Abstract
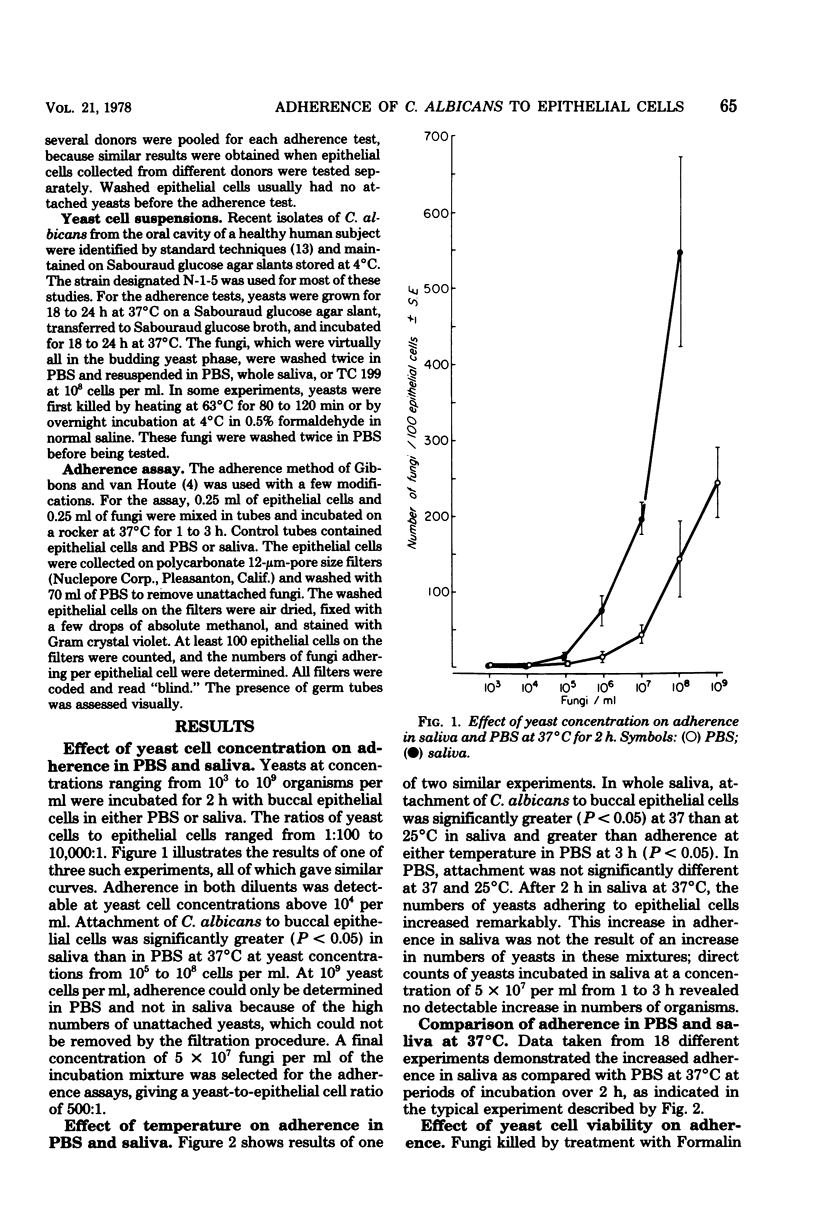
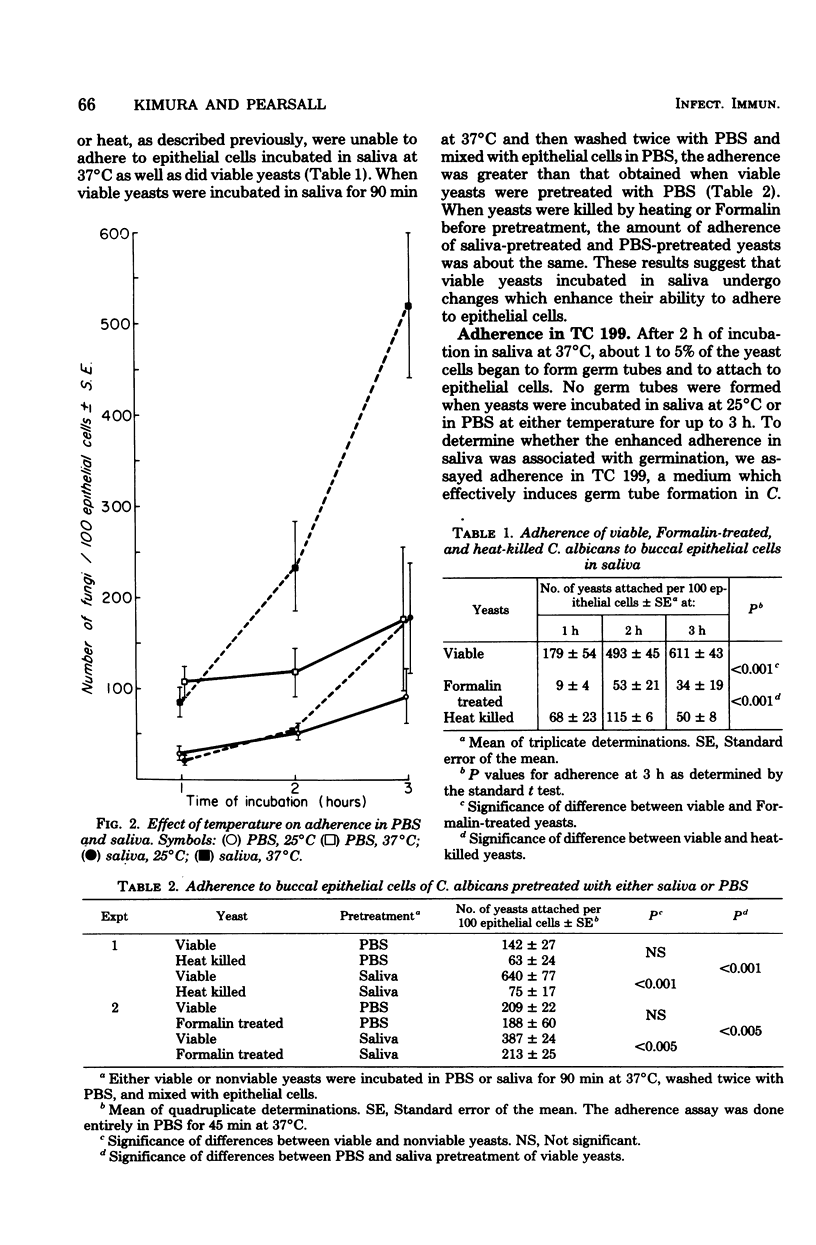
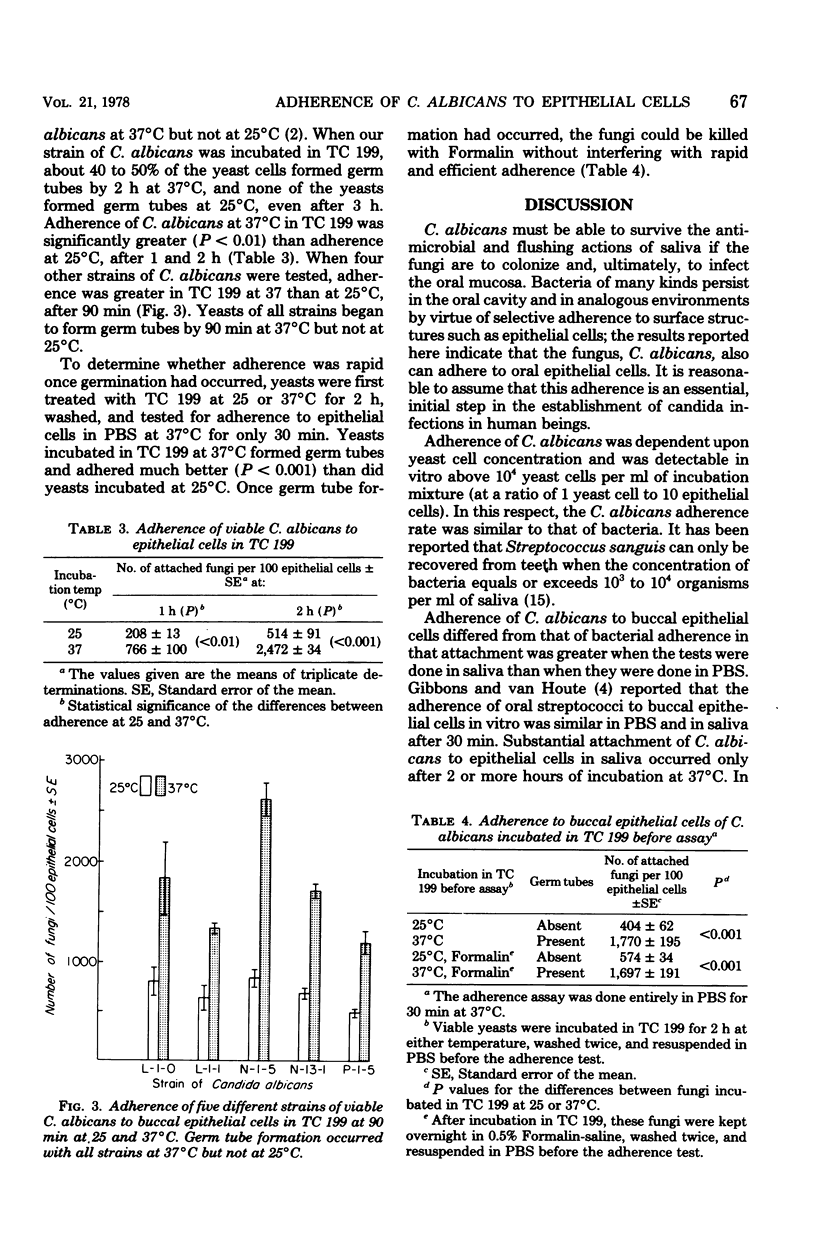
The adherence of Candida albicans to human buccal epithelial cells after 2 h at 37 degrees C was significantly greater in human saliva than in phosphate-buffered saline. in saliva, viable fungi adhered much better than did nonviable fungi, and this adherence was greater at 37 than at 25 degrees C. Viable yeasts, preincubated in saliva for 90 min at 37 degrees C before being washed and mixed with epithelial cells in phosphate-buffered saline, adhered better than nonviable yeasts or yeasts preincubated in phosphate-buffered saline. Enhanced adherence in saliva appeared to be associated with germination of the yeast cells. Conditions permitting germination (growth in tissue culture medium 199 at 37 degrees C but not at 25 degrees C) also supported enhanced adherence. After germination had occurred, the fungi could be killed with Formalin without interfering with their rapid and efficient adherence to epithelial cells. These data indicate that the enhanced adherence of C. albicans observed after incubation in saliva is related to changes in the fungi, rather than to a requirement for prolonged interaction between fungi and epithelial cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchanan T. M., Pearce W. A. Pili as a mediator of the attachment of gonococci to human erythrocytes. Infect Immun. 1976 May;13(5):1483–1489. doi: 10.1128/iai.13.5.1483-1489.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowa N., Howard D. H., Landau J. W., Shechter Y. Synthesis of nueic acids and proteins in the dimorphic forms of Candida albicans. Sabouraudia. 1970 Nov;8(3):163–169. doi: 10.1080/00362177085190831. [DOI] [PubMed] [Google Scholar]
- Freter R., Jones G. W. Adhesive properties of Vibrio cholerae: nature of the interaction with intact mucosal surfaces. Infect Immun. 1976 Jul;14(1):246–256. doi: 10.1128/iai.14.1.246-256.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971 Apr;3(4):567–573. doi: 10.1128/iai.3.4.567-573.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James-Holmquest A. N., Swanson J., Buchanan T. M., Wende R. D., Williams R. P. Differential attachment by piliated and nonpiliated Neisseria gonorrhoeae to human sperm. Infect Immun. 1974 May;9(5):897–902. doi: 10.1128/iai.9.5.897-902.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. Role of the K88 antigen in the pathogenesis of neonatal diarrhea caused by Escherichia coli in piglets. Infect Immun. 1972 Dec;6(6):918–927. doi: 10.1128/iai.6.6.918-927.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner T. Immunofluorescence study of Candida albicans in candidiasis, carriers and controls. J Pathol Bacteriol. 1966 Jan;91(1):97–104. doi: 10.1002/path.1700910114. [DOI] [PubMed] [Google Scholar]
- Liljemark W. F., Gibbons R. J. Suppression of Candida albicans by human oral streptococci in gnotobiotic mice. Infect Immun. 1973 Nov;8(5):846–849. doi: 10.1128/iai.8.5.846-849.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårdh P. A., Westtöm L. Adherence of bacterial to vaginal epithelial cells. Infect Immun. 1976 Mar;13(3):661–666. doi: 10.1128/iai.13.3.661-666.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E. T., Clements J. D., Finkelstein R. A. Vibrio cholerae adherence and colonization in experimental cholera: electron microscopic studies. Infect Immun. 1976 Aug;14(2):527–547. doi: 10.1128/iai.14.2.527-547.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J. Host-parasite interaction in the rat renal pelvis: a possible role for pili in the pathogenesis of pyelonephritis. J Exp Med. 1974 Dec 1;140(6):1696–1711. doi: 10.1084/jem.140.6.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houte J., Green D. B. Relationship between the concentration of bacteria in saliva and the colonization of teeth in humans. Infect Immun. 1974 Apr;9(4):624–630. doi: 10.1128/iai.9.4.624-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


