Abstract
A total of 1,023 environmental surfaces were sampled from 45 rooms with patients infected or colonized with methicillin-resistant Staphylococcus aureus (MRSA) or vancomycin-resistant enterococci (VRE) before terminal room cleaning. Colonized patients had higher median total target colony-forming units (CFU) of MRSA or VRE than did infected patients (median, 25 CFU [interquartile range, 0–106 CFU] vs 0 CFU [interquartile range, 0–29 CFU]; P = .033).
Healthcare-associated infections (HAIs) represent a substantial cause of morbidity, cost, and increased length of stay in the United States.1 The contaminated hospital environment has emerged as a key target area to prevent the spread of HAIs.2,3 For example, patients infected or colonized with methicillin-resistant Staphylococcus aureus (MRSA) or vancomycin-resistant enterococci (VRE) contaminate the surfaces of their rooms. These bacteria can contaminate the gloves and/or hands of healthcare personnel (HCP) and be transferred to other patients.4,5
To our knowledge, environmental contamination from patients infected with MRSA or VRE has not been compared with the environmental contamination from patients colonized by these pathogens. Thus, we examined the difference in hospital room contamination between patients infected versus colonized with MRSA or VRE. Our a priori hypothesis was that patients with infection would lead to more environmental contamination than would patients with colonization by these pathogens.
METHODS
This study was performed at 2 tertiary acute care hospitals, Duke University Medical Center (753 beds) and the University of North Carolina (UNC) Health Care (804 beds). A convenience sample of 45 rooms of patients infected or colonized with MRSA or VRE (target organisms) were tested between July 21, 2009, and February 29, 2012, including 8 rooms at Duke University Medical Center and 37 rooms at UNC Health Care, as previously described.6 Microbiological and infection control databases were used to identify hospital rooms of patients currently under contact precautions due to colonization or infection with MRSA or VRE. The patients discharged from study rooms were assessed for current colonization versus infection via medical chart review, type of infection (if applicable), and the number and type of anatomic sites that were colonized or infected.
After identifying rooms with a target organism, 5–10 high-touch and medium-touch surfaces were sampled once with Rodac plates after patient discharge but before terminal room cleaning by environmental services.7 Each surface was sampled 3–5 times following a specific protocol.8 The following surfaces were chosen for sampling: sink or sink counter, toilet seat, over-bed or bedside table, bed rail, chair arm or seat, bathroom floor, floor by the bed or sink, television remote or computer monitor, medical cart, or laundry bin.
Dey/Engley Neutralizing Agar was used in the Rodac plates (surface area of 33.166 cm2). All plates were incubated at 37°C for 48 hours. Two quantitative microbiologic outcomes were calculated: total colony-forming units (CFU) of MRSA or VRE per room and per location sampled. The number of targeted pathogens was quantified by first identifying morphologies suggestive of the target organisms. These colonies were then subcultured and identified using standard micro-biological methods.
Means or medians were calculated, as appropriate. Median differences and interquartile ranges (IQRs) in room contamination of target organisms between infected and colonized patients were compared using Wilcoxon rank-sum tests; differences in means were determined using the Student t test. Statistical analysis was performed using SAS, version 9.3 (SAS Institute). Differences in room contamination between colonized and infected patients were also analyzed by room type (floor or intensive care unit [ICU]), by the number of days the patient occupied the room, and by the sampled room locations.
RESULTS
A total of 48 room measurements for target pathogens were taken from 45 individual rooms; 30 rooms (62.5%) contained patients who were colonized with either MRSA or VRE, 10 (20.8%) contained patients who had infections with either MRSA or VRE, and 4 contained patients who were colonized or infected with both MRSA and VRE. Of these dual-target rooms, 2 patients were infected with both MRSA and VRE, 1 patient was infected with VRE and colonized with MRSA, and 1 patient was colonized with both MRSA and VRE. Patient infection status was unknown for 1 room.
Nineteen patients (40%) were colonized or infected with MRSA, and 29 patients (60%) were colonized or infected with VRE. Forty-two patients (87.5%) had 1 anatomic site of colonization or infection, 5 (10.4%) had 2 sites, and 1 (2.1%) had 3 sites. Among the 15 infections, there were 3 surgical site infections, 4 urinary tract infections due to VRE (including 3 catheter-associated infections), 2 cases of ventilator-associated pneumonia due to MRSA, and 5 other infections. One patient had an abdominal wound with VRE infection and a urinary tract infection, 1 patient had a pelvic wound with both MRSA and VRE infection, 1 patient had bacteremia due to VRE, and 1 patient had a left ventricular assist device driveline infected with MRSA.
A total of 1,023 total environmental cultures were taken from 26 floor rooms and 22 ICU rooms (Table 1). Twenty-four rooms with colonized patients (73%) had 1 or more environmental sites positive for MRSA or VRE compared with 5 rooms with infected patients (33%; P < .001). Colonized patients were associated with higher median total target CFU (MRSA or VRE) in the study rooms than were infected patients (median, 25 CFU [interquartile range (IQR), 0–106 CFU] vs 0 CFU [IQR, 0–29 CFU]; P = .033; Figure 1). Nineteen rooms with patients infected or colonized with either target organism (40%) contained 0 CFU of VRE or MRSA, including 7 rooms with VRE-colonized patients, 7 rooms with VRE-infected patients, 2 rooms with MRSA-colonized patients, and 3 rooms with MRSA-infected patients. Finally, no significant differences were found between infected or colonized patients by organism, room type, or length of hospitalization, and the amount of contamination at specific environmental locations did not differ between infected versus colonized patients (Table 1). Although a greater number of surfaces were tested in rooms of colonized patients than in rooms of infected patients (6.52 ± 2.47 surfaces vs 4.07 ± 2.12 surfaces; P = .02), the number of target CFU per surfaces tested per room was higher among tests performed in rooms of colonized patients (median, 5 CFU [IQR, 5–10 CFU] vs 5 CFU [IQR, 5–5 CFU]; P = .003).
TABLE 1.
Colonized versus Infected Patient Room Contamination by Organism, Type of Room, and Room Location
| Variable | Colonized patient rooms | Infected patient rooms | Pa |
|---|---|---|---|
| Proportion (%) of rooms | 33/48 (69) | 15/48 (31) | |
| Total target organisms (MRSA and VRE), CFU, median (IQR) | 25 (0–106) | 0 (0–29) | .033 |
| Organism, CFU, median (IQR) | |||
| MRSA | 72 (9–222) | 0 (0–62) | .141 |
| VRE | 8 (0–60) | 0 (0–4) | .142 |
| Type of room, CFU, median (IQR) | |||
| Floor | 35 (8–150) | 0 (0–16.5) | .077 |
| ICU | 9 (0–106) | 0 (0–62) | .236 |
| Room surface, CFU, median (IQR) | |||
| Chair | 0 (0–6) | 0 (0–4) | .887 |
| Over-bed table | 0 (0–5) | 0 (0–3) | .901 |
| Bedrail | 0 (0–3) | 0 (0–0) | .466 |
| Sink | 0 (0–1.5) | 0 (0–0) | .328 |
| Bed table | 0 (0–0) | 0 (0–4) | .106 |
| Supply cart | 0 (0–3) | 0 (0–0) | .643 |
| Toilet | 0 (0–0) | 0 (0–208) | .183 |
| Bathroom floor | 2 (0–46) | 211 (211–211) | .255 |
| Floor (room) | 12.5 (2.5–225.5) | 269 (269–269) | .516 |
| Linen cart | 0 (0–2) | 0 (0–52) | .786 |
| Length of stay, days, median (IQR) | 16 (4–20) | 7 (4–16) | .280 |
NOTE. CFU, colony-forming units; ICU, intensive care unit; IQR, interquartile range; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci.
Wilcoxon rank-sum test.
FIGURE 1.
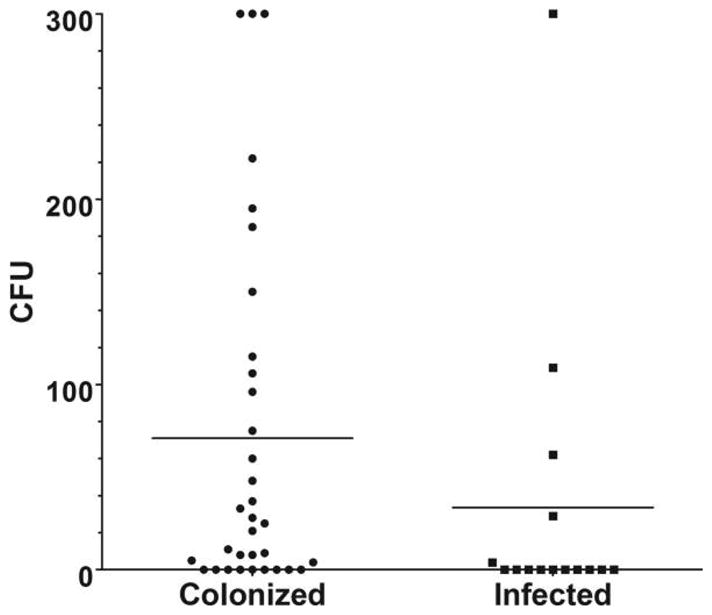
Room contamination with methicillin-resistant Staphylococcus aureus or vancomycin-resistant enterococci by colonized versus infected patients. Target colony-forming units (CFU) were capped at 300 CFU in this figure.
DISCUSSION
Environmental surfaces of patient rooms are a critical component in the spread of healthcare-associated infections.9 Contrary to our a priori hypothesis, MRSA or VRE more frequently contaminated rooms previously occupied by colonized patients than rooms previously occupied by infected patients in our multicenter trial (P = .033). Contamination was not associated with length of hospitalization, room type, or room surface tested.
It is unclear why colonized patients would contaminate their rooms as much as or more than infected patients. This finding requires additional analysis and validation. One potential explanation is that treatment with antibiotics reduces patients’ overall bacterial burden and subsequently decreases room contamination. Another possible reason for less room contamination among infected patients is that colonized patients are likely to be less acutely ill than infected patients and therefore more mobile in their rooms, thus providing more opportunity to contaminate surfaces. Neither hypothesis was specifically assessed in our study. Importantly, contact precautions were used for all patients in this study. Thus, contact precautions do not prevent environmental contamination. Regardless, we believe the current practice of using contact precautions to prevent contamination of healthcare worker clothes and hands from the environment appears to be appropriate for patients colonized or infected with MRSA or VRE.
Our study has limitations. First, this study included 2 tertiary care hospitals, so our results may not be generalizable to community hospitals. Second, this study included samples from 45 rooms, which limited our power to detect differences in environmental contamination of the 2 target organisms of MRSA and VRE. Finally, over one-third of the rooms in our study did not contain any target organism, which further limited our power to determine differences between room contamination associated with infected versus colonized patients.
In conclusion, our multicenter study identifies MRSA-colonized and VRE-colonized patients as significant contributors to hospital room contamination and emphasizes the importance of adequate cleaning techniques to prevent transmission of problem pathogens.
Acknowledgments
Financial support. This study was funded by the Centers for Disease Control and Prevention (CDC) Prevention Epicenters Program (U54CK000164). D.J.A. reports that he has received additional grant support from the National Institutes of Health/National Institute of Allergy and Infectious Diseases (K23AI095357).
Footnotes
Colleagues from the Centers for Disease Control and Prevention assisted in the review of study design but not did participate in manuscript preparation, submission, or review.
Potential conflicts of interest. D.J.A. reports participation on the Speaker’s Bureau for Merck and receipt of royalties from UpToDate Online. D.J.W. reports receipt of honoraria for consulting and speaker’s bureau services from PAI, Germitec, Pfizer, Merck, and Sanofi. W.A.R. reports providing consultation for Advance Sterilization Products and Clorox. All other authors report no conflicts of interest relevant to this article. All authors submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest, and the conflicts that the editors consider relevant to this article are disclosed here.
Presented in part: IDWeek 2013; San Francisco, California; October 5, 2013.
References
- 1.Petti S, Polimeni A, Dancer SJ. Effect of disposable barriers, disinfection, and cleaning on controlling methicillin-resistant Staphylococcus aureus environmental contamination. Am J Infect Control. 2013;41:836–840. doi: 10.1016/j.ajic.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 2.Otter JA, Yezli S, French GL. The role played by contaminated surfaces in the transmission of nosocomial pathogens. Infect Control Hosp Epidemiol. 2011;32:687–699. doi: 10.1086/660363. [DOI] [PubMed] [Google Scholar]
- 3.Carling PC, Huang SS. Improving healthcare environmental cleaning and disinfection: current and evolving issues. Infect Control Hosp Epidemiol. 2013;34:507–513. doi: 10.1086/670222. [DOI] [PubMed] [Google Scholar]
- 4.Weber DJ, Rutala WA. The role of the environment in transmission of Clostridium difficile infection in healthcare facilities. Infect Control Hosp Epidemiol. 2011;32:207–209. doi: 10.1086/658670. [DOI] [PubMed] [Google Scholar]
- 5.Hayden MK, Blom DW, Lyle EA, Moore CG, Weinstein RA. Risk of hand or glove contamination after contact with patients colonized with vancomycin-resistant enterococcus or the colonized patients’ environment. Infect Control Hosp Epidemiol. 2008;29:149–154. doi: 10.1086/524331. [DOI] [PubMed] [Google Scholar]
- 6.Rutala WA, Gergen MF, Weber DJ. Room decontamination with UV radiation. Infect Control Hosp Epidemiol. 2010;31:1025–1029. doi: 10.1086/656244. [DOI] [PubMed] [Google Scholar]
- 7.Huslage K, Rutala WA, Sickbert-Bennett E, Weber DJ. A quantitative approach to defining “high-touch” surfaces in hospitals. Infect Control Hosp Epidemiol. 2010;31:850–853. doi: 10.1086/655016. [DOI] [PubMed] [Google Scholar]
- 8.Anderson DJ, Gergen MF, Smathers E, et al. Decontamination of targeted pathogens from patient rooms using an automated ultraviolet-C-emitting device. Infect Control Hosp Epidemiol. 2013;34:466–471. doi: 10.1086/670215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Datta R, Platt R, Yokoe DS, Huang SS. Environmental cleaning intervention and risk of acquiring multidrug-resistant organisms from prior room occupants. Arch Intern Med. 2011;171:491–494. doi: 10.1001/archinternmed.2011.64. [DOI] [PubMed] [Google Scholar]


