Abstract
Receptors for the Fc portion of nonimmune immunoglobulin G were demonstrated on B103 rat brain neuroma cells infected with herpes simplex virus type 1 (HSV-1) KOS by a radioimmunoassay using 125I-labeled heat-aggregated Fc fragments. Immune F(ab')2 fragments specific for HSV antigens competed efficiently for Fc binding sites, suggesting that the binding of Fc fragments to infected cells is specific for viral cell-surface antigens. It has been suggested that the binding of immune complexes to Fc receptors on the surfaces of tumor cells in vivo plays a role in protecting these cells from immune destruction. In vitro evidence is presented for the ability of aggregated immunoglobulin G molecules bound to cell-surface Fc receptors to protect both HSV-infected and HSV-transformed cells against complement-dependent and cell-mediated immune lysis.
Full text
PDF
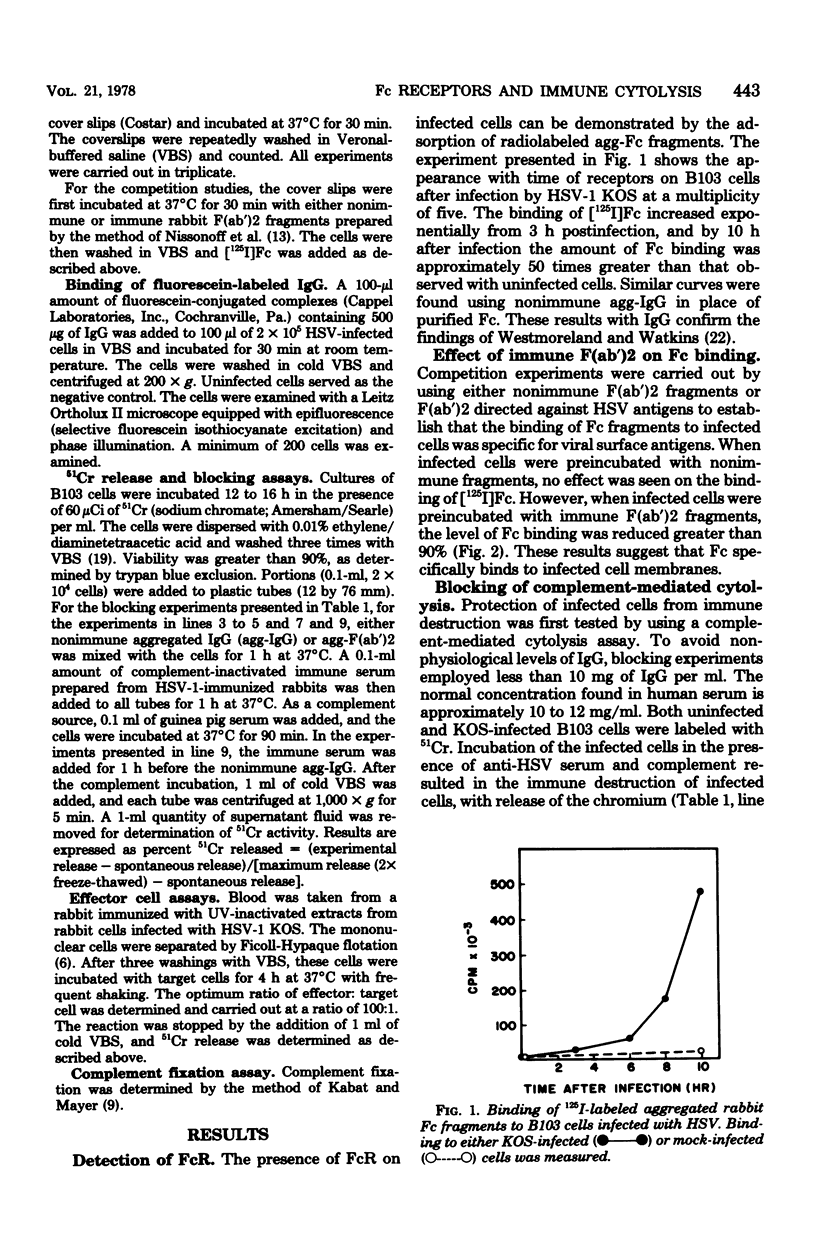
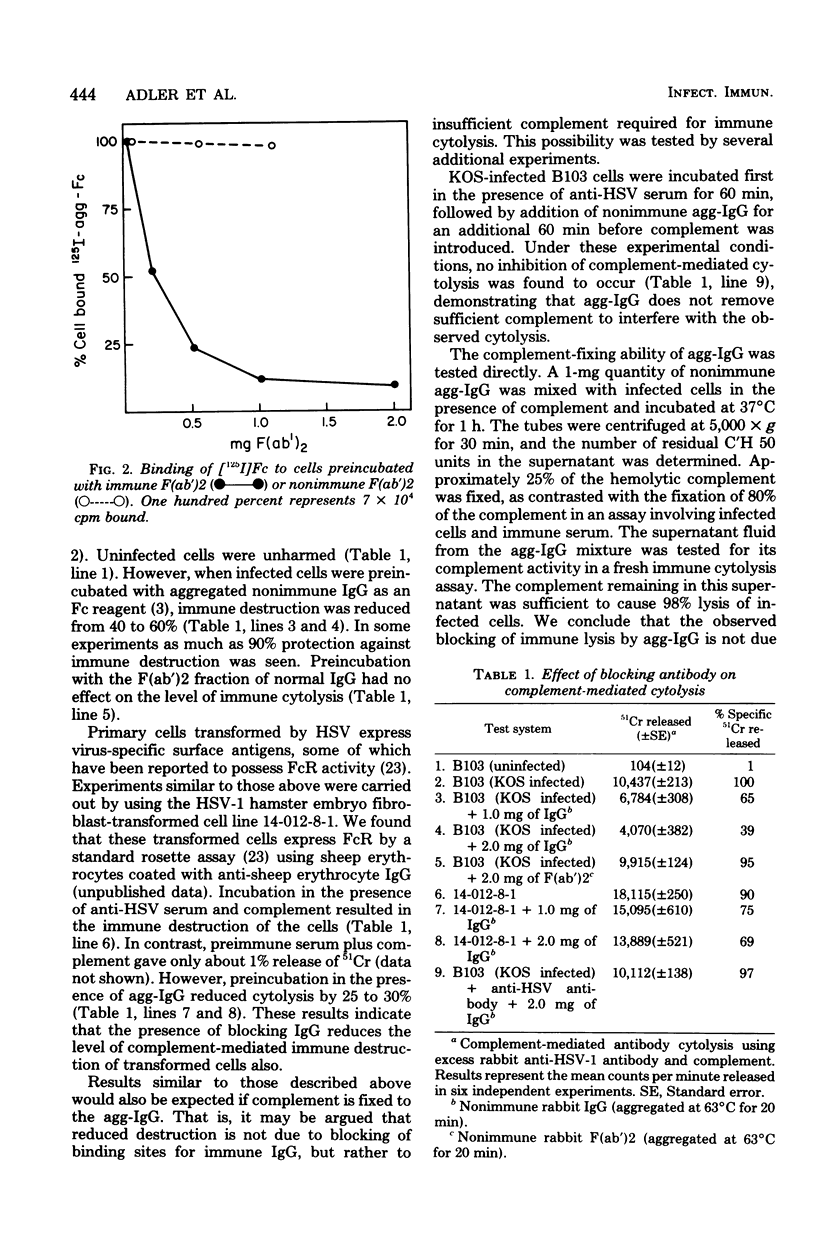



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin R. W., Price M. R., Robins R. A. Blocking of lymphocyte-mediated cytotoxicity for rat hepatoma cells by tumour-specific antigen-antibody complexes. Nat New Biol. 1972 Aug 9;238(84):185–186. doi: 10.1038/newbio238185a0. [DOI] [PubMed] [Google Scholar]
- Cooper S. M., Sambray Y. Isolation of a murine leukemia FC receptor by selective release induced by surface redistribution. J Immunol. 1976 Aug;117(2):511–517. [PubMed] [Google Scholar]
- Dickler H. B., Arbeit R. D., Henkart P. A., Sachs D. H. Association between Ia antigens and the Fc receptors of certain T lymphocytes. J Exp Med. 1976 Jul 1;144(1):282–287. doi: 10.1084/jem.144.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickler H. B. Lymphocyte binding of aggregated immunoglobulin. Scand J Immunol. 1976 Jun;Suppl 5:91–97. doi: 10.1111/j.1365-3083.1976.tb03860.x. [DOI] [PubMed] [Google Scholar]
- Duff R., Rapp F. Oncogenic transformation of hamster embryo cells after exposure to inactivated herpes simplex virus type 1. J Virol. 1973 Aug;12(2):209–217. doi: 10.1128/jvi.12.2.209-217.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensenius J. C., Williams A. F. The binding of anti-immunoglobulin antibodies to rat thymocytes and thoracic duct lymphocytes. Eur J Immunol. 1974 Feb;4(2):91–97. doi: 10.1002/eji.1830040207. [DOI] [PubMed] [Google Scholar]
- Kerbel R. S., Davies A. J. The possible biological significance of Fc receptors on mammalian lymphocytes and tumor cells. Cell. 1974 Oct;3(2):105–112. doi: 10.1016/0092-8674(74)90113-5. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B., Lagrange P. H., Miller T. E., Ishibashi T. Feedback inhibition of specifically sensitized lymphocytes. J Exp Med. 1974 Mar 1;139(3):543–559. doi: 10.1084/jem.139.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgrom F., Humphrey L. J., Tönder O., Yasuda J., Witebsky E. Antibody-mediated hemadsorption by tumor tissues. Int Arch Allergy Appl Immunol. 1968;33(5):478–492. doi: 10.1159/000230063. [DOI] [PubMed] [Google Scholar]
- NISONOFF A., WISSLER F. C., LIPMAN L. N., WOERNLEY D. L. Separation of univalent fragments from the bivalent rabbit antibody molecule by reduction of disulfide bonds. Arch Biochem Biophys. 1960 Aug;89:230–244. doi: 10.1016/0003-9861(60)90049-7. [DOI] [PubMed] [Google Scholar]
- Ommaya A. K., Yarnell P. Subdural haematoma after whiplash injury. Lancet. 1969 Aug 2;2(7614):237–239. doi: 10.1016/s0140-6736(69)90005-1. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A. S., Percy J. S., Kovithavongs T. Cell-mediated immunity to Herpes simplex in humans: lymphocyte cytotoxicity measured by 51-Cr release from infected cells. Infect Immun. 1975 Feb;11(2):355–359. doi: 10.1128/iai.11.2.355-359.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Heinemann S., Carlisle W., Tarikas H., Kimes B., Patrick J., Steinbach J. H., Culp W., Brandt B. L. Clonal cell lines from the rat central nervous system. Nature. 1974 May 17;249(454):224–227. doi: 10.1038/249224a0. [DOI] [PubMed] [Google Scholar]
- Sjögren H. O., Hellström I., Bansal S. C., Hellström K. E. Suggestive evidence that the "blocking antibodies" of tumor-bearing individuals may be antigen--antibody complexes. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1372–1375. doi: 10.1073/pnas.68.6.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Glorioso J. C. Effect of cross-immunization on monotypic antibody responses to herpes simplex virus types 1 and 2. J Immunol. 1976 Apr;116(4):898–903. [PubMed] [Google Scholar]
- Sugamura K., Smith J. B. Reversible blocking of antibody-dependent cell-mediated cytotoxicity. Cell Immunol. 1977 May;30(2):353–357. doi: 10.1016/0008-8749(77)90078-8. [DOI] [PubMed] [Google Scholar]
- Tonder O., Thunold S. Receptors for immunoglobulin Fc in human malignant tissues. Scand J Immunol. 1973;2(3):207–215. doi: 10.1111/j.1365-3083.1973.tb02031.x. [DOI] [PubMed] [Google Scholar]
- Westmoreland D., Watkins J. F., Rapp F. Demonstration of a receptor for IgG in Syrian hamster cells transformed with herpes simplex virus. J Gen Virol. 1974 Oct;25(1):167–170. doi: 10.1099/0022-1317-25-1-167. [DOI] [PubMed] [Google Scholar]
- Westmoreland D., Watkins J. F. The IgG receptor induced by herpes simplex virus: studies using radioiodinated IgG. J Gen Virol. 1974 Jul;24(1):167–178. doi: 10.1099/0022-1317-24-1-167. [DOI] [PubMed] [Google Scholar]
- Wood G. W., Gillespie G. Y., Barth R. F. Receptor sites for antigen-antibody complexes on cells derived from solid tumors: detection by means of antibody sensitized sheep erythrocytes labeled with technetium-99m. J Immunol. 1975 Mar;114(3):950–957. [PubMed] [Google Scholar]


