The developmental tuning process is both protracted and serial. Recent findings indicate that the brain does not complete developmental maturation until early adulthood, and development proceeds in a piecemeal fashion with phylogenetically newer regions undergoing maturation subsequent to older regions29–32. Thus, the fine tuning phase of development proceeds through a serial pattern of distinct sensitive periods and these are likely to extend from the first few years of life well into the third decade in humans15, 29, 33–35.
The pattern of sensitive periods was first demonstrated in the visual system of nonhuman primates and cats by Nobel prize winning work of Hubel and Wiesel, where restriction of visual input for a brief period of development resulted in dramatic alterations in the maturation of the visual system at cellular, morphological, and functional levels26. Many of these alterations persisted throughout life in spite of normative exposure to stimuli through late development and maturity. Similar phenomena have also been observed in humans in the development of the visual and auditory systems36, 37. While the early work focused on visual experience broadly defined during specified developmental windows, more recent studies have revealed sub-domains of specificity within visual development where, for example, visual acuity and motion detection represent different periods of development when emerging circuits are sensitive to different aspects of visual experiences in the environment36.
Although early work focused on the development of primary sensory systems, sensitive periods have been demonstrated in other emerging neural circuits as well including those involved in auditory and visual-spatial mapping38; avian song learning39; filial imprinting40, 41; sexual imprinting42, 43; language acquisition44; cortisol homeostasis10, 11; and face perception45 processes. In all of these examples, the organization of the brain is particularly sensitive to domain-specific stimuli for a limited period of time in development. Exposure to the same stimuli either before or after the sensitive window is either ineffective in inducing organizational change, or more often, the ability to induce such changes is markedly blunted26, 36, 44.
Furthermore, because the maturational sequence is protracted and staged31, different neural circuits undergo functional and structural development during different temporal windows for which only specific environmental conditions are relevant. Thus unique stimulus domains are developmentally relevant at specific periods14, 15. Because of this, the impact of various environmental stimuli will depend on which circuits are concurrently undergoing maturation.
While the most robust evidence for sensitive periods in development has been found in the early postnatal period, mounting evidence suggests that sensitive periods for different domains also occur later in development46–48. For example animal based studies have shown that exposure to chemosensory signals for a limited period of time around puberty are uniquely capable of organizing neural circuits and behavioral responses related to reproduction46. In both humans and animal models recent studies indicate that both brain and behavioral responses to many drugs of abuse appear to peak during the peri-pubertal period48. Furthermore, in humans epidemiological studies have pointed to the adolescent period as a time when many emotional disorders initially emerge which has led some to suggest that this is a sensitive period for affective organization47, 48. Finally, neuroimaging and histological investigations over the last several years have indicated that maturational changes continue to take place well beyond puberty31, 32. Because periods of heightened sensitivity are likely to emerge whenever circuits are undergoing developmental fine-tuning, these findings suggest that sensitive periods are likely to extend into early adult life as well.
An important corollary to the thesis that development is marked by periods of heightened sensitivity is that development is also likely to be punctuated by periods during which the brain is relatively insensitive to particular sensory domains or periods of domain specific insensitivity, where environmental stimuli may be less efficacious in inducing learned adaptations, or produce distinct outcomes in children and adults. Several examples of such an effect have also been found. For instance, infant rats are incapable of developing an aversion to an odor that is associated with either an illness49 or a shock41. This developmentally specific deficit in inhibitory learning may be an adaptive response because infants do not have many options when it comes to caregiver, nest location, or food; developing an aversion to any of these would be maladaptive, and tend to reduce survival rates41, 49. Indeed Sullivan and colleagues have demonstrated that infant rats develop a preference for odors that are paired with shocks during the early infantile period41. Recently, developmentally specific deficits have also been demonstrated in fear extinction learning in both human and rodent models50, 51. These studies have shown that relative to both juveniles and adults, adolescents are selectively impaired in the ability to extinguish an acquired fear in a pavlovian conditioning paradigm50. Furthermore adolescents demonstrate a unique suppression of some types of fear learning that was acquired early in life51. While it is unclear what adaptive function such a fear inflexibility might convey, these findings do underscore the point that development is marked by periods of both heightened and diminished sensitivity to selective stimuli. As such an important feature of such pervasive emotional fluctuations development may be to accentuate the features that are relevant during the specific and time sensitive windows of sensitivity, and thereby match the maturational state of the organism with the environmental conditions in which development is taking place.
THE FUNCTIONAL ROLE OF EMOTION IN DEVELOPMENT
On a daily basis we are bombarded by stimuli. While some of the stimuli we encounter are significant, the vast majority are not. An important aspect of optimizing behavioral responses in a particular environment is to highlight the significant stimuli, and rapidly generate an appropriate response. Emotions serve an important role in this process. Emotion selectively enhances the sensory processing of stimuli thereby amplifying the signal imprint of select stimuli5. Emotions also coordinate and direct responses among a number of systems (like attention, endocrine, and locomotor responses) which tend otherwise to be orthogonal52,1, 53. Finally emotions promote the long term memory of specific stimuli5, 54, 55,6, and often result in consistent learned behavior patterns5, 54–56,7. Thus, through numerous means emotional experiences augment the imprint specific stimuli leave on the nervous system.
In a developmental context selective engagement of emotional experience may help guide the nervous system to seek out and incorporate specific stimulus domains that are most relevant for concurrent brain circuit maturation. In this way shifts in emotional sensitivity may be an intrinsic signal of development that highlights developmentally relevant features in the environment. One domain in which developmentally linked dynamic shifts in emotionality is particularly apparent is in response to social affiliative stimuli. Here, affective intensity varies in systematic ways for discrete social categories and events across development.
An example of such fluctuating emotional reactivity across development can be seen in results from a recent study that examined developmental changes in response of infant monkeys to maternal separation (see Fig 1). Across the first 6 months of life, there was a steady decline in emotional distress (vocalization frequency), in response to maternal separation57. While this pattern could be viewed as the emergence of cortical systems involved in affect regulation58 we believe that findings from other domains of affiliative behavior which indicate peaks and troughs of emotional responsiveness at different points in development support a more nuanced interpretation.
Fig One.
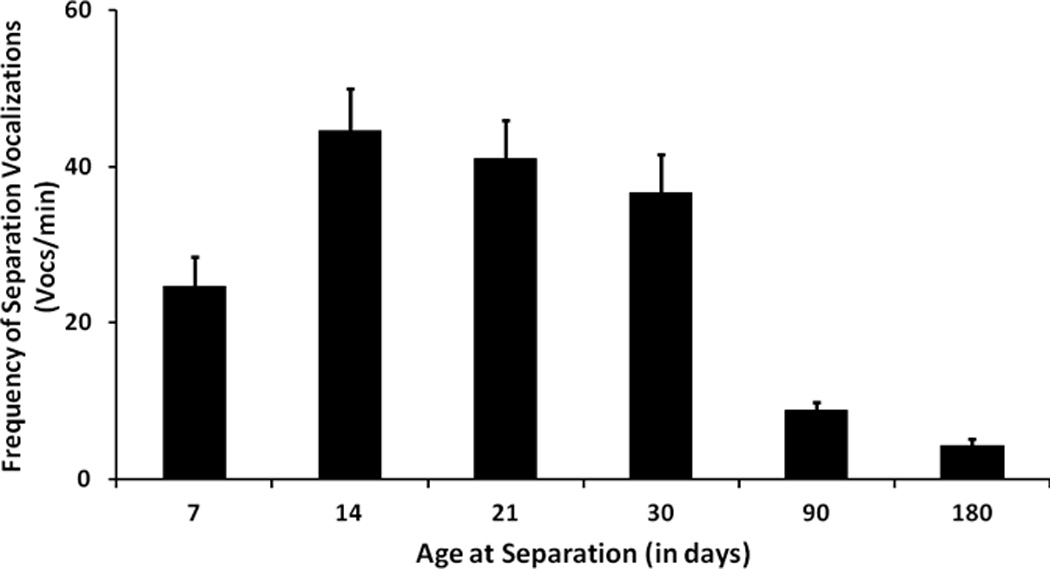
Figure One is a depiction of the frequency of cries emitted by rhesus monkey infants across the first 6 months of life during a period of separation from the mother. The systematic decline in this index of distress is similar to what has been observed in humans and we believe is a reflection of a shift in motivational state across development. Reprinted from58
For example, in rodent studies of play, motivation to engage in bouts of free play with peers displays a marked inverted u across development with a peak occurring in the mid juvenile period between weaning and puberty59, 60. At a slightly later point, during early puberty and adolescence, integration with peer groups rather than play becomes most motivationally salient61 and slightly later yet, interaction with romantic partners62 elicits stronger emotional reactions than at other points in development. Indeed emotional reactions to opposite sex peers is the most common sources of positive affect in adolescence and is also a leading cause of negative affective responses including depression62. Finally, sensory stimuli related to infants evoke strong emotional responses among mammalian mothers, including humans, immediately after they give birth63. Although the postpartum period is not typically considered a developmental phase, the orchestrated shift in emotional responsiveness during this period is remarkably similar to other developmental shifts described above and so is included here.
While the examples cited above focus on changing emotional attributions within the social context, developmental fluctuations of emotional responsiveness are not limited to interpersonal or social processes. For example emotional responses to stimulus color64; darkness65; novelty66; risky situations67; psychoactive substances48; and other stimuli and contexts that are not explicitly social68 vary across development, with peak responsiveness occurring at different points in maturation. We believe these shifts in emotional reactivity are an important and regulated aspect of development that serve to orient, enhance, and orchestrate brain maturation by highlighting the significant stimuli in the environment that will guide fine tuning of the nervous system at key sensitive periods.
THE ROLE OF THE AMYGDALA
Emotions are clearly complex phenomena and as indicated above, emotional experience likely results from the orchestrated response of multiple neuronal regions rather than being embedded in any single structure1, 53. That being said, however, it is clear that regions in the limbic system and related subcortical sturctures play a particularly prominent role in generating emotional responses because they are strongly interconnected with multiple sensory and effector systems1, 2, 69. A particularly important limbic region in the present context is the amygdala. The amygdala is prominently featured in many models of emotion3, 16, 17, 54, 70, 71 In addition the amygdala has been specifically implicated in many of the processes we highlight here including emotionally mediated shifts in attention, enhanced perceptual processing, and long term consolidation of emotional experiences5, 6, 17, 72.
Recent conceptualizations of amygdala function suggest a primary role is assigning salience to stimuli5, 6, 17, 72 Importantly, amygdala activity signifies salience for stimuli which are both appetitive and aversive, and for stimuli whose salience is both innate (snakes) or acquired (ie CS+)3, 16, 17, 70, 72, 73. Another important feature of amygdala function in several recent models is the flexibility of salience attribution to the same stimulus. The magnitude of mygdala response can vary to the same stimulus at different points in time or in different contexts, and this variance tracks the concurrent value of the stimulus to the organism3, 17 These features of the amygdala make it ideally suited to selectively track the importance of different stimulus domains across development as well. As we have argued above, dynamic shifts in the value of different stimulus domains take place across normative development. As development progresses, some stimuli (eg a kiss from mother) lose value while other stimuli (eg a kiss from a potential mate) gain in value. We propose that in development, just as in learning, the amygdala plays the specific role of enhancing the value of stimuli during sensitive periods of development and this then functions to highlight specific environmental features that are used in the fine tuning phase of neural circuit maturation.
There are several indications from recent developmental studies suggesting that the amygdala may play such a role. Although the amygdala begins to mature early in fetal life and is fully functional at birth, many studies have demonstrated dynamic changes in amygdala structure and function across development. For example, volumetric changes in the amygdala continue through puberty,71, 74 and dysregulation in the trajectory of this growth are linked with neurodevelopmental disorders such as autism74 whose symptoms include deficits in social development. In addition, developmental changes in gene expression75 and differential patterns of activity in amygdala induced by similar stimulus conditions have been observed in both human and animal models of development41, 71 Moreover, functional changes in amygdala responsiveness have been linked to changes in circulating steroid levels that are associated with specific developmental milestones such as weaning41 and puberty71. Thus physiological indices of developmental transitions may mediate behavior shifts via effects on amygdala function.
Several neuroimaging studies have also noted differences in functional activation of the amygdala during normative development. Across a wide developmental span of 4–17 years of age, a recent study reported declining amygdala activation to images of unfamiliar adults which tracked the decline in measures of stranger anxiety23. This finding suggests that the normative reduction in stranger anxiety that occurs across development may be mediated by declines in salience that the amygdala ascribes to unfamiliar adults. A number of studies have also reported developmental shifts in amygdala response to unfamiliar faces across later stages of development. When viewing emotional faces, and fearful faces in particular, activity in amygdala increases from childhood to adolescence, and then declines from adolescence to adulthood19, 21. These findings suggest that the amygdala may highlight the salience of social expressions during a developmental period when monitoring emotional experiences of peers is highly relevant for development.22, 61
Finally, more direct evidence for a functional role in development has been obtained from studies that show lesions to the amygdala produce fundamentally different patterns of behavior as a function of the developmental stage during which the lesion occurred. In non-human primates, young monkeys who received amygdala lesions as neonates displayed a pattern of extreme fearfulness when they interacted with peers76. This contrasts with findings from animals who sustained the same lesion later in development. Monkeys who received amygdala lesions as juveniles or young adults displayed a pattern of social behavior that is marked by reduced levels of fear24, 77. The interpretation of these discrepant findings is that the amygdala plays a developmentally specific role in guiding social learning. Animals that had very little social experience with an intact amygdala were unable to use the amygdala to guide behavior during early social interactions, and therefore failed to acquire rudimentary patterns of social behavior or social expectations and consequently were chronically socially dysfunctional, fearful and submissive76, 78. In contrast, juvenile animals who had a functional amygdala during early peer interactions established basic rules and expectations of peer behavior, and therefore were not fearful in a social context. In fact the low levels of fear exhibited by the juvenile-lesioned animals along with other differences in social behavior suggest deficits in completely different domains of behavior (such as wariness and dominance) which are typically acquired during adolescence77, 79. Moreover, although the magnitude of these effects diminished over the course of development in both groups of monkeys distinct patterns in most functions did persist into adulthood suggesting that amygdala modulated social behavior in development has persistent effects78, 80
Finally there have been similar findings of developmental specificity of deficits observed in humans who received amygdala lesions either early in life or as adults25. Relative to the adult-lesioned patients, those who sustained amygdala damage either neonatally or during early childhood demonstrated specific deficits in interpreting nuanced social language like ironic or figurative statements and were significantly impaired in interpreting emotional signals of other individuals. The authors suggest that this impairment highlights a specific role of the amygdala in acquiring social knowledge in early development. A process which is facilitated by amygdala guided salience of social cues16.
LONG TERM EFFECTS OF EMOTION IN DEVELOPMENT
An important function of emotionally guided developmental change that we are postulating here is that the changes persist beyond the emotional experience. Limbic regions, like the amygdala and hippocampus, play a key role in orchestrating emotional responses and facilitating learning during the initial exposures to stimuli. However, over time, both the emotional response and limbic activity evoked by a particular stimulus wanes, even as a behavior patterns persist. In other words, as emotionally mediated memories become consolidated, they promote flexible behavior patterns that are largely independent of the original emotional context, or the emotionally mediated neuronal structures originally engaged by this context.
Emotional learning studies have found that long term emotionally modulated memories are consolidated in distributed neocortical networks, which may be engaged in the absence of the initial emotional experience6, 81. For example, learning-based plasticity generated by fear conditioning has been detected in the amygdala, but also in local connectivity and functional activation patterns within the neocortex82. Recent animal models of amygdala mediated pavlovian conditioning have revealed functional modifications in responsiveness of neocortical circuits that are generated when a neutral conditioned stimulus is paired with electrical stimulation of the amygdala6, 81. These cortical changes persist for a long period of time and are subsequently independent of amygdala activity. A similar pattern of persistent changes in brain and behavior that persist independently of the original emotional context or neural underpinnings has been demonstrated in other paradigms as well83,84. Thus in traditional learning paradigms emotional modulation of stimuli generates a pattern of learning in which long term memories are instantiated in cortical rather than limbic function and the behavior persists independent of original emotional modulatory effects.
We recently observed a developmental effect that resembles this phenomenon in development20 (see Fig 2). In this task adolescent and adult participants underwent neuroimaging as they viewed faces paired (CS+) or unpaired (CS−) with an aversive sound. Adolescents were unable to accurately label the two CS stimuli, but engaged the amygdala significantly more to CS+ than CS− images. In contrast, adults were able to rapidly categorize the two stimulus classes but displayed relatively little differential amygdala activity. Importantly adults but not adolescents engaged a region of lateral prefrontal cortex when making safety assessment (CS−>Cs+). In the present context we believe these findings highlight two themes of this review. First, because associating emotions with faces is a developmentally pertinent task for adolescents, such an experience is likely to be more salient, and engage the amygdala to a greater extent in adolescents than adults. Second, because of previous developmental experiences, adults have undergone a cortical transfer of this process. Such a corticalization has enabled adults to explicitly categorize facial stimuli much more easily than adolescents. Thus amygdala-guided experiences in development generated an amygdala independent capability in mature adults
Fig Two.
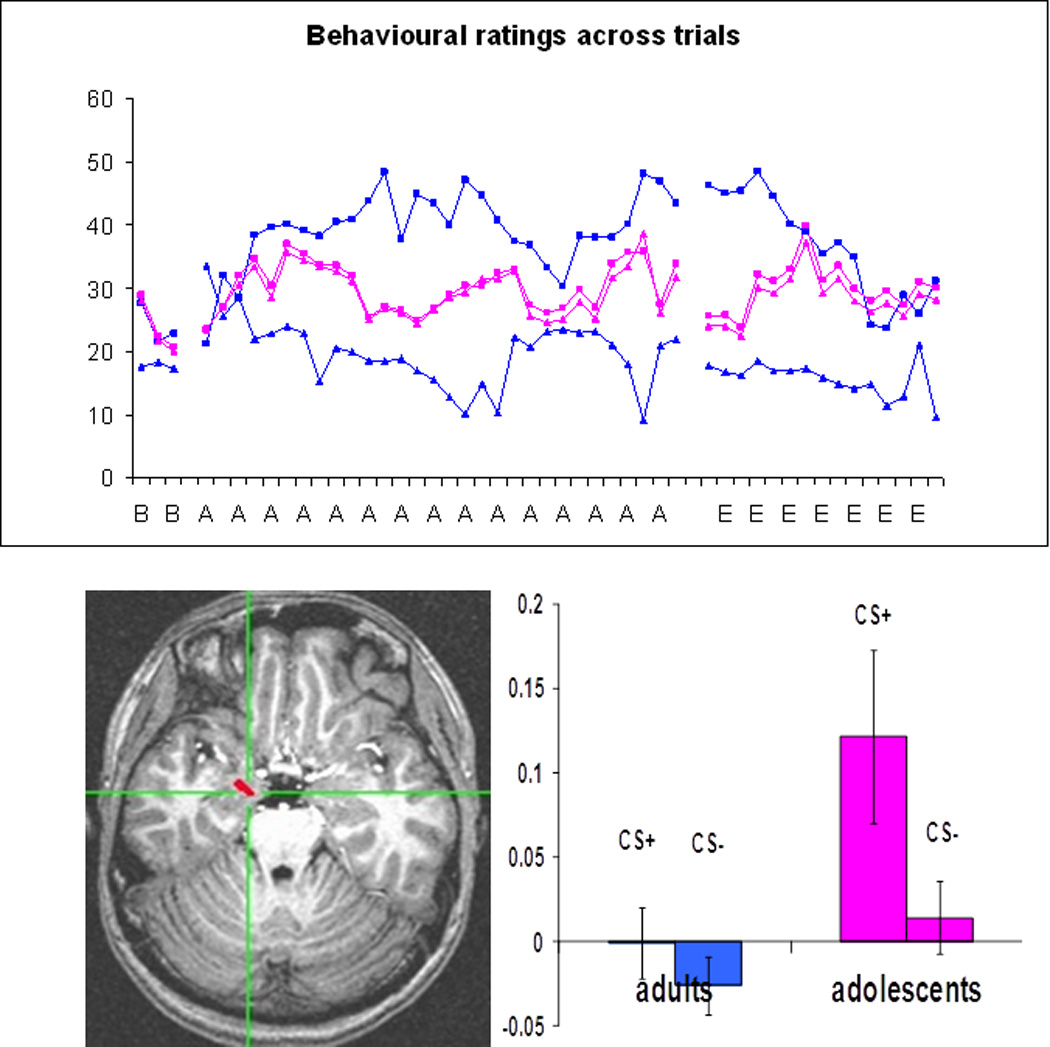
Figure Two (a top panel) is a depiction of self reported fear of a CS+ (squares) and CS− (triangles) during an aversive conditioning paradigm in a group of psychiatrically healthy adolescents and young adults. Stimuli and ratings were collected during fMRI across acquisition (A) and non-reinforced extinction (E) trials. Concurrent activity in the amygdala is depicted in Fig 2b (bottom) in this same group of subjects. While adults rapidly develop a differential pattern of categorizing these two stimuli into fear and no fear groups, no such difference is apparent in the adolescents. In contrast adolescents but not adults displayed a differential pattern of amygdala activation when viewing these same stimuli during fear acquisition training. Adults displayed a pattern of differential activation in the dorsolateral prefrontal cortex that tracked safety ratings. These data demonstrate the transient nature of amygdala activity across development and suggest that once domain specific categorical skills are acquired amygdala dependent functions are transferred to neocortex. Reprinted from20
This feature of emotional memory, which permits emotion to act as a temporary guide for long term adaptive patterns of responding, is ideally suited for developmental adaptations that persist across the lifespan. Indeed, many studies have shown that extreme emotional experiences during specific and brief periods of development can generate persistent alterations in perceptual processing12; endocrine homeostasis85; social behavior13, 86 and a number of other nervous system functions35, 87, 88 that persist well beyond the original emotional experience. If specific stimulus domains (i.e., peers) evoke emotional attributions while distinct neural networks are being formed, long-term adaptations to specific environmental conditions may be facilitated. Internally mediated shifts in emotional attributions may therefore be an important mechanism for highlighting developmentally relevant components of the environment and guiding maturation of specific neural networks (see Fig 3).
Fig Three.
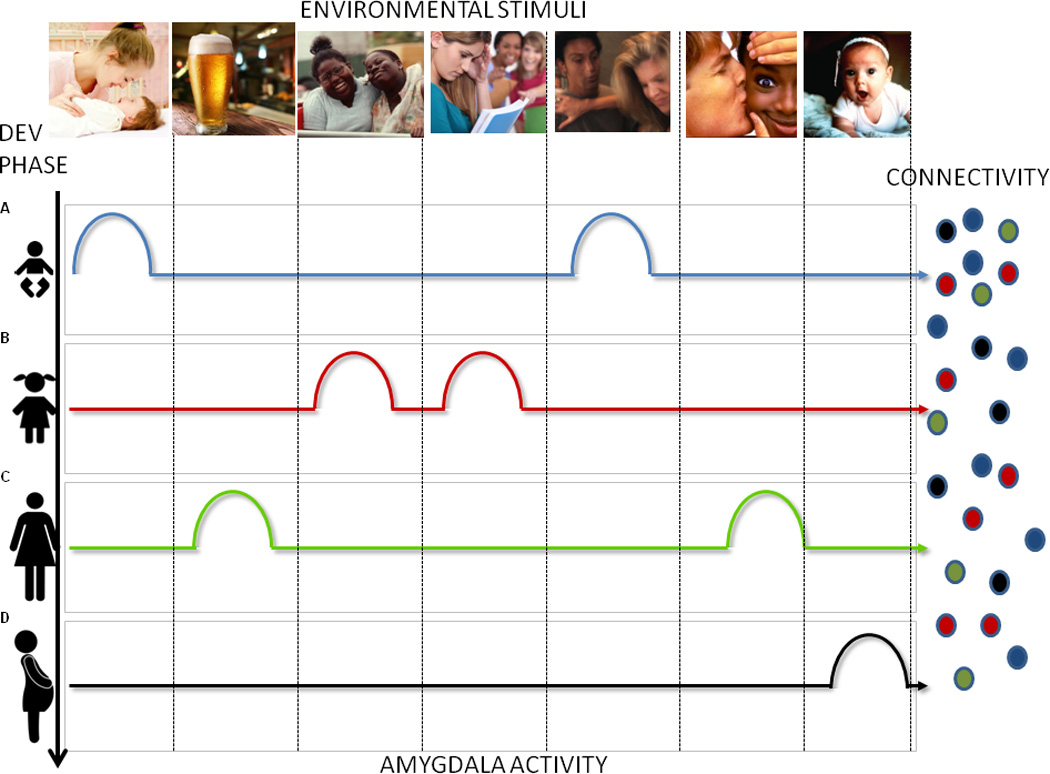
Figure three is a schematic representation of the proposed role for the amygdala in development. Different domains in the environment (ie caretakers, peers, babies) are depicted as images across the top while developmental phases are depicted in vertical rows on the left. Selective amygdala responses within each developmental phase to each stimulus domain are indicated by rows of colored bumps. Amygdala activation in response to each stimulus domain highlights the presence of this stimulus class in the environment and generates long term patterns of cortical connectivity depicted as small circular arrays on the right. Amygdala modulated circuit maturation that occurs during these specified developmental windows ultimately function independently of amygdala activity.
Our model is depicted schematically in Figure Three in which different stimulus domains are depicted across the top and developmental phases are represented as vertical rows. Differential amygdala activation in response to select stimulus domains occurs across development which fine tunes emerging circuits and results in long term stable patterns of brain function.
COMPLIMENTARY PERSPECTIVES
Finally while we do present unique perspectives, we note that several complimentary viewpoints particularly in regards to a functional role of emotion in development have recently appeared in the literature. Galvan9 observed the similar mechanisms involved in learning and developmentally mediated plasticity and Lourenco and Casey89 discussed the role of non-linear patterns of emotion and learning in development; Pfeifer and Allen90, and Crone and Dahl33, have also suggested ways in which emotion may facilitate flexibility and adaptation across development; and Bjork and colleagues discussed how contextual variability may relate to developmental differences in emotion91. Finally, Scherf et al proposed that steroid hormones released at puberty may lead to a re-organization of behavior via their effects on the amygdala and consequent shifts in emotional behavior71. Although each of these perspectives vary somewhat in details and primary focus, they all present a perspective in which emotion and learning are central to the developmental experience.
HIGHLIGHTS.
Learning and development enable adaptive changes in brain via similar neurobiological mechanisms
Emotion plays important role in facilitating both learning and development
Amygdala-mediated motional sensitivity and stimulus salience change across development in domain specific ways.
Emotional variability may help guide learning during developmentally sensitive periods
FUTURE DIRECTIONS.
An important remaining question is what triggers shifts in salience attribution across development. Intrinsic signals associated with developmental milestones like puberty and weaning have been linked to functional changes in the amygdala, yet other intrinsic signals are also likely and yet to be indentified.
Future work is needed to determine the extent to which systematic fluctuations in emotional responsiveness play a functional role in maturation of brain and behavior or simply reflect a non functional by-product of other maturational processes
Structures besides the amygdala are likely involved in emotional modulation of learning. Expanding the developmental focus beyond the amygdala will also be important. For example recent studies suggest a similar developmental profile within the striatum may relate to risk taking and substance use in adolescence.
Could emotional induction modify neuronal and behavioral patterns acquired during the critical periods, even after they have passed?
Does emotionally modulated learning during sensitive periods inform onset trajectory or treatment of psychopathology?
Box 3: ADAPTATION MECHANISMS UNIQUE TO DEVELOPMENT.
While many of the same mechanisms are invoked to adapt the nervous system in development and learning, there are also important differences. One is the scale on which these changes occur. Traditional learning typically involves relatively modest modifications to synaptic strength, while developmental adaptations are typically large in scale26, 28. The amount of experience needed to drive learning and developmental changes is also quite different Marked developmental changes usually result from limited stimulus exposure, but learning generally requires more exposure26. Organization of cortical space is one example of such differences. In development, limited exposure to sensory experiences can induce dramatic changes such as ocular dominance columns or tonotopic organization of the auditory cortex36, 37. While changes to cortical architecture have also been noted following adult learning26, 98, the changes are much more modest than those that occur during development.
While learning and development share many mechanisms of change There are also some that are specific to development. Synaptic Pruning (SP) is one such mechanism in which synaptic contacts are eliminated in a systematic fashion27, 32. SP occurs at various developmental periods in different brain regions, and is more drawn out in evolutionarily newer regions like granular prefrontal cortex and multi-modal association areas27, 32. Indeed, in humans, SP appears to continue throughout the third decade of life, and may be one of the primary contributors to decline in gray matter that occurs between late childhood and early adulthood27, 31, 32
In addition to NMDA- and GABA-mediated stablilzation of synapses seen in both learning and development, epigenetics is another mechanism by which adaptive neuronal changes may be facilitated. Epigenetics refers to modifications to the DNA, or the surrounding chromatin scaffolding (on which the DNA sits), that alters DNA transcription11. While some modifications like acetylation tend to be dynamic across the lifespan99, others, like methylation, occur primarily during early periods of maturation, and tend to result in lifelong (and sometimes trans-generational) inhibition of genetic expression11, 100. Recently it has been shown that psychological experiences during a specified developmental window can also alter patterns of DNA methylation and these patterns generally persist throughout the life of the animal11. In short some but not all of the mechanisms of neural plasticity are shared between ‘traditional’ and ‘developmental’ learning; and the magnitude and duration of plastic changes may not be comparable in both forms of adaptation.
Acknowledgements
EN and JJ were funded by the intramural research program of the National Institute of Mental Health. JL received funding from the Economic and Social Research Council and from the Calleva Centre for Evolution and Human Science.
REFERENCES
- 1.Damasio A, Carvalho GB. The nature of feelings: evolutionary and neurobiological origins. Nat Rev Neurosci. 2013;14:143–152. doi: 10.1038/nrn3403. [DOI] [PubMed] [Google Scholar]
- 2.Berridge KC, Kringelbach ML. Neuroscience of affect: brain mechanisms of pleasure and displeasure. Curr Opin Neurobiol. 2013;23:294–303. doi: 10.1016/j.conb.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray EA. The amygdala, reward and emotion. Trends Cogn Sci. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Bergado JA, et al. Emotional tagging--a simple hypothesis in a complex reality. Prog Neurobiol. 2011;94:64–76. doi: 10.1016/j.pneurobio.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Pourtois G, et al. Brain mechanisms for emotional influences on perception and attention: what is magic and what is not. Biol Psychol. 2013;92:492–512. doi: 10.1016/j.biopsycho.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Roozendaal B, McGaugh JL. Memory modulation. Behav Neurosci. 2011;125:797–824. doi: 10.1037/a0026187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gruber AJ, McDonald RJ. Context, emotion, and the strategic pursuit of goals: interactions among multiple brain systems controlling motivated behavior. Front Behav Neurosci. 2012;6:50. doi: 10.3389/fnbeh.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis BJ, et al. Differential susceptibility to the environment: an evolutionary--neurodevelopmental theory. Dev Psychopathol. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- 9.Galvan A. Neural plasticity of development and learning. Hum Brain Mapp. 2010;31:879–890. doi: 10.1002/hbm.21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Meaney MJ. Epigenetics and the biological definition of gene x environment interactions. Child Dev. 2010;81:41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- 12.Pollak SD, et al. Development of perceptual expertise in emotion recognition. Cognition. 2009;110:242–247. doi: 10.1016/j.cognition.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rutter M, et al. Longitudinal studies using a "natural experiment" design: the case of adoptees from Romanian institutions. J Am Acad Child Adolesc Psychiatry. 2012;51:762–770. doi: 10.1016/j.jaac.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Bischof HJ. Behavioral and neuronal aspects of developmental sensitive periods. Neuroreport. 2007;18:461–465. doi: 10.1097/WNR.0b013e328014204e. [DOI] [PubMed] [Google Scholar]
- 15.Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- 16.Adolphs R. What does the amygdala contribute to social cognition? Ann N Y Acad Sci. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pessoa L. Emotion and cognition and the amygdala: from "what is it?" to "what's to be done?". Neuropsychologia. 2010;48:3416–3429. doi: 10.1016/j.neuropsychologia.2010.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forbes EE, et al. Neural systems of threat processing in adolescents: role of pubertal maturation and relation to measures of negative affect. Dev Neuropsychol. 2011;36:429–452. doi: 10.1080/87565641.2010.550178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guyer AE, et al. A developmental examination of amygdala response to facial expressions. J Cogn Neurosci. 2008;20:1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lau JY, et al. Distinct neural signatures of threat learning in adolescents and adults. Proc Natl Acad Sci U S A. 2011;108:4500–4505. doi: 10.1073/pnas.1005494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore WE, 3rd, et al. Facing puberty: associations between pubertal development and neural responses to affective facial displays. Soc Cogn Affect Neurosci. 2012;7:35–43. doi: 10.1093/scan/nsr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfeifer JH, et al. Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron. 2011;69:1029–1036. doi: 10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tottenham N, et al. Amygdala response to mother. Dev Sci. 2012;15:307–319. doi: 10.1111/j.1467-7687.2011.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bachevalier J, Malkova L. The amygdala and development of social cognition: theoretical comment on Bauman, Toscano, Mason, Lavenex, and Amaral (2006) Behav Neurosci. 2006;120:989–991. doi: 10.1037/0735-7044.120.4.989. [DOI] [PubMed] [Google Scholar]
- 25.Shaw P, et al. The impact of early and late damage to the human amygdala on 'theory of mind' reasoning. Brain. 2004;127:1535–1548. doi: 10.1093/brain/awh168. [DOI] [PubMed] [Google Scholar]
- 26.Levelt CN, Hubener M. Critical-period plasticity in the visual cortex. Annu Rev Neurosci. 2012;35:309–330. doi: 10.1146/annurev-neuro-061010-113813. [DOI] [PubMed] [Google Scholar]
- 27.Stiles J. The Fundamentals of Brain Development Integrating Nature and Nurture. Harverd University Press; 2008. [Google Scholar]
- 28.Sur M, Rubenstein JL. Patterning and plasticity of the cerebral cortex. Science. 2005;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- 29.Blakemore SJ. Development of the social brain in adolescence. J R Soc Med. 2012;105:111–116. doi: 10.1258/jrsm.2011.110221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giedd JN, et al. Trajectories of anatomic brain development as a phenotype. Novartis Found Symp. 2008;289:101–112. doi: 10.1002/9780470751251.ch9. discussion 112–108, 193–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gogtay N, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petanjek Z, et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat Rev Neurosci. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- 34.Steinberg L. Cognitive and affective development in adolescence. Trends Cogn Sci. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Teicher MH, et al. Hurtful words: association of exposure to peer verbal abuse with elevated psychiatric symptom scores and corpus callosum abnormalities. Am J Psychiatry. 2010;167:1464–1471. doi: 10.1176/appi.ajp.2010.10010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maurer D, et al. Missing sights: consequences for visual cognitive development. Trends Cogn Sci. 2005;9:144–151. doi: 10.1016/j.tics.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Sharma A, et al. Cortical development, plasticity and re-organization in children with cochlear implants. J Commun Disord. 2009;42:272–279. doi: 10.1016/j.jcomdis.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keuroghlian AS, Knudsen EI. Adaptive auditory plasticity in developing and adult animals. Prog Neurobiol. 2007;82:109–121. doi: 10.1016/j.pneurobio.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Brainard MS, Doupe AJ. What songbirds teach us about learning. Nature. 2002;417:351–358. doi: 10.1038/417351a. [DOI] [PubMed] [Google Scholar]
- 40.Horn G. Pathways of the past: the imprint of memory. Nat Rev Neurosci. 2004;5:108–120. doi: 10.1038/nrn1324. [DOI] [PubMed] [Google Scholar]
- 41.Sullivan RM, et al. Developmental neurobiology of olfactory preference and avoidance learning. In: Blumberg MS, et al., editors. Oxford Handbook of Developmental Behavioral Neuroscience. Oxford: 2010. pp. 573–586. [Google Scholar]
- 42.Adkins-Regan E. Neuroendocrine contributions to sexual partner preference in birds. Front Neuroendocrinol. 2011;32:155–163. doi: 10.1016/j.yfrne.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Fillion TJ, Blass EM. Infantile experience with suckling odors determines adult sexual behavior in male rats. Science. 1986;231:729–731. doi: 10.1126/science.3945807. [DOI] [PubMed] [Google Scholar]
- 44.Kuhl PK. Brain mechanisms in early language acquisition. Neuron. 2010;67:713–727. doi: 10.1016/j.neuron.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pascalis O, et al. Plasticity of face processing in infancy. Proc Natl Acad Sci U S A. 2005;102:5297–5300. doi: 10.1073/pnas.0406627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romeo RD, et al. Puberty and the maturation of the male brain and sexual behavior: recasting a behavioral potential. Neurosci Biobehav Rev. 2002;26:381–391. doi: 10.1016/s0149-7634(02)00009-x. [DOI] [PubMed] [Google Scholar]
- 47.Paus T, et al. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spear LP, Varlinskaya EI. Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: implications for prevention science? Dev Psychobiol. 2010;52:236–243. doi: 10.1002/dev.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alberts JR, Pickler RH. Evolution and development of dual ingestion systems in mammals: notes on a new thesis and its clinical implications. Int J Pediatr. 2012;2012:730673. doi: 10.1155/2012/730673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pattwell SS, et al. Altered fear learning across development in both mouse and human. Proc Natl Acad Sci U S A. 2012;109:16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pattwell SS, et al. Selective early-acquired fear memories undergo temporary suppression during adolescence. Proc Natl Acad Sci U S A. 2011;108:1182–1187. doi: 10.1073/pnas.1012975108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jacobs RH, et al. The amygdala, top-down effects, and selective attention to features. Neurosci Biobehav Rev. 2012;36:2069–2084. doi: 10.1016/j.neubiorev.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 53.Panksepp J. The basic emotional circuits of mammalian brains: do animals have affective lives? Neurosci Biobehav Rev. 2011;35:1791–1804. doi: 10.1016/j.neubiorev.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 54.Richter-Levin G, Akirav I. Emotional tagging of memory formation--in the search for neural mechanisms. Brain Res Brain Res Rev. 2003;43:247–256. doi: 10.1016/j.brainresrev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Watanabe N, et al. Reward prediction error signal enhanced by striatum-amygdala interaction explains the acceleration of probabilistic reward learning by emotion. J Neurosci. 2013;33:4487–4493. doi: 10.1523/JNEUROSCI.3400-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mineka S, Ohman A. Phobias and preparedness: the selective, automatic, and encapsulated nature of fear. Biol Psychiatry. 2002;52:927–937. doi: 10.1016/s0006-3223(02)01669-4. [DOI] [PubMed] [Google Scholar]
- 57.Zhang B, et al. Developmental changes of rhesus monkeys in response to separation from the mother. Dev Psychobiol. 2012;54:798–807. doi: 10.1002/dev.21000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Casey B, et al. Braking and Accelerating of the Adolescent Brain. J Res Adolesc. 2011;21:21–33. doi: 10.1111/j.1532-7795.2010.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Panksepp J, et al. The psychobiology of play: theoretical and methodological perspectives. Neurosci Biobehav Rev. 1984;8:465–492. doi: 10.1016/0149-7634(84)90005-8. [DOI] [PubMed] [Google Scholar]
- 60.Varlinskaya EI, Spear LP. Social interactions in adolescent and adult Sprague-Dawley rats: impact of social deprivation and test context familiarity. Behav Brain Res. 2008;188:398–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steinberg L, Monahan KC. Age differences in resistance to peer influence. Dev Psychol. 2007;43:1531–1543. doi: 10.1037/0012-1649.43.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Collins WA, et al. Adolescent romantic relationships. Annu Rev Psychol. 2009;60:631–652. doi: 10.1146/annurev.psych.60.110707.163459. [DOI] [PubMed] [Google Scholar]
- 63.Olazabal DE, et al. New theoretical and experimental approaches on maternal motivation in mammals. Neurosci Biobehav Rev. 2013;37:1860–1874. doi: 10.1016/j.neubiorev.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 64.Franklin A, et al. Biological components of colour preference in infancy. Dev Sci. 2010;13:346–354. doi: 10.1111/j.1467-7687.2009.00884.x. [DOI] [PubMed] [Google Scholar]
- 65.Meltzer H, et al. Children's specific fears. Child Care Health Dev. 2009;35:781–789. doi: 10.1111/j.1365-2214.2008.00908.x. [DOI] [PubMed] [Google Scholar]
- 66.Clinton SM, et al. Developmental underpinnings of differences in rodent novelty-seeking and emotional reactivity. Eur J Neurosci. 2011;34:994–1005. doi: 10.1111/j.1460-9568.2011.07811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith SS. alpha 4 beta delta GABA receptors and tonic inhibitory current during adolescence: effects on mood and synaptic plasticity. Front Neural Circuits. 2013;7:135. doi: 10.3389/fncir.2013.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Forbes EE, et al. Healthy adolescents' neural response to reward: associations with puberty, positive affect, and depressive symptoms. J Am Acad Child Adolesc Psychiatry. 2010;49:162–172. e161–e165. doi: 10.1097/00004583-201002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lindquist KA, Barrett LF. A functional architecture of the human brain: emerging insights from the science of emotion. Trends Cogn Sci. 2012;16:533–540. doi: 10.1016/j.tics.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mahler SV, Berridge KC. What and when to "want"? Amygdala-based focusing of incentive salience upon sugar and sex. Psychopharmacology (Berl) 2012;221:407–426. doi: 10.1007/s00213-011-2588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Scherf KS, et al. The amygdala: An agent of change in adolescent neural networks. Horm Behav. 2013 doi: 10.1016/j.yhbeh.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.LeDoux J. The amygdala. Curr Biol. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 73.Kalin NH, et al. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schumann CM, et al. Abnormal structure or function of the amygdala is a common component of neurodevelopmental disorders. Neuropsychologia. 2011;49:745–759. doi: 10.1016/j.neuropsychologia.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sabatini MJ, et al. Amygdala gene expression correlates of social behavior in monkeys experiencing maternal separation. J Neurosci. 2007;27:3295–3304. doi: 10.1523/JNEUROSCI.4765-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bauman MD, et al. The development of social behavior following neonatal amygdala lesions in rhesus monkeys. J Cogn Neurosci. 2004;16:1388–1411. doi: 10.1162/0898929042304741. [DOI] [PubMed] [Google Scholar]
- 77.Kalin NH, et al. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. J Neurosci. 2001;21:2067–2074. doi: 10.1523/JNEUROSCI.21-06-02067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bliss-Moreau E, et al. The Impact of Early Amygdala Damage on Juvenile Rhesus Macaque Social Behavior. J Cogn Neurosci. 2013 doi: 10.1162/jocn_a_00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2006;120:761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- 80.Malkova L, et al. Long-term effects of neonatal medial temporal ablations on socioemotional behavior in monkeys (Macaca mulatta) Behav Neurosci. 2010;124:742–760. doi: 10.1037/a0021622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chavez CM, et al. Activation of the basolateral amygdala induces long-term enhancement of specific memory representations in the cerebral cortex. Neurobiol Learn Mem. 2013;101:8–18. doi: 10.1016/j.nlm.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johansen JP, et al. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Euston DR, et al. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76:1057–1070. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clark JJ, et al. Dopamine encoding of Pavlovian incentive stimuli diminishes with extended training. J Neurosci. 2013;33:3526–3532. doi: 10.1523/JNEUROSCI.5119-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heim C, et al. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol. 2010;52:671–690. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- 86.Bell HC, et al. Juvenile peer play experience and the development of the orbitofrontal and medial prefrontal cortices. Behav Brain Res. 2009;207:7–13. doi: 10.1016/j.bbr.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 87.Brown DW, et al. Adverse childhood experiences and the risk of premature mortality. Am J Prev Med. 2009;37:389–396. doi: 10.1016/j.amepre.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 88.Schneider P, et al. Adolescent peer-rejection persistently alters pain perception and CB1 receptor expression in female rats. Eur Neuropsychopharmacol. 2013 doi: 10.1016/j.euroneuro.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 89.Lourenco F, Casey BJ. Adjusting behavior to changing environmental demands with development. Neurosci Biobehav Rev. 2013 doi: 10.1016/j.neubiorev.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pfeifer JH, Allen NB. Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends Cogn Sci. 2012;16:322–329. doi: 10.1016/j.tics.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bjork JM, et al. Brain maturation and risky behavior: the promise and challenges of neuroimaging-based accounts. Child Development Perspectives. 2012;6:385–391. [Google Scholar]
- 92.Karmiloff-Smith A. Precis of Beyond modularity: a developmental perspective on cognitive science. Behavioral and Brain Sciences. 1994;17:693–745. [Google Scholar]
- 93.Cheour M, et al. Development of language-specific phoneme representations in the infant brain. Nat Neurosci. 1998;1:351–353. doi: 10.1038/1561. [DOI] [PubMed] [Google Scholar]
- 94.Kandel ER. The molecular biology of memory: cAMP PKA CRE, CREB-1, CREB-2, and CPEB. Mol Brain. 2013;5:14. doi: 10.1186/1756-6606-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lohmann C, Kessels H. The Developmental Stages of Synaptic Plasticity. J Physiol. 2013 doi: 10.1113/jphysiol.2012.235119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang ZJ, Scheiffele P. GABA and neuroligin signaling: linking synaptic activity and adhesion in inhibitory synapse development. Curr Opin Neurobiol. 2008;18:77–83. doi: 10.1016/j.conb.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maurer D, et al. Repeated measurements of contrast sensitivity reveal limits to visual plasticity after early binocular deprivation in humans. Neuropsychologia. 2006;44:2104–2112. doi: 10.1016/j.neuropsychologia.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 98.May A. Experience-dependent structural plasticity in the adult human brain. Trends Cogn Sci. 2011;15:475–482. doi: 10.1016/j.tics.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 99.Peixoto L, Abel T. The role of histone acetylation in memory formation and cognitive impairments. Neuropsychopharmacology. 2013;38:62–76. doi: 10.1038/npp.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bohacek J, et al. Transgenerational epigenetic effects on brain functions. Biol Psychiatry. 2013;73:313–320. doi: 10.1016/j.biopsych.2012.08.019. [DOI] [PubMed] [Google Scholar]


