Abstract
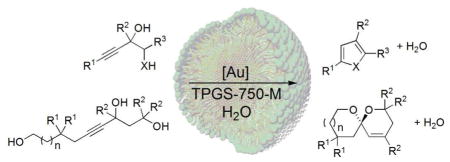
The first examples of gold-catalyzed cyclizations of diols and triols to the corresponding hetero- or spirocycles in a bulk aqueous medium are presented. These reactions take place within nanomicelles, where the hydrophobic effect is operating, thereby driving the dehydrations, notwithstanding the surrounding water. By the addition of simple salts such as sodium chloride, reaction times and catalyst loadings can be significantly decreased.
Dehydrative reactions that take place in organic media are typically driven by the presence of a dehydrating agent, such as molecular sieves. As an alternative strategy, micellar catalysis offers an opportunity to take advantage of the hydrophobic effect that exists within the lipophilic cores of nanomicelles formed upon dissolution of a surfactant in water.1 Since the surfactant is providing a very limited amount of an organic medium in which these reactions can take place, reactions occur under typically high internal concentrations and under mild, usually room temperature conditions. Water is expelled for entropic reasons; it cannot solvate the hydrocarbon present on the inside of these nanoparticles. Nonetheless, solvent effects can be large, as with any organic reaction run in an organic medium, and hence, the nature of the amphiphile can play a significant role in the outcome of reactions run under such aqueous conditions. This has been extensively demonstrated in transition metal-catalyzed reactions, including several name reactions that are Nobel-prize winning, such as olefin metathesis,2 Heck reactions,3 and Suzuki-Miyaura couplings.4 The preclusion of water from the inside of such nanoreactors has also been used, contrary to textbook thinking, to effect both net Negishi-type couplings of highly moisture sensitive organozinc reagents,5 as well as conjugate additions via water-intolerant organocopper complexes, in the absence of pre-formed organometallics.6 These latter two cases provide strong evidence that the surrounding water does not easily penetrate the hydrophobic micellar pockets. On the basis of these observations, it was anticipated that the hydrophobic effect would act to drive gold-catalyzed dehydrative reactions to cyclic products7 in bulk water.
Our investigations began with acetylenic diol 1 which undergoes dehydrative cyclization to furan 2. Previously, cationic or neutral gold(I) catalysts in THF or toluene was used for this purpose, sometimes in the presence of activated molecular sieves for scavenging water.8 Treatment of 1 with 5 mol % AuBr3 in 2 weight % aqueous PTS (poly(oxyethyl)-α-tocopheryl sebacate; global concentration 0.5 M) for 2 h afforded furan 2 in excellent yield (90%; Table 1, entry 1). In accordance with previous work by Aponick et al.8a, a control experiment done “on water” (i.e., without amphiphile) led to no product formation under identical conditions, documenting that these are reactions taking place under micellar catalysis conditions (entry 2). With second generation surfactant TPGS-750-M the yield was slightly higher (entry 3). Other surfactants such as TPGS-1000, Brij 30, and Nok9 gave inferior levels of conversion (entries 4–6). It was possible to decrease the gold catalyst loading to 2 mol % but with somewhat elongated reaction times (entries 7 and 8). Addition of silver salts or use of cationic gold catalyst A (entry 13) did not lead to higher yields or shorter reaction times (entries 9–13). All subsequent experiments were performed in aqueous TPGS-750-M.
Table 1.
Gold-catalyzed Dehydrative Cyclization of Diol 1.

| |||||
|---|---|---|---|---|---|
| entry | [Au] | surfactant | time (h) | convb (%) | yieldc (%) |
| 1 | AuBr3 | PTS | 2 | 94 | 90 |
| 2 | AuBr3 | none | 7 | 0 | nd |
| 3 | AuBr3 | TPGS-750-M | 2 | 87 | 91 (2.5 h) |
| 4 | AuBr3 | TPGS-1000 | 2 | 79 | nd |
| 5 | AuBr3 | Brij 30 | 2 | 50 | nd |
| 6 | AuBr3 | NOK | 2 | 63 | nd |
| 7 | AuBr3c | PTS | 2 | 35 | 88 (4 h) |
| 8 | AuBr3d | TPGS-750-M | 2 | 16 | 86 (6 h) |
| 9 | AuCl3/AgOTf | TPGS-750-M | 6 | nd | 70 |
| 10 | AuCl3/AgSbF6 | TPGS-750-M | 6 | nd | 68 |
| 11 | AuBr3/AgOTf | TPGS-750-M | 2 | nd | 92 |
| 12 | AuBr3/AgSbF6 | TPGS-750-M | 2 | nd | 90 |
| 13 | Ae | TPGS-750-M | 3 | nd | 84 |
PTS = poly(oxyethyl)-α-tocopheryl sebacate; TPGS-750-M = D-α-tocopherol-polyethyleneglycol-750-succinate monomethyl ether; TPGS-1000 = D-α-tocopherol-polyethyleneglycol-1000-succinate; Brij 30 = tetraethyleneglycol-monododecyl ether; NOK = β-sitosterol-polyethyleneglycol-600-succinate monomethyl ether.
Conversion determined by GC-MS.
Isolated yield.
2 mol %.
A = [t-Bu2(o-biphenyl)]PAu(MeCN)SbF6.
After this first example (1 to 2) we explored other diols as well as amino alcohols bearing secondary amino groups. Primary amines have shown poor reactivity in gold catalyzed cyclizations due to deactivation of the gold catalyst by the Lewis basic amine.10 Under these micellar conditions, however, pyrroles 4a and 4b were formed smoothly and quickly; after 1 h, with high yields of 91% and 93%, respectively (Table 2, entries 1, 2). Diols 3c–3e were also cyclized readily with different gold(I) and gold(III) catalysts (entries 3–14); the latter seem to be best suited for these substrates with reaction times of 5–20 min and result in high yields (83–92%; entries 3, 7, 11). This may reflect a limited stability of gold(I) salts in aqueous media. Diols 3f–3i with different substitution patterns were subjected to 2 mol % AuBr3/AgOTf to form furans with various substituents at the 2-, 3-, and 5-positions. With this lower catalyst loading the products could be obtained in 2 h with consistently good yields. It is worth noting that with increasing steric hindrance at the 2- and/or 3-positions, the yield was increased (compare entries 16 and 17). Moreover, these reactions tolerate the presence of a nitrile (entries 11–14) and an olefin (entry 15).
Table 2.
Gold-catalyzed Dehydrative Cyclization of Diols and Amino Alcohols 3.

| ||||||||
|---|---|---|---|---|---|---|---|---|
| entry | 3 | X | R1 | R2 | R3 | [Au] | time | 4/yielda (%) |
| 1 | 3a | NBu | Ph | H | H | AuBr3 | 1 h | 4a (91) |
| 2 | 3b | NBn | Ph | H | H | AuBr3 | 1 h | 4b (93) |
| 3 | 3c | O | Ph | H | H | AuBr3 | 20 min | 4c (92) |
| 4 | 3c | O | Ph | H | H | AuCl | 2 h | 4c (79) |
| 5 | 3c | O | Ph | H | H | Ph3PAuCl/AgOTf | 40 min | 4c (82) |
| 6 | 3c | O | Ph | H | H | Ph3PAuCl/AgBF4 | 4 h | 4c (84) |
| 7 | 3d | O | n-C8H17 | H | H | AuBr3 | 5 min | 4d (90) |
| 8 | 3d | O | n-C8H17 | H | H | AuCl | 3 h | 4d (76) |
| 9 | 3d | O | n-C8H17 | H | H | Ph3PAuCl/AgOTf | 1 h | 4d (85) |
| 10 | 3d | O | n-C8H17 | H | H | Ph3PAuCl/AgBF4 | 6 h | 4d (86) |
| 11 | 3e | O | (CH2)3CN | H | H | AuBr3 | 10 min | 4e (83) |
| 12 | 3e | O | (CH2)3CN | H | H | AuCl | 2 h | 4e (80) |
| 13 | 3e | O | (CH2)3CN | H | H | Ph3PAuCl/AgOTf | 1 h | 4e (82) |
| 14 | 3e | O | (CH2)3CN | H | H | Ph3PAuCl/AgBF4 | 4 h | 4e (80) |
| 15 | 3f | O | cyclohexen-1-yl | H | H | AuBr3/AgOTf b | 2 h | 4f (83) |
| 16 | 3g | O | Ph | Ph | Ph | AuBr3/AgOTf b | 2 h | 4g (95) |
| 17 | 3h | O | Ph | Me | Me | AuBr3/AgOTf b | 2 h | 4h (85) |
| 18 | 3i | O | n-Hex | Me | Me | AuBr3/AgOTf b | 2 h | 4i (85) |
Isolated yield.
2 mol %.
Further optimization focused on decreasing the time associated with these dehydrative cyclization reactions. From previous work it had been shown that dissolution of salts, such as NaCl, in the aqueous reaction medium can lead to beneficial increases in micellar diameter, thereby extending the time for substrate/catalyst exposure to the inner lipophilic core.1,11 The outgrowth of this simple modification, in addition to a further reduction in catalyst loading to 1 mol %, is an enhanced reaction rate leading to shorter reaction times (Table 3, entries 4, 6, 8–10).
Table 3.
Gold-catalyzed Dehydrative Cyclization of Diols 3 in the Presence of NaCl.

| ||||||
|---|---|---|---|---|---|---|
| entry | 3 | R | AuBr3 (mol %) | additive | time (min) | 4/yielda (%) |
| 1 | 3c | Ph | 5 | - | 20 | 4c (92) |
| 2 | 3c | Ph | 2 | - | 50 | 4c (91) |
| 3 | 3c | Ph | 1 | - | 120 | 4c (88) |
| 4 | 3c | Ph | 1 | NaClb | 30 | 4c (90) |
| 5 | 3d | n-C8H17 | 5 | - | 5 | 4d (90) |
| 6 | 3d | n-C8H17 | 1 | NaClb | 20 | 4d (87) |
| 7 | 3e | (CH2)3CN | 5 | - | 10 | 4e (83) |
| 8 | 3e | (CH2)3CN | 1 | NaClb | 40 | 4e (91) |
| 9 | 3j | n-Hex | 2 | NaClb | 30 | 4j (89) |
| 10 | 3k | (CH2)3Cl | 2 | NaClb | 30 | 4k (86) |
Isolated yield.
2 M.
Previous experiments run in D2O have shown that the proton that replaces the gold on the ring derives from the surrounding water, rather than the starting alcohol. If iodine (one equivalent) is present in the reaction of diol 3g, iodinated furan 5 is formed in 93% isolated yield (Scheme 1). With the iodinated furan in hand, it is possible to use it in palladium-catalyzed cross-coupling reactions.12
Scheme 1.
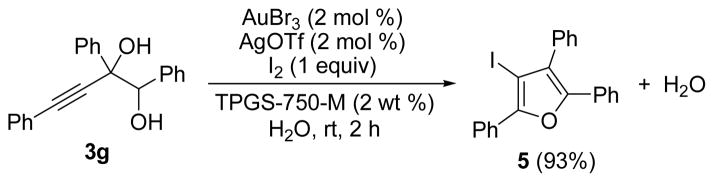
Gold-catalyzed dehydrative cyclization of diol 3g in the presence of iodine.
Further application of this technology beyond diols was made to triols, thereby forming various spirocycles (Table 4). Previously, cationic gold(I) catalysts in THF were used for this transformation, in the presence of activated molecular sieves for scavenging water.13 Triol 6a was smoothly cyclized with Ph3PAuCl/AgOTf in water containing 2 wt % TPGS-750-M at room temperature over 5 h to the corresponding spirocycle 7a (89% yield; entry 1). Under the same conditions, increased steric hindrance at the 1- and 3-positions dramatically slowed the conversion (triol 6b; entry 2). An increased rate of protodeauration after the initial cyclization led to formation of a complex mixture containing unsaturated monocyclization products13a together with the desired spirocycle 7b. With a cationic gold(I) catalyst formed from B and AgOTf the yield of 7b could be doubled (entry 3), but it was only after the use of precatalyst C/AgOTf that a moderate yield of product 7b could be isolated (46%; entry 4). The related triol 6c bearing the same steric hindrance at the 1- and 3-positions, but with a longer carbon chain (albeit without phenyl substituents) could be cyclized in 64% yield to the corresponding spirocycle 7c upon heating to 40 °C for 12 h.
Table 4.
Gold-catalyzed Dehydrative Spirocyclization of Triols 6.

| |||||||
|---|---|---|---|---|---|---|---|
| entry | 6 | R1 | R2 | n | [Au] | time (h) | 7/yielda (%) |
| 1 | 6a | Ph | H | 0 | Ph3PAuCl | 5 | 7a (89) |
| 2 | 6b | Ph | Me | 0 | Ph3PAuCl | 4 | 7b (11) |
| 3 | 6b | Ph | Me | 0 | Bb | 4 | 7b (22)c |
| 4 | 6b | Ph | Me | 0 | Cd | 4 | 7b (46) |
| 5 | 6c | H | Me | 1 | Ph3PAuCl | 12 | 7c (64)e |
Isolated yield.
B = [c-Hex2(o-biphenyl)]PAuCl.
40 °C.
C = [t-Bu2(o-biphenyl)]PAuCl.
19% reisolated starting material.
In summary, acetylenic vicinal diols and amino alcohols can be cyclodehydrated to the corresponding furans and pyrroles, respectively, using nanoparticle technology in water. Nanomicelles composed of the designer surfactant TPGS-750-M, which is an item of commerce, enable these gold-catalyzed reactions to take place at ambient temperatures and in high isolated yields. Appropriately substituted alkynes containing three hydroxyl residues can be converted to spirocyclic products in the presence of a Au(I) catalyst. Further work on developing an enantioselective version of this route to spirocycles is currently under study.
Supplementary Material
Acknowledgments
Financial support for our programs in green chemistry provided by the NIH (GM 86485) to BHL is warmly acknowledged with thanks.
Footnotes
Supporting Information Available: Experimental procedures and data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Contributor Information
Norbert Krause, Email: norbert.krause@tu-dortmund.de.
Bruce H. Lipshutz, Email: lipshutz@chem.ucsb.edu.
References
- 1.a) Lipshutz BH, Ghorai S. Aldrichimica Acta. 2008;41:59–72. [PMC free article] [PubMed] [Google Scholar]; b) Lipshutz BH, Ghorai S. Aldrichimica Acta. 2012;45:3–16. [PMC free article] [PubMed] [Google Scholar]; c) Lipshutz BH, Isley NA, Fennewald JC, Slack ED. Angew Chem Int Ed. 2013;52:10952–10958. doi: 10.1002/anie.201302020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a) Lipshutz BH, Aguinaldo GT, Ghorai S, Voigtritter K. Org Lett. 2008;10:1325–1328. doi: 10.1021/ol800028x. [DOI] [PubMed] [Google Scholar]; b) Lipshutz BH, Ghorai S, Aguinaldo GT. Adv Synth Catal. 2008;350:953–956. [Google Scholar]; c) Lipshutz BH, Ghorai S. Tetrahedron. 2010;66:1057–1063. [Google Scholar]
- 3.Lipshutz BH, Taft BR. Org Lett. 2008;10:1329–1332. doi: 10.1021/ol702755g. [DOI] [PubMed] [Google Scholar]
- 4.a) Lipshutz BH, Petersen TB, Abela AR. Org Lett. 2008;10:1333–1336. doi: 10.1021/ol702714y. [DOI] [PubMed] [Google Scholar]; b) Lipshutz BH, Abela AR. Org Lett. 2008;10:5329–5332. doi: 10.1021/ol801712e. [DOI] [PubMed] [Google Scholar]; c) Nishikata T, Lipshutz BH. J Am Chem Soc. 2009;131:12103–12105. doi: 10.1021/ja905082c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Krasovskiy A, Duplais C, Lipshutz BH. J Am Chem Soc. 2009;131:15592–15593. doi: 10.1021/ja906803t. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Krasovskiy A, Duplais C, Lipshutz BH. Org Lett. 2010;12:4742–4744. doi: 10.1021/ol101885t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipshutz BH, Huang S, Leong WWY, Zhong G, Isley NA. J Am Chem Soc. 2012;134:19985–19988. doi: 10.1021/ja309409e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biannic B, Aponick A. Eur J Org Chem. 2011:6605–6617.See also: Manabe K, Iimura S, Sun X-M, Kobayashi S. J Am Chem Soc. 2002;124:11971–11978. doi: 10.1021/ja026241j.
- 8.a) Aponick A, Li CY, Malinge J, Marques EF. Org Lett. 2009;11:4624–4627. doi: 10.1021/ol901901m. [DOI] [PubMed] [Google Scholar]; b) Egi M, Azechi K, Akai S. Org Lett. 2009;11:5002–5005. doi: 10.1021/ol901942t. [DOI] [PubMed] [Google Scholar]; c) Egi M, Azechi K, Akai S. Adv Synth Catal. 2011;353:287–290. [Google Scholar]
- 9.Klumphu P, Lipshutz BH. J Org Chem. doi: 10.1021/jo401744b. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a) Morita N, Krause N. Org Lett. 2004;6:4121–4123. doi: 10.1021/ol0481838. [DOI] [PubMed] [Google Scholar]; b) Morita N, Krause N. Eur J Org Chem. 2006:4634–4641. [Google Scholar]
- 11.Minkler SRK, Lipshutz BH, Krause N. Angew Chem Int Ed. 2011;50:7820–7823. doi: 10.1002/anie.201101396. [DOI] [PubMed] [Google Scholar]
- 12.a) Gockel B, Krause N. Eur J Org Chem. 2010:311–316. [Google Scholar]; b) Bew SP, El-Taeb GMM, Jones S, Knight DW, Tan WF. Eur J Org Chem. 2007:5759–5770. [Google Scholar]
- 13.Aponick A, Li CY, Palmes JA. Org Lett. 2009;11:121–124. doi: 10.1021/ol802491m.See also: Fang C, Pang Y, Forsyth CJ. Org Lett. 2010;12:4528–4531. doi: 10.1021/ol101833h.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


