Abstract
Infections by opportunistic bacteria have significant contributions to morbidity and mortality of hospitalized patients and also lead to high expenses in healthcare. In this setting, one of the major clinical problems is caused by Gram-positive bacteria such as enterococci and staphylococci. In this study we extract, purify, identify and characterize immunogenic surface-exposed proteins present in the vancomycin resistant enterococci (VRE) strain Enterococcus faecium E155 using three different extraction methods: trypsin shaving, biotinylation and elution at high pH. Proteomic profiling was carried out by gel-free and gel-nanoLC-MS/MS analyses. The total proteins found with each method were 390 by the trypsin shaving, 329 by the elution at high pH, and 45 using biotinylation. An exclusively extracytoplasmic localization was predicted in 39 (10%) by trypsin shaving, in 47 (15%) by elution at high pH, and 27 (63%) by biotinylation. Comparison between the three extraction methods by Venn diagram and subcellular localization predictors (CELLO v.2.5 and Gpos-mPLoc) allowed us to identify six proteins that are most likely surface-exposed: the SCP-like extracellular protein, a low affinity penicillin-binding protein 5 (PBP5), a basic membrane lipoprotein, a peptidoglycan-binding protein LysM (LysM), a D-alanyl-D-alanine carboxypeptidase (DdcP) and the peptidyl-prolyl cis-trans isomerase (PpiC). Due to their close relationship with the peptidoglycan, we chose PBP5, LysM, DdcP and PpiC to test their potential as vaccine candidates. These putative surface-exposed proteins were overexpressed in Escherichia coli and purified. Rabbit polyclonal antibodies raised against the purified proteins were able to induce specific opsonic antibodies that mediated killing of the homologous strain E. faecium E155 as well as clinical strains E. faecium E1162, Enterococcus faecalis 12030, type 2 and type 5. Passive immunization with rabbit antibodies raised against these proteins reduced significantly the colony counts of E. faecium E155 in mice, indicating the effectiveness of these surface-related proteins as promising vaccine candidates to target different enterococcal pathogens.
Introduction
Enterococci have emerged as important nosocomial pathogens due to their multiple antibiotic resistances [1]. E. faecalis and E. faecium are the third and fourth most commonly isolated nosocomial pathogens worldwide, causing up to 14% and 9,6% of hospital acquired infections in the US and Europe, respectively [2]–[4]. Especially E. faecium infections have become a major concern, since resistance to vancomycin and ampicillin have increased to almost 100% in some institutions in the US, and a similar rise of resistances has been observed recently also in Europe [5]–[7]. The ability of this species to survive under a range of adverse environmental conditions, and its dramatic increase in antibiotic resistance worldwide highlights the need for the development of alternative treatment and prevention strategies [8], [9]. To date, many different surface antigens have been identified in E. faecalis and E. faecium, but only a few of these may be promising vaccine candidates [10].
In Gram-positive bacteria, the cell wall is composed of a peptidoglycan macromolecule that protects bacteria against environmental conditions and serves as anchor for the attachment of capsular polysaccharides, teichoic acids, and proteins that are covalently or non-covalently attached to peptidoglycan [11]. Surface proteins have an important role in the interactions between the bacterial cell and its environment. They are involved in adhesion and invasion of the host cell, sensing the physicochemical conditions of the environment and sending signals to the cytoplasm, in mounting defenses against the host responses and toxicity [12]–[15]. Therefore, surface proteins have become attractive targets for drug development [16]–[18]. Their ability to interact with the host immune system makes them interesting vaccine candidates, since protein based vaccines may overcome some of the challenges encountered by polysaccharide-based vaccines, like serotype-dependent coverage, high production costs, and low immunogenicity [19], [20]. Despite these advantages, only few surface and secreted proteins have been studied in clinically relevant enterococci. Aggregation substance (AS) protein and the collagen adhesin Ace have been examined in E. faecalis [21], [22] and enterococcal surface protein Esp, secreted antigen protein SagA and two ABC transporters have been tested for antigenicity in E. faecium [23]–[25]. Using appropriate in vitro and in vivo models to confirm protective efficacy, only SagA, Ace and an ABC transporter were identified as potential vaccine candidates [10], [23].
There are several strategies for the identification of surface proteins. The most widely used techniques are in silico analysis of the genome (“reverse vaccinology”), bacterial cell wall fraction analysis by Two-dimensional gel electrophoresis (2-DE) coupled to mass spectrometry, partial enzymatic digestion of cell wall proteins by trypsin (trypsin shaving) and biotinylation [26], [27]. New bioinformatic approaches have been developed and these strategies have significantly improved the prediction of bacterial protein localization. These include the pipelines SLEP (Surface Localization Extracellular Proteins), developed by Giombini et al [28], LocateP developed by Zhou et al [29], and SurfG+ developed by Barinov et al [30]. However, these in silico approaches are still not fully reliable and do not provide detailed surface protein localization in the bacterial cell wall [26]. Separations of the membrane and cell wall fractions are analyzed by 2-DE, gel excision of the protein spots and analysis by mass- spectrometry (MS). This strategy has been used in other Gram-positive bacteria [31], [32] and is fairly well established. However, the preparations are usually contaminated with cytoplasmic proteins and often give insufficient information regarding surface exposure, similar to the in silico approach [26]. Recently, trypsin shaving has been used in E. faecalis, group A Streptococci, Bacillus subtilis and Staphylococcus aureus [26], [27], [33], [34]. This strategy is based on the proteolytic digestion of surface-exposed proteins from intact cells and the analysis of the resulting peptides by liquid chromatography/tandem-mass-spectrometry (LC-MS/MS). The principal advantage of this technique is that it allows a rapid and more selective identification of the surface-exposed proteins. However, it leads to the identification of many cytoplasmic proteins and further verification of location of the identified proteins is necessary [26], [27], [34]. Using biotinylation, intact cells are treated with Sulfo-NHS-SS-Biotin, to which the cell membrane is impermeable. It reacts specifically with the ε-amino-group of lysine residues of surface-exposed proteins. Subsequently, labeled proteins can be separated by affinity chromatography with streptavidin from whole-cell lysates and these can be analyzed by LC-MS/MS or 2-DE [27], [32]. Despite the advantages of being relatively simple to use and facilitating the identification of more predicted surface-exposed proteins compared to cytoplasmic proteins, this method has the major disadvantage that biotin has poor affinity to sortase-attached surface proteins leading to low detection for these important protein antigens [27].
In the current study, we compared the above-described gel-free methods, i.e. trypsin shaving and biotinylation, to correlate the results between them and identify surface protein candidates with greater accuracy. We describe the subsequent overexpression, purification and immunological characterization of surface protein candidates present in hospital-associated vancomycin-resistant E. faecium E155 [35] to evaluate their potential role as targets for immunotherapy.
Materials and Methods
Bacterial strains and sera
The bacterial strains and sera used for the present study are listed in the Table 1. For the production of polyclonal antibodies against the recombinant proteins, New Zealand white rabbits were immunized with two subcutaneous injections of 10 µg protein given 2 weeks apart; in the third week, three injections of 5 µg were given intravenously. Finally, in the fifth week two injections of 5 µg were given intravenously and the terminal bleeding was collected in the seventh week. Serum from terminal bleedings was heat inactivated at 56°C for 30 min and frozen at −20°C before being used in experiments.
Table 1. Bacterial strains and sera used for this study.
| Strain or serum | Description* | Reference or source |
| Strains | ||
| E. faecium E155 | ARE, VRE strain isolated from a patient in the USA (Chicago), CC17 | [35] |
| E. faecium E1162 | ARE strain isolated from blood in the Netherlands, CC17 | [72] |
| E. faecalis 12030 | isolated from a patient in the USA (Cleveland) | [44] |
| E. faecalis type 2 | isolated from a patient in Japan (Sapporo) | [73] |
| E. faecalis type 5 | isolated from a patient in Japan (Kobe) | [73] |
| E coli M15pRep4 | M15 harboring pREP4 plasmid | (INVITROGEN) |
| E. coli M15/pQE30LysM | M15 harboring pREP4 and pQE30LysM plasmids | This study |
| E. coli M15/pQE30PpiC | M15 harboring pREP4 and pQE30PpiC plasmids | This study |
| E. coli M15/pQE30DdcP | M15 harboring pREP4 and pQE30DdcP plasmids | This study |
| E. coli M15/pQE30PBP5 | M15 harboring pREP4 and pQE30PBP5 plasmids | This study |
| Sera | ||
| NRS | Preimmune sera from rabbit | This study |
| αSagA | Rabbit serum raised against the recombinant SagA | [23] |
| αLysM | Rabbit serum raised against the recombinant LysM | This study |
| αPpiC | Rabbit serum raised against the recombinant PpiC | This study |
| αDdcP | Rabbit serum raised against the recombinant DdcP | This study |
| αPBP5 | Rabbit serum raised against the recombinant PBP5 | This study |
*ARE, ampicillin resistant enterococci; CC17, clonal linage complex 17; DdcP, D-alanyl-D-alanine carboxypeptidase; PBP5, low affinity penicillin-binding protein 5; PpiC, peptidyl-prolyl cis-trans isomerase; SagA; major secreted antigen; VRE, vancomycin resistant enterococci.
Protein extraction by trypsin shaving
Extractions were performed as described by Tjalsma et al. [34]. Briefly, two aliquots of 50 mL of bacterial cultures of E. faecium E155 grown in brain heart infusion (BHI) were harvested at an OD600 nm = 0.4 by centrifugation (10.000 r.p.m., 2 min) and washed twice with 4 mL Bicam (triethylammonium bicarbonate buffer 100 mM pH 8.0). Then the cells were resuspended in 600 µL of Bicam. The first aliquot was mixed with trypsin (Promega) at a final concentration of 10 µg/mL in Bicam. The second aliquot was resuspended in Bicam without any trypsin. All the samples were incubated for 1 h at 37°C with gentle shaking. After centrifugation (7500 r.p.m., 5 min), the cell pellets were removed and the supernatants were treated with 1 mM dithiothreitol (DTT) for 30 min, followed by 1 mM iodoacetamide (IAA), also for 30 min at room temperature. Finally, fresh trypsin (0.5 µg) was added to all samples and tryptic cleavage was continued for 18 h at 37°C. Proteins identified from the extraction of the second aliquot were digested with trypsin overnight and considered as ‘controls’ to be subtracted from the proteins identified in the cells treated with trypsin after mass spectrometry identification.
Protein extraction by biotinylation
Surface-exposed proteins were labeled and extracted by exposure of cells to Sulfo-NHS-SS-Biotin using a protocol described by Hempel et al. [36] with the following modifications: 100 mL of bacterial cultures of E. faecium E155 grown in BHI at OD600 nm = 0.5 harvested at 8000× g for 5 min at 4°C. About 0.2 g of wet cell pellet was resuspended in 5 mL ice-cold phosphate buffered saline (PBS pH 8.0) with 1 mM phenylmethylsulfonyl fluoride (PMSF) and mixed with 0.6 mg of sulfo-NHS-SS-Biotin (Thermo Scientific) previously dissolved in 100 µL of PBS. The mixture was incubated by gentle shaking for 1 h on ice. Unbound biotinylation reagent was removed by centrifugation at 8000× g for 1 min at 4°C and washed three times with ice cold PBS (pH 8.0)/500 mM glycine. Disruption of cells was performed mechanically in a FastPrep cell disrupter (Zymo Research) at 6 m/s2 twice for 30 s. The cell debris was recovered from the glass beads with a total of 3 mL of PBS (pH 8.0). The lysate was centrifuged (100.000× g for 1 h at 4°C), the cell debris resuspended in a total of 400 µL of PBS (pH 8.0), supplemented with 5% IAA and homogenized in the cell disrupter at 6 m/s2 twice for 30 s with 0.25 mL of glass beads. The proteins were then solubilized by addition of 100 µL of PBS (pH 8.0) with 1 mM PMSF, 4% CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate) and 2% ASB-14 (amidosulfobetaine-14). A second homogenization step was done after detergent addition under the same conditions as mentioned above. Cell debris was removed by centrifugation (14000 r.p.m., 15 min) after 1 h of incubation with the detergent. The biotinylated proteins were isolated and purified by NeutrAvidin (Thermo Scientific) agarose affinity-purification. For a reaction volume of 500 µL protein mixture 150 µL of NeutrAvidin agarose resin was washed twice with PBS (pH 8.0)/1% NP-40 and centrifuged at 1000 r.p.m. for 1 min at 4°C. The resin was mixed with the cell lysate for 1 h by gently shaking on ice. The supernatant was removed and the resin-bound complex washed. Biotinylated proteins were eluted twice by incubation with 1 mL of elution buffer (5% mercaptoethanol in H2O) for 1 h with gentle shaking. Supernatant was then recovered after centrifugation at 1000 r.p.m. for 1 min and mixed with 8 mL of cold acetone (−20°C, overnight). The precipitated proteins were harvested by centrifugation (8500 r.p.m., 30 min, 4°C) and washed twice with 1 mL of cold 98% ethanol (4°C). Finally the pellets were dried in a Concentrator 5301 (Eppendorf) for 2 min and dissolved in 15 µL 6M urea/2M thiourea for 2 min at 80°C.
Elution of cell-wall-associated proteins at high pH
Surface-exposed proteins were extracted by exposure of cells to high pH using a protocol described by Morsczeck et al. [37]. A cell pellet from a 50 mL culture of E. faecium E155 grown in BHI to OD600 = 0.5 was washed with a PBS sucrose solution (100 mM NaCl, 60 mM sucrose, 55 mM sodium phosphate, pH 7.2), and then gently shaken for 1 h at room temperature in 2 mL NaOH glycine sucrose (glycine 50 mM, sucrose 60 mM, pH 12.4). After centrifugation (30 min, 10.000× g), 108 µL 1 M HCl and 100 µL 1 M Tris-HCl (pH 7.0) were added to 1 mL supernatant. Proteins were precipitated at 4°C by addition of 8 mL cold acetone overnight. The protein pellet obtained after centrifugation (10 min, 10.000× g) was resuspended in 200 µL of Tris-HCl (pH 7.5). After an aliquot of 25 µL of the protein solution was run through SDS-PAGE and Coomassie blue staining, each gel line with the protein-containing region was cut in five pieces. After each piece was digested with trypsin as is described in the mass-spectrometry section.
Mass-spectrometry analyses
Overnight tryptic digestion of the obtained proteins (or peptides) was performed after each extraction method, and subsequently, MS analyses were performed as described elsewhere [38]. In brief, the samples extracted by trypsin shaving, biotinylation and alkaline extraction were treated with 0.5 µg trypsin (Promega) overnight at 37°C. Trypsin-cleaved samples were desalted and concentrated to obtain 1–2 µg of peptides on a tipmicroC18 Omix (Agilent) before nano-liquid chromatography nanoLC-MS/MS analysis. The chromatography step was performed on a nano-LC system (Prominence, Shimadzu). Peptides were concentrated on a Zorbax 5×0.3 mm C18 precolumn (Agilent) and separated onto a Zorbax 150×75 µm C18 column (Agilent). Mobile phases consisted of 0.1% trifluoroacetic acid, 99.9% water (v/v) (A) and 0.1% trifluoroacetic acid, 20% water in 79.9% ACN (v/v/v) (B). The nanoflow rate was set at 300 nL/min, and the gradient profile was as follows: constant 7% B for 5 min, from 7 to 70% B in 183 min, from 70 to 100% B in 5 min, and return to 7% B. The 300 nL/min volume of the peptide solution was mixed with 1.2 µL/min volumes of solutions of 5 mg/mL CHCA matrix prepared in a diluent solution of 50% ACN with 0.1% TFA. Twenty nine second fractions were spotted by an AccuSpot spotter (Shimadzu) on a stainless steel Opti-TOF 384 targets. MS experiments were performed on an AB SCIEX 5800 proteomics analyzer equipped with TOF ion optics and OptiBeam on-axis laser irradiation with a 1000 Hz repetition rate. The resulting fragmentation patterns were used to determine the sequences of the peptides. Database searching was performed using the mascot 2.3.02 program (Matrix Science). A database corresponding to an updated compilation download from the NCBI database was used with E. faecium as selected species (including 169 998 entries). The variable modifications allowed were as follows: C-Carbamidomethyl, K-acetylation, methionine oxidation, and dioxidation. Trypsin was selected as the enzyme, with three miss cleavages also allowed. Mass accuracy was set to 200 p.p.m. and 0.6 Da for MS and MS/MS modes, respectively. Finally, to confirm the identity of the recombinant proteins after affinity purification, SDS-PAGE and Coomassie blue staining, the protein-containing regions (bands) were excised, and washed twice with ultrapure water and once with acetonitrile/50 mM ammonium bicarbonate (1∶1, v/v). Samples were stirred for 15 min and vacuum-dried for 30 min. In-gel digestion of the excised protein bands was carried out using 0.5 µg trypsin, incubating overnight at 37°C. MS analysis was performed as described above.
Determination of protein subcellular localization
The subcellular localization of the proteins was determined using two different in silico approaches as follows. The sequence of the identified proteins given by the MS analyses were retrieved from the NCBI data base and analyzed with two Web-server predictors: CELLO v.2.5 (http://cello.life.nctu.edu.tw/) [39] and Gpos-mPLoc (http://www.csbio.sjtu.edu.cn/bioinf/Gpos-multi/) [40]–[42].
General molecular methods
PCR was performed with Phusion highfidelity DNApolymerase (Finnzymes). The primers used are listed in Table 2. PCR products and plasmids were purified using the NucleoSpin plasmid kit (Macherey-Nagel). Restriction enzymes and T4 DNA ligase were purchased from Promega and used as recommended by the manufacturer. Genomic DNA extraction and other standard techniques were carried out as described by Sambrook et al. [43].
Table 2. Primers used in this study.
| Primer name | 5′-3′sequence+ | Restriction site |
| LysM-5-BamHI-2 | aggcGGATCCGATGAAGTTTATACAGTAAAATC | BamH I |
| LysM-3-PstI | aggcCTGCAGGGCTTAGTACCAGCCGTTTG | Pst I |
| DdcP-5-BamHI-2 | aggcGGATCCGAAGATACTTTCAAAGTAAATG | BamH I |
| DdcP -3-PstI | aggcCTGCAGCAATTAAAACAAGTTACCGAAAA | Pst I |
| PpiC-5-BamHI-2 | aggcGGATCCTGTTCAGGCGATACTAATAAAG | BamH I |
| PpiC-3-SacI | aggcGAGCTCCTTTTATTTTGATGAATCAGTTG | Sac I |
| PBP5-5-BamHI | aggcGGATCCATGAAAAGAAGTGACAAGCACG | BamH I |
| PBP5-3-SacI | aggcGAGCTCAGCAATTTTTTATTGATAATTTTGGS | Sac I |
Bases in lowercase letters are not complementary to the target sequence.
Underlined bases correspond to restriction sites.
Construction of E. coli strains M15/pQE30LysM, M15/pQE30PpiC, M15/pQE30PBP5 and M15/pQE30DdcP
The proteins were recombinantly expressed to raise antibodies against the different antigens. The respective genes were amplified without the signal peptide using primers listed in Table 2 and genomic DNA from the E. faecium E155 as template. The amplified genes were then inserted downstream of the IPTG (Isopropyl β-D-1-thiogalactopyranoside)-inducible promoter into the pQE30 expression vector (QIAexpressionist kit; Qiagen) to obtain an N-terminal His6-tagged recombinant protein. The resulting construct was electroporated into the E. coli M15pREP4, creating the different M15/pQE30protein strains (see Table 1). Recombinant proteins were overproduced and purified under denaturing conditions using the Protino Ni-NTA Agarose (Macherey-Nagel) resin, following the manufacture instructions. Finally, the purified recombinant proteins were desalted by diafiltration using the Amicon Ultra-15 Centrifugal Filter Units of 3 KDa (Merck-Millipore).
Opsonophagocytic assay (OPA) and opsonophagocytic inhibition assay (OPIA)
An in vitro opsonophagocytic assay (OPA) was performed as described elsewhere [23], [44]. Briefly, four components were prepared: (a) baby rabbit serum (Cedarlane Laboratories) absorbed with the target bacterial strain as a source of complement, (b) the different rabbit sera (see table 1), (c) polymorphonuclear neutrophils (PMNs) freshly prepared from human blood collected from healthy adult volunteers, and (d) the bacterial strains grown to OD650 nm = 0.4 in tryptic soy Broth (TSB). For the assay, the four components were mixed: 100 µL of PMNs (2.5×104 µL−1); 100 µL of the appropriate serum dilution, 100 µL of complement (1∶30 dilution for E. faecium strains and 1∶15 for E. faecalis strains), and 100 µL of an appropriate dilution of bacteria to yield the desired colony counts (i.e. 1∶1 relation PMNs/bacteria). The mixture was incubated on a rotor rack at 37°C for 90 min, and samples were plated on TSA plates in quadruplicate at time 0 and after 90 min. Percent killing was calculated by comparing the colony counts of a control without PMN's to the colony counts after a 90-minute incubation at 37°C (T90). For inhibition studies, rabbit serum was diluted 1∶50 and incubated for 60 min at 4°C with an equal volume of a diluted sera containing 100 µg of the corresponding protein. Subsequently, the absorbed-serum was used in the OPA as described above. Inhibition assays were performed at serum dilutions yielding 50–60% killing of the inoculum without the addition of the inhibitor. The percentage of inhibition of opsonophagocytic killing was compared to controls without inhibitor.
Animal model
A mouse bacteremia model was performed to evaluate the passive protection conferred by antibodies raised against the recombinant proteins as described elsewhere [45], [46] with some modifications. In brief, Five female Balb-C mice 6 to 8 weeks-old (Charles River) received intravenously 200 µL of NRS, serum raised against the recombinant proteins or serum raised against recombinant protein SagA as a positive control, 48 and 24 h before the challenge. Bacterial inoculum of E. faecium E155 (5.2×108 c.f.u per mouse) was injected via the tail vein (i.v.). 24 h after challenge, mice were sacrificed and colony counts in kidneys were determined by homogenizing and plating of serial dilutions.
Statistical Analysis
The software program GraphPad PRISM version 5.00 was used for the statistical analyses. The percentage of organisms killed using immune sera in the OPA was expressed as geometrical mean ± the standard error of the means. Statistical significance for the OPA and OPIA was determined by ANOVA and Dunnett's Multiple Comparison Test. A p value of <0.05 was considered significant. Significance of the bacterial counts in the animal experiment was determined by analysis of variance for multi-group comparisons using log-transformed data, and Dunnett post hoc test. A p value of <0.05 was considered significant.
Ethics Statement
All animal experiments were performed in compliance with the German animal protection law (TierSchG). The mice were housed and handled in accordance with good animal practice as defined by FELASA and the national animal welfare body GV-SOLAS. The animal welfare committees of the University of Freiburg (Regierungspraesidium Freiburg Az 35/9185.81/G-12/070) approved all animal experiments.
Results
Identification of surface related proteins in E. faecium E155
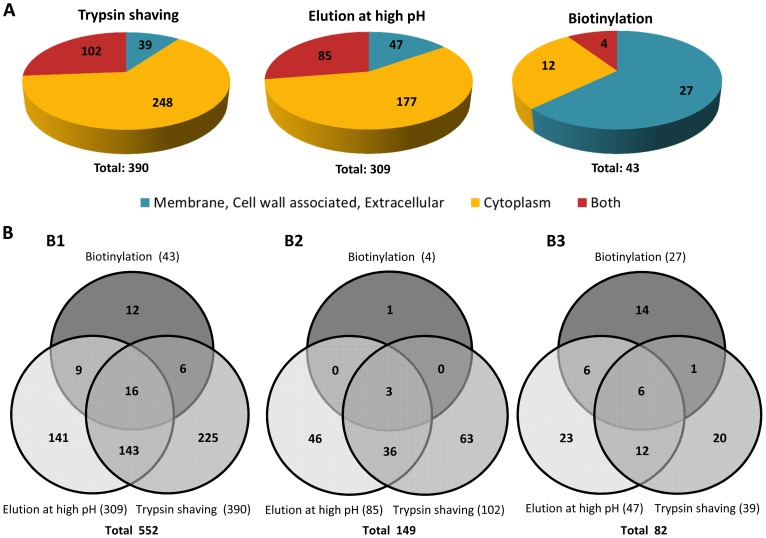
For a more accurate identification of surface proteins in the E. faecium E155 strain, three different approaches were used: trypsin shaving, biotinylation and high pH elution. The number of proteins identified by MS analysis containing at least one unique peptide in at least two sample replicates (see supplementary tables S1 to S3) for the different methods were 390 for trypsin shaving, 309 for elution at high pH, and 45 for biotinylation. We analyzed the sequence of each protein through two Web-server predictors (CELLO v.2.5 and Gpos-mPLoc) to evaluate their sub-cellular localization. For each of the three methods, proteins were then classified in three main groups: a) Inside: If a protein was predicted to have an exclusively cytoplasmic location by both algorithms we considered it to be inside of the cell. b) Both: If one of the algorithms predicted that the subcellular localization of a protein is intracellular (cytoplasmic) and the other predicted that is outside of the cytoplasm (i.e. membrane, cell wall associated and/or extracellular) OR if the algorithms predicts two locations inside and outside of the cytoplasm (Cytoplasm-membrane or cytoplasm-extracellular) at the same time, the protein was considered to be both inside and outside of the cytoplasm. c) Surface-associated: If a protein was predicted to have an exclusively extracytoplasmic location (i.e. membrane, cell wall associated and/or extracellular) by both algorithms we considered these proteins as surface-associated. Among all the proteins identified, 39 (10%), 47 (15%) and 27 (63%) polypeptides were predicted to be extracytoplasmic by trypsin shaving, elution at high pH, and biotinylation, respectively (see figure 1A). On the other hand, we observed that 102 (26%) proteins obtained by trypsin shaving, 85 (27%) by elution at high pH and 4 (9%) by biotinylation were predicted to have both cytoplasmic and extracytoplasmic location. The data were then compared using Venn-diagrams (figure 1B1) to identify proteins classified as surface-associated by more than one method. A total of 552 proteins with at least one unique peptide were uncovered and among them 16 proteins were identified by all three methods; 158 proteins appeared at least in two of the three different extraction procedures. We compared the proteins predicted to have cytoplasmic and extracytoplasmic localizations (see figure 1B2). Three of them were part of those polypeptides identified by all three methods, while 36 appeared at least in two (see supplementary table S4). Finally, we compared the proteins that were predicted to have an extracytoplasmic location (figure 1B3), showing that six of them appeared in all the extraction methods and 23 were identified by at least two of the three methods. Extracytoplasmic proteins identified by more than one method and their subcellular localization are summarized in table 3. Considering these results, we assumed that the six extracytoplasmic proteins identified by all three extraction methods were the most promising candidates to study immunogenicity and protective efficacy. Among the six proteins, we finally decided to focus on four that interact with peptidoglycan (PG) and are more likely to be surface-exposed: (a) the 21.6 kDa peptidoglycan-binding protein LysM (LysM) that has been reported to be non-covalently attached to PG [47]; (b) the 73.7 kDa low-affinity penicillin-binding protein 5 (PBP5) that is involved in polymerization of PG [48]–[50], (c) the 47.7 kDa D-alanyl-D-alanine carboxypeptidase (DdcP) - a low molecular weight penicillin binding protein (LMW-PBP) cross-linking PG chains to form rigid cell walls [49], [51] and (d) the 37.3 kDa PpiC-type peptidyl-prolyl cis-trans isomerase (PpiC) also involved in PG cross-linking [52].
Figure 1. Distribution of E. faecium E155 proteins identified by the different extraction methods.
(A) Rate of E. faecium E155 proteins identified with one or more unique peptides in at least two biological replicates by trypsin shaving, elution at high pH and biotinylation and their corresponding subcellular localization predicted by Cellov.2.5 (http://cello.life.nctu.edu.tw) and Gpos-mPLoc (http://www.csbio.sjtu.edu.cn/bioinf/Gpos-multi). (B) Venn-diagram of all the proteins identified by the different extraction methods. (B1) Correlation between the proteins extracted by the different extraction methods. (B2) Correlation between the proteins predicted to have both cytoplasmic and extracytoplasmic location by CELLO v.2.5 and Gpos-mPLoc. (B3) Correlation between the Proteins predicted to have exclusively an extracytoplasmic location by CELLO v.2.5 and Gpos-mPLoc.
Table 3. Summary of the proteins identified by at least two of the three extraction methods and predicted to have an extracytoplasmic location.
| Subcellular localizationb | Extraction method | |||||
| Protein name | Gene Locusa | CELLO v.2.5 | Gpos-mPLoc | Biot* | Tryp§ | HpH$ |
| Peptidoglycan-binding protein LysM | EFF34034 | Ext-CW | Ext-Cw | + | + | + |
| Low affinity penicillin-binding protein 5 | EFF35784 | Ext | Ext-CW | + | + | + |
| D-alanyl-D-alanine carboxypeptidase | EFF35669 | Mem | Mem | + | + | + |
| PpiC-type peptidyl-prolyl cis-trans isomerase | EFF34785 | Ext-Mem | Ext-Mem | + | + | + |
| SCP-like extracellular protein | EFF35540 | Ext | Ext | + | + | + |
| Basic Membrane lipoprotein | EFF34523 | Ext | Mem | + | + | + |
| Glycosyl transferase | EEV52587 | Ext | Ext | + | + | − |
| DNA-entry nuclease | EEI59681 | Ext | Ext | + | − | + |
| Extracellular solute-binding protein, family 5 | EAN09846 | Ext | Ext | + | − | + |
| NLPA lipoprotein | EAN09985 | Mem | Mem | + | − | + |
| Peptidase M41, FtsH | EAN10268 | Mem | Mem | + | − | + |
| Extracellular solute-binding protein, family 3 | EAN08986 | Mem | Mem | + | − | + |
| Periplasmic solute binding protein | EAN10630 | Mem | Mem | + | − | + |
| Cell envelope-related transcriptional attenuator | EAN08970 | Ext | Ext | − | + | + |
| Peptidase S1, chymotrypsin | EAN09870 | Ext-Mem | Ext | − | + | + |
| Beta-ketoacyl-acyl carrier protein synthase III (FabH) | EAN10058 | Mem | Ext | − | + | + |
| 50S ribosomal protein L2 | EEI61156 | Mem | Ext | − | + | + |
| Peptidylprolyl isomerase | EEI59596 | Mem | Ext | − | + | + |
| Metallo-beta-lactamase superfamily protein | EEV42569 | Mem | Ext | − | + | + |
| Penicillin-binding protein | EEV43240 | Ext | Mem | − | + | + |
| ABC superfamily ATP binding cassette transporter | EEI61138 | Mem | Mem | − | + | + |
| Family 2 glycosyltransferase | EEI61366 | Mem | Mem | − | + | + |
| VANA ligase | CAA40215 | Mem | Mem | − | + | + |
| PilT protein, N-terminal | EAN10184 | Mem | Mem | − | + | + |
| Helicase, C-terminal: DEAD/DEAH box helicase | EAN08953 | Mem | Mem | − | + | + |
Gene locus given by blast in the NCBI (http://www.ncbi.nlm.nih.gov/);
subcellular localization predicted by Cellov.2.5 (http://cello.life.nctu.edu.tw) and Gpos-mPLoc (http://www.csbio.sjtu.edu.cn/bioinf/Gpos-multi).
CW, cell wall. Ext, extracellular. Mem, membrane.
*Biot; Biotinylation.
Tryp; Trypsin shaving.
HpH; Elution at high pH.
The target proteins induce opsonic and cross-reactive antibodies
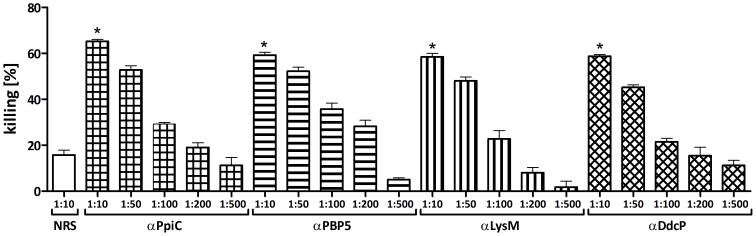
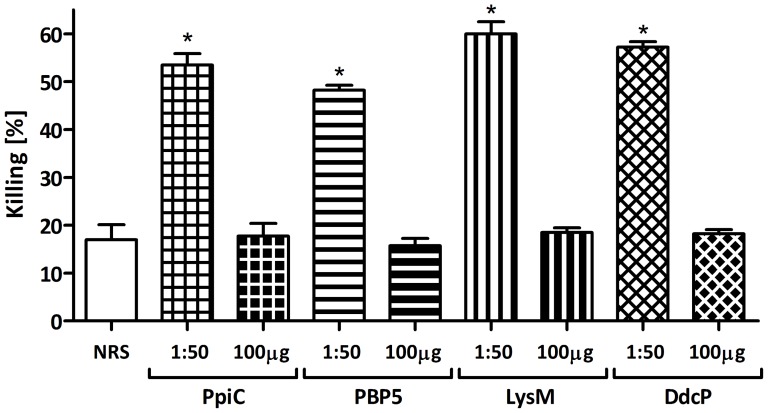
The genes encoding the four candidate proteins were amplified without their signal peptides, cloned into the pQE30 expression vector and transformed into E. coli. The recombinant proteins were then purified under denaturing conditions. The purity of the proteins was assessed by SDS-PAGE and their identity was confirmed by LC-MS/MS (data not shown). New Zeeland white rabbits were immunized with purified proteins and exsanguinated two weeks after the last injection. The obtained polyclonal antibodies raised against the different proteins were tested in an OPA against the corresponding strain E. faecium E155 showing that all the proteins were able to induce opsonic antibodies. Different concentrations were tested to titer out the opsonic activity of the sera. Maximum opsonic activity of the antibodies was between 58–65% of killing with a 1∶10 serum dilution, and a reduction of killing was observed in a dose dependent fashion using increasingly higher dilutions of sera (see figure 2). To verify the specificity of the killing against the respective recombinant protein, opsonophagocytic inhibition assays (OPIA) were carried out by pre-incubating the sera with 100 µg/mL of the corresponding recombinant protein. These sera were then tested in an OPA using E. faecium strain E155 which showed that opsonic killing is inhibited by more than 85% in all cases (see figure 3).
Figure 2. Opsonophagocytic assay against the homologous strain E. faecium E155.
Opsonophagocytic assay used to test the ability to mediate opsonic killing in the strain E. faecium E155 by antibodies raised against the recombinant proteins at different dilutions. αPpiC (square grid), αPBP5 (horizontal stripes), αLysM (vertical stripes) and αDdcP (rhombic grid), compare with the activity of the preimune rabbit serum (NRS, white bar). Bars represent the mean of data and the error bars represent the standard error of the mean. Statistical significance was determined by ANOVA and Dunnett's Multiple Comparison Test. Comparing killing rates of similar dilutions (i.e. 1∶10) with the NRS, all comparisons were significant at p<0.001 (indicated by asterisk).
Figure 3. Specificity of the antibodies raised against the recombinant proteins.
The sera were used at final dilution of 1∶50, PpiC (square grid), PBP5 (horizontal stripes), LysM (vertical stripes) and DdcP (rhombic grid) and the strain tested was E. faecium E155. Purified recombinant proteins were used as inhibitors at concentration of 100 µg/mL, and were preincubated with the corresponding sera dilution for 1 h at 4°C prior to OPA. Opsonic killing of the target strain with non-absorbed antibodies was used to assess the reduction of opsonic killing produced by each inhibitor, using preimune rabbit serum (NRS, white bar) as a Control. Bars represent the mean of data and the error bars represent the standard error of the mean. Statistical significance was determined by ANOVA and Dunnett's Multiple Comparison Test. Comparing killing rates of similar dilutions (i.e. 1∶50) with the NRS, all comparisons were significant at p<0.001 (indicated by asterisk).
Specific and opsonic antibodies against the recombinant proteins are cross-reactive with different E. faecium and E. faecalis isolates
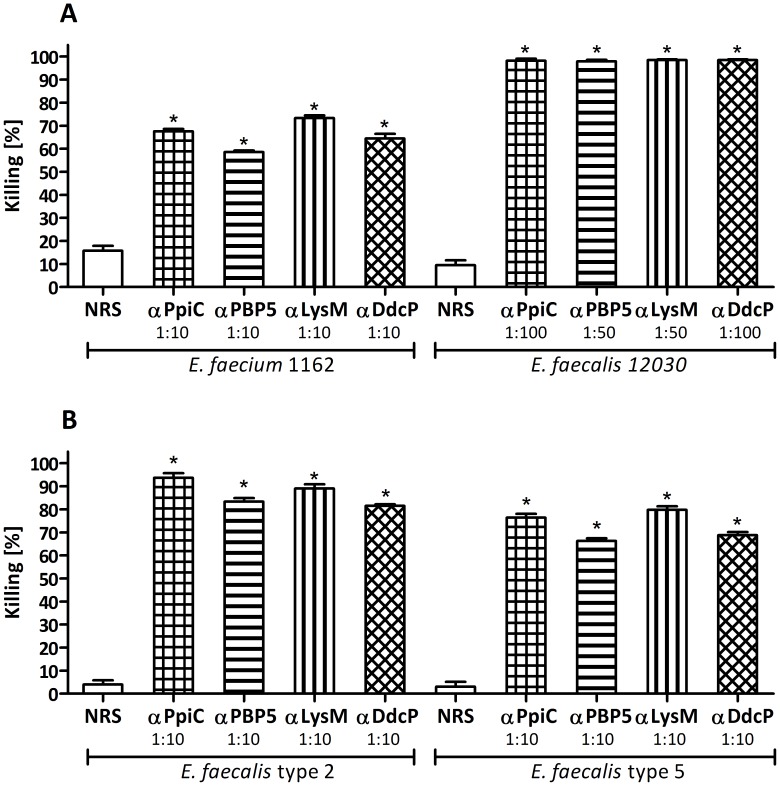
To determine if the antibodies directed against the recombinant proteins were able to opsonize different strains, serum dilutions between 1∶10 and 1∶100 were tested in OPAs against E. faecium E1162 and E. faecalis 12030, type 2 and type 5 [46], [53]. The four sera were able to opsonize all strains exhibiting killing above 60% (see Figure 4A and 4B). Passive immunization with antibodies directed against the different proteins promotes clearance of bacteria in mice
Figure 4. Cross-reactivity of the sera against different enterococcal strains.
Opsonophagocytic assay used to test the ability to mediate opsonic killing of different enterococcal strains by antibodies raised against the recombinant proteins. A) Opsonophagocytic killing of strains E. faecium E1162 and E. faecalis 12030 by antibodies raised against the recombinant proteins at dilutions between 1∶10 and 1∶100. αPpiC (square grid), αPBP5 (horizontal stripes), αLysM (vertical stripes) and αDdcP (rhombic grid), compared with the activity of the preimune rabbit serum (NRS, white bar). B) Opsonophagocytic killing in E. faecalis type 2 and E. faecalis type 5 by antibodies raised against the recombinant proteins at dilution 1∶10. αPpiC (square grid), αPBP5 (horizontal stripes), αLysM (vertical stripes) and αDdcP (rhombic grid), compared with the activity of the preimune rabbit serum (NRS, white bar). Bars represent the mean of data and the error bars represent the standard error of the mean. Statistical significance was determined by ANOVA and Dunnett's Multiple Comparison Test. Comparing killing rates of similar dilutions (i.e. 1∶10, 1∶50 or 1∶100) with the NRS, all comparisons were significant at p<0.001 (indicated by asterisk).
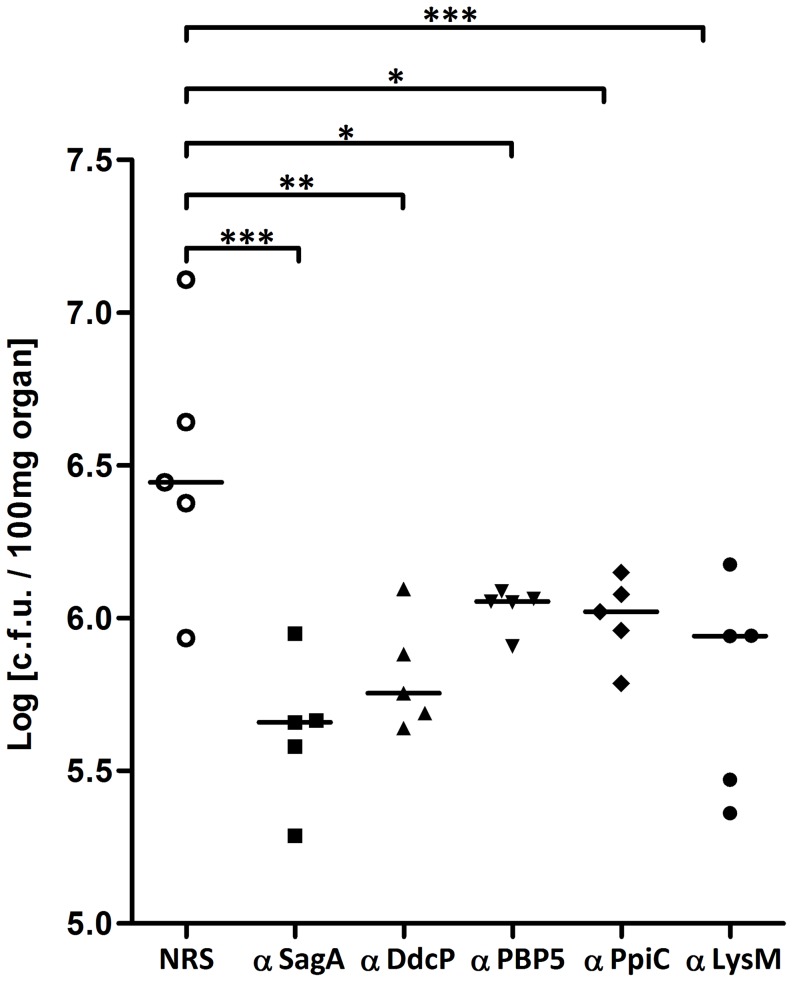
To determine if antibodies directed against the recombinant proteins are protective in a mouse bacteremia model, mice were passively immunized twice within 48 h before bacterial infection. Sera raised against the four recombinant proteins significantly reduced E. faecium E155 colony counts in the kidneys. These results are comparable to the protection achieved by antibodies raised against the previously reported antigen SagA [23]. Immunization with the sera raised against PpiC and PBP5 proteins resulted in higher viable counts (i.e. less protection) (P value≤0.05) compared to serum raised against DdcP (P value≤0.01) and LysM (P value≤0.001) (see Figure 5).
Figure 5. Protection against bacteremia in mice.
Passive Immunization with the antibodies raised against the recombinant proteins promotes clearance of E. faecium E155 in mouse kidney in comparison with the normal rabbit serum. 24 h after the bacterial challenge mice were killed and kidneys were removed to assess viable counts. Each point represents the bacterial counts from a single mouse. Bars indicate the median CFU/100 mg of kidney for the group. P value was <0.05 (*P≤0.05, **P≤0.01, ***P≤0.001) for comparison between the animals immunized with the antibodies raised against the recombinant proteins and control animals immunized with preimune rabbit serum (NRS) determined by analysis of variance for multi-group comparisons using on log-transformed data, and Dunnett post hoc test.
Discussion
It has been reported by in silico analysis that between 30 to 40% of the bacterial proteome corresponds to surface-associated proteins. However, few of these proteins have been physicochemically and immunologically characterized [26], although surface-exposed and secreted proteins have been shown to be promising vaccine candidates in some pathogenic bacterial species [23], [54], [55]. Surface-exposed proteins can be identified more or less successfully by in silico approaches or with different extraction methods, such as trypsin shaving and biotinylation [15], [26], [27], [36], [54]. Maione et al. used multiple genome screening approaches in group B Streptococcus, identifying 589 predicted surface-exposed proteins. They overexpressed and tested 312 of these candidates, but only four were found to be potential vaccine candidates [54]. In group A Streptococcus, trypsin shaving has been shown to be a useful technique to extract surface-exposed proteins. Rodríguez-Ortega and coworkers were able to identify 72 proteins and demonstrate that 95% corresponded to extracytoplasmic proteins and around 86% of them were effectively surface-exposed [26]. However, in our study trypsin shaving was not the most efficient method. Indeed, only 36% of the identified proteins were predicted to have an extracytoplasmic location. This is in agreement with the findings of Hempel et al. in Staphylococcus aureus, showing that by trypsin shaving only 41% of the extracted proteins corresponded to surface-exposed proteins [27]. It is important to point out that some of these proteins that we classified as cytoplasmic proteins by different web-server predictors (e.g. enolase, Inosine-5′-monophosphate dehydrogenase, glyceraldehyde-3-phosphate dehydrogenase, pyruvate dehydrogenase, triosephosphate isomerase, elongation factor Tu, and GroEL) have been described as “moonlight proteins” since they perform more than one function on the cell [56], [57] and have been identified on the cell surface of some gram-positive bacterial pathogens [33], [58]–[67]. Although the proteins mentioned above are predicted to be cytoplasmic we cannot be ruled out their possible surface location and should be consider as good candidates for further immunological studies. The combined results may indicate that the efficiency of trypsin shaving may be species-dependent. In the case of E. faecium, we demonstrated that biotinylation was the most accurate and specific procedure for the identification of extracytoplasmic proteins with a yield of 72%. In the present study the killing of bacteria in the opsonophagocytic assay indicates that the respective target is accessible for antibodies and complement. However, additional methods (such as immuno-electron microscopy or confocal microscopy) are necessary to confirm surface exposure.
Only one protein has been identified so far as a potential vaccine target in E. faecium. The major secreted protein SagA induced opsonic and protective antibodies in rabbit, that were able to mediate in vitro opsonophagocytic killing against the homologous strain and to reduce colony counts in mice [23]. Additionally, an antibody isolated from a phage display antibody library, directed against an epitope present in an ABC transporter protein has been described to promote clearance of E. faecium in mice, suggesting its possible use in immunotherapy [24]. The peptidoglycan-associated proteins tested as vaccine candidates in the present study have been implicated in antibiotic resistance and virulence. Penicillin-binding proteins, such as PBP5 and Ddcp, have been reported to play a key role in intrinsic resistance to β-lactams, being the major contributors to ampicillin resistance in E. faecium [51]. In E. faecalis, the homologue to protein PpiC has been characterized as a potential virulence factor that confers resistance to high NaCl concentrations and ampicillin, because this protein is involved in the folding and trafficking of extracellular proteins, especially PBPs [52], [68]. LysM, which is non-covalently attached to peptidoglycan, has been reported to be involved in early stages of erythromycin resistance in E. faecalis, but its precise function has not been elucidated yet [69]. All these proteins are clearly potential targets for drug development and we show here that they could also be interesting for vaccine development.
We were able to demonstrate that all four proteins induced opsonic antibodies in rabbits, which mediate effectively in vitro opsonophagocytic killing (higher than 50%) not only of the homologous strain but also of other enterococcal strains, i.e. E. faecium E1162 (belonging to clonal complex 17 [70]), E. faecalis 12030, E. faecalis type 2 and E. faecalis type 5 [53]. The broad cross-reactivity of the sera indicates that these protein antigens may effectively supplement serotype-dependent coverage of polysaccharide-based vaccines. The lower opsonophagocytic killing observed against the homologous strain compared with E. faecium E1162 and E. faecalis 12030, may be attributed to the surface accessibility of the protein antigens that vary from strain to strain, even if the antigen's encoding genes are conserved [54]. Such variability may be due to differences in gene expression, antigen masking by other cell wall components, protein degradation, or other factors [26], [54]. The presence of a putative antiphagocytic polysaccharide capsule in E. faecium E155, similar to the one found in E. faecalis serotypes C and D, may mask protein antigens, making them less available for binding or less accessible for complement components or phagocytes [46]. A similar effect was observed for E. faecalis Type 2 and Type 5 since these strains were killed to a lesser extent than E. faecalis 12030. Compared to this strain, 10 times higher serum concentrations were necessary to observe similar killing of these strains, strengthening the suggestion that masking may be the reason for the reduced opsonophagocytic killing observed in E. faecium E155. Specificity of the sera raised against the different proteins was demonstrated by the reduction of the opsonophagocytic killing elicited by serum absorbed with the corresponding recombinant protein. The OPA is known to correlate well with in vivo immune response and is considered a surrogate for the human protective immune response [10]. This assay is an indicator for the bacteria's ability to survive in the human blood and to cause infections [71]. We observed a good correlation between the ability of the antibodies raised against the different recombinant proteins to mediate opsonic killing in vitro and promote a statistically significant reduction of bacteria in mice after i.v. challenge.
In summary, we compared three existing extraction methods for bacterial surface proteins that are likely to interact with the host immune system. These proteins can be targets for drugs aimed at preventing bacterial infections and diseases, or could be used as components for conjugate vaccines. We demonstrate that the four peptidoglycan associated proteins identified by this approach, i.e. LysM, DdcP, PpiC and PBP5 elicit specific, opsonic and protective antibodies, with a broad cross-reactivity and serotype-independent coverage among E. faecalis and E. faecium. These antigens are interesting targets to be used as single component or as carrier proteins together with polysaccharide antigens in vaccine development against enterococcal infections.
Supporting Information
Summary of all the proteins identified by trypsin shaving.
(DOCX)
Summary of all the proteins identified by elution at high pH.
(DOCX)
Summary of all the proteins identified by biotinylation.
(DOCX)
Summary of all the proteins identified by at least two of the three extraction methods and predicted to have both cytoplasmic and extracytoplasmic location.
(DOCX)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Murray BE (2000) Vancomycin-resistant enterococcal infections. N Engl J Med 342: 710–721 10.1056/NEJM200003093421007 [DOI] [PubMed] [Google Scholar]
- 2. Zarb P, Coignard B, Griskeviciene J, Muller A, Vankerckhoven V, et al. (2012) The European Centre for Disease Prevention and Control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Euro Surveill 17. [DOI] [PubMed] [Google Scholar]
- 3. Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, et al. (2013) Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 34: 1–14 10.1086/668770 [DOI] [PubMed] [Google Scholar]
- 4. Werner G, Coque TM, Hammerum AM, Hope R, Hryniewicz W, et al. (2008) Emergence and spread of vancomycin resistance among enterococci in Europe. Euro Surveill 13. [PubMed] [Google Scholar]
- 5. Arias C a, Murray BE (2009) Antibiotic-resistant bugs in the 21st century–a clinical super-challenge. N Engl J Med 360: 439–443 10.1056/NEJMp0804651 [DOI] [PubMed] [Google Scholar]
- 6. Top J, Willems R, van der Velden S, Asbroek M, Bonten M (2008) Emergence of clonal complex 17 Enterococcus faecium in The Netherlands. J Clin Microbiol 46: 214–219 10.1128/JCM.01351-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Treitman A, Yarnold P (2005) Emerging incidence of Enterococcus faecium among hospital isolates (1993 to 2002). J Clin Microbiol 43: 462–463 10.1128/JCM.43.1.462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fisher K, Phillips C (2009) The ecology, epidemiology and virulence of Enterococcus. Microbiology 155: 1749–1757 10.1099/mic.0.026385-0 [DOI] [PubMed] [Google Scholar]
- 9. Sava IG, Heikens E, Huebner J (2010) Pathogenesis and immunity in enterococcal infections. Clin Microbiol Infect 16: 533–540 10.1111/j.1469-0691.2010.03213.x [DOI] [PubMed] [Google Scholar]
- 10.Repp C, Huebner J (2012) Protective Immune Response. In: Semedo-Lemsaddek T, Barreto-Crespo MT, Tenreiro R, editors. Enterococcus and Safety. Lisbone: Nova Science Publishers, Incorporated. pp. 305–317. [Google Scholar]
- 11. Schneewind O, Missiakas DM (2012) Protein secretion and surface display in Gram-positive bacteria. Philos Trans R Soc Lond B Biol Sci 367: 1123–1139 10.1098/rstb.2011.0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lindahl G (2005) Surface proteins of Streptococcus agalactiae and related proteins in other bacterial pathogens. Clin Microbiol Rev 18: 102–127 10.1128/CMR.18.1.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ton-That H, Marraffini LA, Schneewind O (2004) Protein sorting to the cell wall envelope of Gram-positive bacteria. Biochim Biophys Acta 1694: 269–278 10.1016/j.bbamcr.2004.04.014 [DOI] [PubMed] [Google Scholar]
- 14. Lin J, Huang S, Zhang Q (2002) Outer membrane proteins: key players for bacterial adaptation in host niches. Microbes Infect 4: 325–331 10.1016/S1286-4579(02)01545-9 [DOI] [PubMed] [Google Scholar]
- 15. Janulczyk R, Rasmussen M (2001) Improved Pattern for Genome-Based Screening Identifies Novel Cell Wall-Attached Proteins in Gram-Positive Bacteria. Infect Immun 69: 4019–4026 10.1128/IAI.69.6.4019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zervosen A, Sauvage E, Frère J-M, Charlier P, Luxen A (2012) Development of new drugs for an old target: the penicillin binding proteins. Molecules 17: 12478–12505 10.3390/molecules171112478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kumar P, Arora K, Lloyd JR, Lee IY, Nair V, et al. (2012) Meropenem inhibits D,D-carboxypeptidase activity in Mycobacterium tuberculosis. Mol Microbiol 86: 367–381 10.1111/j.1365-2958.2012.08199.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Olaya-Abril A, Jiménez-Munguía I, Gómez-Gascón L, Obando I, Rodríguez-Ortega MJ (2013) Identification of potential new protein vaccine candidates through pan-surfomic analysis of pneumococcal clinical isolates from adults. PLoS One 8: e70365 10.1371/journal.pone.0070365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Assaad U, El-Masri I, Porhomayon J, El-Solh AA (2012) Pneumonia immunization in older adults: review of vaccine effectiveness and strategies. Clin Interv Aging 7: 453–461 10.2147/CIA.S29675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mond JJ, Lees A, Snapper CM (1995) T cell-independent antigens type 2. Annu Rev Immunol 13: 655–692 10.1146/annurev.iy.13.040195.003255 [DOI] [PubMed] [Google Scholar]
- 21. Singh K V, Nallapareddy SR, Sillanpää J, Murray BE (2010) Importance of the collagen adhesin ace in pathogenesis and protection against Enterococcus faecalis experimental endocarditis. PLoS Pathog 6: e1000716 10.1371/journal.ppat.1000716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McCormick JK, Hirt H, Waters CM, Tripp TJ, Dunny GM, et al. (2001) Antibodies to a surface-exposed, N-terminal domain of aggregation substance are not protective in the rabbit model of Enterococcus faecalis infective endocarditis. Infect Immun 69: 3305–3314 10.1128/IAI.69.5.3305-3314.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kropec A, Sava IG, Vonend C, Sakinc T, Grohmann E, et al. (2011) Identification of SagA as a novel vaccine target for the prevention of Enterococcus faecium infections. Microbiology 157: 3429–3434 10.1099/mic.0.053207-0 [DOI] [PubMed] [Google Scholar]
- 24. Burnie J, Carter T, Rigg G, Hodgetts S, Donohoe M, et al. (2002) Identification of ABC transporters in vancomycin-resistant Enterococcus faecium as potential targets for antibody therapy. FEMS Immunol Med Microbiol 33: 179–189 10.1111/j.1574-695X.2002.tb00589.x [DOI] [PubMed] [Google Scholar]
- 25. Sava IG, Heikens E, Kropec A, Theilacker C, Willems R, et al. (2010) Enterococcal surface protein contributes to persistence in the host but is not a target of opsonic and protective antibodies in Enterococcus faecium infection. J Med Microbiol 59: 1001–1004 10.1099/jmm.0.020578-0 [DOI] [PubMed] [Google Scholar]
- 26. Rodríguez-Ortega MJ, Norais N, Bensi G, Liberatori S, Capo S, et al. (2006) Characterization and identification of vaccine candidate proteins through analysis of the group A Streptococcus surface proteome. Nat Biotechnol 24: 191–197 10.1038/nbt1179 [DOI] [PubMed] [Google Scholar]
- 27. Hempel K, Herbst F-A, Moche M, Hecker M, Becher D (2011) Quantitative proteomic view on secreted, cell surface-associated, and cytoplasmic proteins of the methicillin-resistant human pathogen Staphylococcus aureus under iron-limited conditions. J Proteome Res 10: 1657–1666 10.1021/pr1009838 [DOI] [PubMed] [Google Scholar]
- 28. Giombini E, Orsini M, Carrabino D, Tramontano A (2010) An automatic method for identifying surface proteins in bacteria: SLEP. BMC Bioinformatics 11: 39 10.1186/1471-2105-11-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou M, Boekhorst J, Francke C, Siezen RJ (2008) LocateP: genome-scale subcellular-location predictor for bacterial proteins. BMC Bioinformatics 9: 173 10.1186/1471-2105-9-173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barinov A, Loux V, Hammani A, Nicolas P, Langella P, et al. (2009) Prediction of surface exposed proteins in Streptococcus pyogenes, with a potential application to other Gram-positive bacteria. Proteomics 9: 61–73 10.1002/pmic.200800195 [DOI] [PubMed] [Google Scholar]
- 31. Cole JN, Ramirez RD, Currie BJ, Stuart J, Djordjevic SP, et al. (2005) Surface Analyses and Immune Reactivities of Major Cell Wall-Associated Proteins of Group A Streptococcus. Infect Immun 75: 3137–3146 10.1128/IAI.73.5.3137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gatlin CL, Pieper R, Huang S-T, Mongodin E, Gebregeorgis E, et al. (2006) Proteomic profiling of cell envelope-associated proteins from Staphylococcus aureus. Proteomics 6: 1530–1549 10.1002/pmic.200500253 [DOI] [PubMed] [Google Scholar]
- 33. Benachour A, Morin T, Hébert L, Budin-Verneuil A, Le Jeune A, et al. (2009) Identification of secreted and surface proteins from Enterococcus faecalis. Can J Microbiol 55: 967–974 10.1139/w09-052 [DOI] [PubMed] [Google Scholar]
- 34. Tjalsma H, Lambooy L, Hermans PW, Swinkels DW (2008) Shedding & shaving: disclosure of proteomic expressions on a bacterial face. Proteomics 8: 1415–1428 10.1002/pmic.200700550 [DOI] [PubMed] [Google Scholar]
- 35. Leavis HL, Willems RJL, van Wamel WJB, Schuren FH, Caspers MPM, et al. (2007) Insertion sequence-driven diversification creates a globally dispersed emerging multiresistant subspecies of E. faecium. PLoS Pathog 3: e7 10.1371/journal.ppat.0030007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hempel K, Pané-Farré J, Otto A, Sievers S, Hecker M, et al. (2010) Quantitative cell surface proteome profiling for SigB-dependent protein expression in the human pathogen Staphylococcus aureus via biotinylation approach. J Proteome Res 9: 1579–1590 10.1021/pr901143a [DOI] [PubMed] [Google Scholar]
- 37. Morsczeck C, Prokhorova T, Sigh J, Pfeiffer M, Bille-Nielsen M, et al. (2008) Streptococcus pneumoniae: proteomics of surface proteins for vaccine development. Clin Microbiol Infect 14: 74–81 10.1111/j.1469-0691.2007.01878.x [DOI] [PubMed] [Google Scholar]
- 38. Reffuveille F, Serror P, Chevalier S, Budin-Verneuil A, Ladjouzi R, et al. (2012) The prolipoprotein diacylglyceryl transferase (Lgt) of Enterococcus faecalis contributes to virulence. Microbiology 158: 816–825 10.1099/mic.0.055319-0 [DOI] [PubMed] [Google Scholar]
- 39. Yu C, Chen Y, Lu C, Hwang J (2006) Prediction of Protein Subcellular Localization. Proteins 651: 643–651 10.1002/prot [DOI] [PubMed] [Google Scholar]
- 40. Chou K-C, Shen H-B (2008) Cell-PLoc: a package of Web servers for predicting subcellular localization of proteins in various organisms. Nat Protoc 3: 153–162 10.1038/nprot.2007.494 [DOI] [PubMed] [Google Scholar]
- 41. Shen H-B, Chou K-C (2009) Gpos-mPLoc: A Top-Down Approach to Improve the Quality of Predicting Subcellular Localization of Gram-Positive Bacterial Proteins. Protein Pept Lett 16: 1478–1484 10.2174/092986609789839322 [DOI] [PubMed] [Google Scholar]
- 42. Shen H-B, Chou K-C (2007) Gpos-PLoc: an ensemble classifier for predicting subcellular localization of Gram-positive bacterial proteins. Protein Eng Des Sel 20: 39–46 10.1093/protein/gzl053 [DOI] [PubMed] [Google Scholar]
- 43.Sambrook J, Russell DW (2001) Molecular Cloning: A Laboratory Manual, Volume 1. 3rd ed. Cold Spring Harbor, NY: CSHL Press. [Google Scholar]
- 44. Huebner J, Wang Y, Krueger WA, Madoff LC, Martirosian G, et al. (1999) Isolation and chemical characterization of a capsular polysaccharide antigen shared by clinical isolates of Enterococcus faecalis and vancomycin-resistant Enterococcus faecium. Infect Immun 67: 1213–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huebner J, Quaas A, Krueger WA, Goldmann DA, Pier GB (2000) Prophylactic and Therapeutic Efficacy of Antibodies to a Capsular Polysaccharide Shared among Vancomycin-Sensitive and -Resistant Enterococci. Infect Immun 68: 4631–4636 10.1128/IAI.68.8.4631-4636.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Theilacker C, Kaczyński Z, Kropec A, Sava I, Ye L, et al. (2011) Serodiversity of opsonic antibodies against Enterococcus faecalis–glycans of the cell wall revisited. PLoS One 6: e17839 10.1371/journal.pone.0017839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Buist G, Steen A, Kok J, Kuipers OP (2008) LysM, a widely distributed protein motif for binding to (peptido)glycans. Mol Microbiol 68: 838–847 10.1111/j.1365-2958.2008.06211.x [DOI] [PubMed] [Google Scholar]
- 49. Sauvage E, Kerff F, Terrak M, Ayala J a, Charlier P (2008) The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol Rev 32: 234–258 10.1111/j.1574-6976.2008.00105.x [DOI] [PubMed] [Google Scholar]
- 50. Sauvage E, Kerff F, Fonzé E, Herman R, Schoot B, et al. (2002) The 2.4-Å crystal structure of the penicillin-resistant penicillin-binding protein PBP5fm from Enterococcus faecium in complex with benzylpenicillin. Cell Mol Life Sci 59: 1223–1232 10.1007/s00018-002-8500-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang X, Paganelli FL, Bierschenk D, Kuipers A, Bonten MJM, et al. (2012) Genome-wide identification of ampicillin resistance determinants in Enterococcus faecium. PLoS Genet 8: e1002804 10.1371/journal.pgen.1002804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hyyryläinen H-L, Marciniak BC, Dahncke K, Pietiäinen M, Courtin P, et al. (2010) Penicillin-binding protein folding is dependent on the PrsA peptidyl-prolyl cis-trans isomerase in Bacillus subtilis. Mol Microbiol 77: 108–127 10.1111/j.1365-2958.2010.07188.x [DOI] [PubMed] [Google Scholar]
- 53. Hufnagel M, Hancock LE, Koch S, Theilacker C, Gilmore MS, et al. (2004) Serological and genetic diversity of capsular polysaccharides in Enterococcus faecalis. J Clin Microbiol 42: 2548–2557 10.1128/JCM.42.6.2548-2557.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Maione D, Margarit I, Rinaudo CD, Masignani V, Mora M, et al. (2005) Identification of a universal Group B streptococcus vaccine by multiple genome screen. Science 309: 148–150 10.1126/science.1109869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pizza M (2000) Identification of Vaccine Candidates Against Serogroup B Meningococcus by Whole-Genome Sequencing. Science (80-) 287: 1816–1820 10.1126/science.287.5459.1816 [DOI] [PubMed] [Google Scholar]
- 56. Jeffery CJ (2003) Moonlighting proteins: old proteins learning new tricks. Trends Genet 19: 415–417 10.1016/S0168-9525(03)00167-7 [DOI] [PubMed] [Google Scholar]
- 57. Olaya-Abril A, Jiménez-Munguía I, Gómez-Gascón L, Rodríguez-Ortega MJ (2014) Surfomics: shaving live organisms for a fast proteomic identification of surface proteins. J Proteomics 97: 164–176 10.1016/j.jprot.2013.03.035 [DOI] [PubMed] [Google Scholar]
- 58. Carneiro CRW, Postol E, Nomizo R, Reis LFL, Brentani RR (2004) Identification of enolase as a laminin-binding protein on the surface of Staphylococcus aureus. Microbes Infect 6: 604–608 10.1016/j.micinf.2004.02.003 [DOI] [PubMed] [Google Scholar]
- 59. Pancholi V, Fischetti VA (1998) {alpha}-Enolase, a Novel Strong Plasmin(ogen) Binding Protein on the Surface of Pathogenic Streptococci. J Biol Chem 273: 14503–14515 10.1074/jbc.273.23.14503 [DOI] [PubMed] [Google Scholar]
- 60. Baums CG, Valentin-Weigand P (2009) Surface-associated and secreted factors of Streptococcus suis in epidemiology, pathogenesis and vaccine development. Anim Health Res Rev 10: 65–83 10.1017/S146625230999003X [DOI] [PubMed] [Google Scholar]
- 61. Modun B, Williams P (1999) The staphylococcal transferrin-binding protein is a cell wall glyceraldehyde-3-phosphate dehydrogenase. Infect Immun 67: 1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dallo SF, Kannan TR, Blaylock MW, Baseman JB (2002) Elongation factor Tu and E1 beta subunit of pyruvate dehydrogenase complex act as fibronectin binding proteins in Mycoplasma pneumoniae. Mol Microbiol 46: 1041–1051 10.1046/j.1365-2958.2002.03207.x [DOI] [PubMed] [Google Scholar]
- 63. Schaumburg J, Diekmann O, Hagendorff P, Bergmann S, Rohde M, et al. (2004) The cell wall subproteome of Listeria monocytogenes. Proteomics 4: 2991–3006 10.1002/pmic.200400928 [DOI] [PubMed] [Google Scholar]
- 64. Zysk G, Bongaerts RJM, ten Thoren E, Bethe G, Hakenbeck R, et al. (2000) Detection of 23 Immunogenic Pneumococcal Proteins Using Convalescent-Phase Serum. Infect Immun 68: 3740–3743 10.1128/IAI.68.6.3740-3743.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vytvytska O, Nagy E, Blüggel M, Meyer HE, Kurzbauer R, et al. (2002) Identification of vaccine candidate antigens of Staphylococcus aureus by serological proteome analysis. Proteomics 2: 580–590 [DOI] [PubMed] [Google Scholar]
- 66. Gillis TP, Miller RA, Young DB, Khanolkar SR, Buchanan TM (1985) Immunochemical characterization of a protein associated with Mycobacterium leprae cell wall. Infect Immun 49: 371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hennequin C, Collignon A, Karjalainen T (2001) Analysis of expression of GroEL (Hsp60) of Clostridium difficile in response to stress. Microb Pathog 31: 255–260 10.1006/mpat.2001.0468 [DOI] [PubMed] [Google Scholar]
- 68. Reffuveille F, Connil N, Sanguinetti M, Posteraro B, Chevalier S, et al. (2012) Involvement of peptidylprolyl cis/trans isomerases in Enterococcus faecalis virulence. Infect Immun 80: 1728–1735 10.1128/IAI.06251-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Aakra A, Vebø H, Snipen L, Hirt H, Aastveit A, et al. (2005) Transcriptional response of Enterococcus faecalis V583 to erythromycin. Antimicrob Agents Chemother 49: 2246–2259 10.1128/AAC.49.6.2246-2259.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Willems RJL, Top J, van Santen M, Robinson DA, Coque TM, et al. (2005) Global spread of vancomycin-resistant Enterococcus faecium from distinct nosocomial genetic complex. Emerg Infect Dis 11: 821–828 10.3201/eid1106.041204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hufnagel M, Koch S, Kropec A, Huebner J (2003) Opsonophagocytic assay as a potentially useful tool for assessing safety of enterococcal preparations. Int J Food Microbiol 88: 263–267 10.1016/S0168-1605(03)00189-2 [DOI] [PubMed] [Google Scholar]
- 72. Van den Bogaard A (1997) High prevalence of colonization with vancomycin- and pristinamycin- resistant enterococci in healthy humans and pigs in The Netherlands: is the addition of antibiotics to animal feeds to blame? J Antimicrob Chemother 40: 454–456 10.1093/jac/40.3.454 [DOI] [PubMed] [Google Scholar]
- 73. Maekawa S, Yoshioka M, Kumamoto Y (1992) Proposal of a New Scheme for the Serological Typing of Enterococcus faecalis Strains. Microbiol Immunol 36: 671–681 10.1111/j.1348-0421.1992.tb02070.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of all the proteins identified by trypsin shaving.
(DOCX)
Summary of all the proteins identified by elution at high pH.
(DOCX)
Summary of all the proteins identified by biotinylation.
(DOCX)
Summary of all the proteins identified by at least two of the three extraction methods and predicted to have both cytoplasmic and extracytoplasmic location.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.