Abstract
The heart is a mechanically-active organ that dynamically senses its own mechanical environment. This environment is constantly changing, on a beat-by-beat basis, with additional modulation by respiratory activity and changes in posture or physical activity, and further overlaid with more slowly occurring physiological (e.g. pregnancy, endurance training) or pathological challenges (e.g. pressure or volume overload). Far from being a simple pump, the heart detects changes in mechanical demand and adjusts its performance accordingly, both via heart rate and stroke volume alteration. Many of the underlying regulatory processes are encoded intracardially, and are thus maintained even in heart transplant recipients. Over the last three decades, molecular substrates of cardiac mechanosensitivity have gained increasing recognition in the scientific and clinical communities. Nonetheless, the processes underlying this phenomenon are still poorly understood. Stretch-activated ion channels (SAC) have been identified as one contributor to mechanosensitive autoregulation of the heartbeat. They also appear to play important roles in the development of cardiac pathologies – most notably stretch-induced arrhythmias. As recently discovered, some established cardiac drugs act, in part at least, via mechanotransduction pathways suggesting SAC as potential therapeutic targets. Clearly, identification of the molecular substrate of cardiac SAC is of clinical importance and a number of candidate proteins have been identified. At the same time, experimental studies have revealed variable–and at times contrasting–results regarding their function. Further complication arises from the fact that many ion channels that are not classically defined as SAC, including voltage and ligand-gated ion channels, can respond to mechanical stimulation. Here, we summarise what is known about the molecular substrate of the main candidates for cardiac SAC, before identifying potential further developments in this area of translational research.
Keywords: heart, mechanotransduction, mechano-electric feedback
Introduction
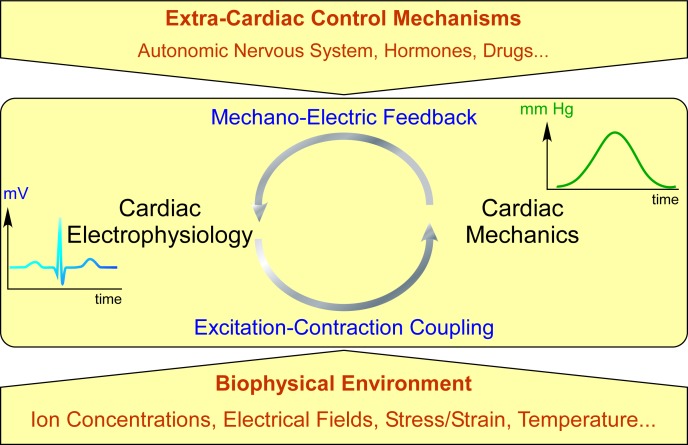
Mechanosensitivity is an intrinsic property of cardiac functional autoregulation (Figure 1), affecting mechanical activity (e.g. via the Frank-Starling effect 1,2 : an acute stretch-induced increase in cardiac contractility in the absence of raised intracellular calcium) and electrical behaviour (e.g. through the Bainbridge effect 3 : the stretch-induced increase in spontaneous pacemaker rate) of the heart. These mechano-electric feedback (MEF) responses are sustained in denervated (e.g. isolated 4–6 or transplanted 7–9 ) hearts, in isolated tissue 10,11 and even single cells – in both cardiac myocytes 12–16 and non-myocytes. 17–19
Figure 1.
Simplified diagram of cardiac electromechanical integration. Cardiac electrophysiology controls cardiac mechanics via excitation-contraction coupling. Changes in the heart's mechanical environment from contraction or external interventions affect electrophysiology via mechano-electric feedback. This intra-cardiac regulatory loop (middle) is subject to modulation by extra-cardiac control (top) and environmental parameters (bottom).
Adaptation to a highly dynamic mechanical environment is a crucial feature of normal cardiac function. It is involved in the regulation of beat-by-beat physiology, 4,20,21 and implicated in the progression of cardiac diseases, including rhythm disturbances. 22–25 For a compendium of current insight into cardiac mechano-electric coupling and arrhythmias, from pipette to patient, see 26 . Although the mechanisms underlying cardiac mechanotransduction are not completely understood, key players are thought to include mechanosensitive ion channels (MSC). MSC are defined in the broadest sense by their ability to change ion channel open probability in response to mechanical stimuli, thereby converting mechanical energy into the modification of an electrochemical signal. 27 MSC have been demonstrated to act as functional mechanotransducers in a number of different tissues, including the heart, and their block is capable of preventing or terminating certain mechanically-induced arrhythmias. 28,29
MSC can be subcategorised by the type of mechanical stimulation required for channel activation. Although these boundaries are far from clear-cut, it is useful to make this conceptual distinction. In this review we shall focus on stretch-activated ion channels (SAC), which are those MSC whose switching from ‘closed’ to ‘open’ state can be driven over their full dynamic range by stretch alone, for example through direct mechanical membrane deformation (Figure 2).
Figure 2.
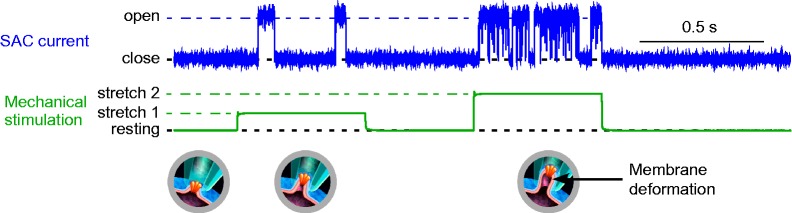
SAC current recording (top) and patch pipette suction (middle) used for membrane deformation (bottom). Using the patch clamp technique, the most common way to cause mechanical stimuli is to apply negative pressure to the inside of the pipette; this induces membrane patch deformation (inserts). At the beginning of the protocol, after seal formation but in the absence of additional suction, channels are closed (no current). If negative pressure is applied (first pulse), channels open (here two openings of a single SAC). After a brief release, a higher level of suction is applied. This increases the open probability of the SAC (second pulse), but not the single channel conductance (same current amplitude). The original current trace shown here was taken from a potassium selective SAC; insets adapted from F. Aguila (CNRS), with permission.
Another subcategory of MSC, cell volume-activated ion channels (VAC), are also considered to respond to mechanical stimuli, 30 but these differ in their micro-mechanical deformation properties (cell swelling increases cell diameter with less dominant effects on length, while axial stretch increases cell length and reduces diameter), time course of response (VAC usually show a lag-time of tens of seconds to minutes between the onset of cell swelling and channel opening, while SAC activate instantaneously), and pathophysiological context (it is assumed that – in contrast to ischaemia or hypertrophy – the normal cycle of contraction and relaxation is not associated with cell volume changes). For these reasons, VAC are unlikely to be main contributors to acute and/or beat-by-beat responses of the heart to mechanical stimuli, and they will not be considered in detail here (for a review on VAC, see 30 ).
SAC were discovered in 1984 in embryonic chick skeletal myocytes by Guharay and Sachs. 31 In subsequent years, SAC have been identified in many other cell types 32,33 including cardiomyocytes. 34 Cardiac SAC can be either cation non-selective (SACNS) 34 or potassium-selective (SACK) 35 (Figure 3). The development of the patch clamp technique was vital for the study of cardiac SAC, and it revealed, in addition to stretch-activated whole-cell currents, evidence of single-channel activity in atrial myocytes, 35 foetal 34 and (for SACK at least) adult ventricular myocytes, 36 as well as cardiac non-myocytes. 19 That said, formation of membrane patches is associated with significant alterations in local mechanical and structural properties, especially in complex and densely ‘crowded’ cells such as cardiac myocytes. This leaves the potential for false-positive (e.g. channels that would normally be protected from opening, such as by cytoskeletal interaction) and false-negative observations (channels that are constitutively activated by patch formation may not be identified as mechano-sensitive upon additional patch deformation). This highlights the importance of multi-level investigations, combining a range of electrophysiological recording techniques, from lipid bilayers to sub-cellular and cellular studies in expression systems and native cells, to cultures, tissue slices, native tissue and organs, right through to whole animal or patient research. As pointed out elsewhere, much of this hinges on the availability of improved pharmacological agents, and it requires quantitative structure-based integration, such as by computational modelling.
Figure 3.
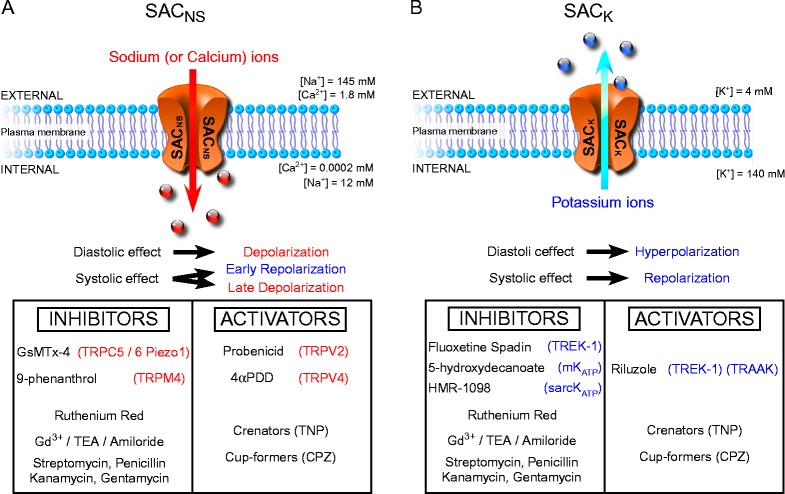
Overview of cation non-selective (SACNS) and potassium-selective (SACK) channel function, effects, and pharmacological modulators. A. SACNS opening leads to sodium and possibly calcium entry (in addition to also present potassium fluxes); this depolarises the plasma membrane during diastole, repolarises it during early systole, and may cause after-depolarisation-like behaviour in later stages of action potential repolarisation, depending on the current's reversal potential (usually in the mid-region between systolic and diastolic peak potentials). B. SACK opening leads to potassium exit from the cytoplasm; this acts to hyperpolarize the plasma membrane during diastole, and to repolarize it during systole. SAC modulators are summarized in boxes, with the more selective ones at the top of each table. Channels: mKATP: mitochondrial KATP, sarcKATP: sarcolemmal KATP, Gd3+: gadolinium ions, TEA: tetraethylammonium, TNP: trinitrophenol, CPZ: chlorpromazine.
First insights into the structure and possible mechanisms of operation of these channels were provided by the cloning and crystallization of two bacterial SAC. 37,38 However, even after an exhaustive search, no sequence homologues of these particular ion channels were found in mammals. The first cloned mammalian SAC was the TREK channel (a ‘tandem of two-pore K+ domains in a weak inwardly rectifying K+ channel’ = TWIK-related potassium channel). 39 Despite these significant steps, the molecular identities of mammalian cardiac SAC have yet to be determined.
In spite of a lack of firm molecular identification, there are several prominent candidates for mammalian cardiac SAC, and these will be reviewed here. For SACNS these include transient receptor potential (TRP) channels, and Piezo1. SACK candidates are TREK-1, the large-conductance calcium-activated K+ channel (BKCa; a member of the ‘Big K+’ channel family), and the ATP-sensitive potassium channel (KATP); see Table 1.
Table 1.
Summary of the currently known main molecular candidates for stretch-activated ion channels in cardiac myocytes. TT: T-tubules, S: sarcolemma; N/S: not specified.
| Location | |||
| Ion channels | Species | Cellular | Sub-cellular |
| SACNS | |||
| TRPC1 | Human 48 | Myocardium | N/S |
| Rat 42 | Atrial myocyte | S | |
| Ventricular myocyte | TT | ||
| TRPC6 | Human 48 | Myocardium | N/S |
| Mouse 58 | Ventricular myocyte | TT | |
| TRPV2 | Mouse 62 | Myocardium | N/S |
| TRPV4 | Neonatal rat 64 | Ventricular myocytes | Nucleus |
| TRPM4 | Rat 158 | Myocardium | N/S |
| Piezo1 | Rat 74 | Myocardium | N/S |
| SACK | |||
| TREK-1 | Rat 80 | Atrial myocyte | S |
| Ventricular myocyte | N/S | ||
| BK ca | Chick embryo 94,129 | Ventricular myocyte | S |
| K ATP | Neonatal rat 159 | Atrial myocyte | S |
In the following, we evaluate the available evidence for presence and contributions of these main cardiac SAC candidates, including their sensitivity to pharmacological interventions, highlight some of the present experimental challenges, and conclude with a consideration of anticipated further developments in this exciting and dynamic field of translational heart research. We will not discuss alternative mechano-sensors, detailed signalling pathways, or protein-protein interactions, all of which form deserving topics for separate reviews.
Sacns
Whole-cell currents with a linear current-voltage relationship attributed to SACNS (ISAC,NS), were first identified in cardiac cells by Craelius et al., 34 using whole-cell patch clamp recordings from neonatal rat ventricular myocytes. By applying a voltage clamp, Zeng et al. 40 later described the properties of this current further, including a lack of inactivation and a pronounced sensitivity to block by gadolinium ions (Gd3+). The channel's reversal potential is positive to the resting potential of working cardiomyocytes, so that activation of SACNS will depolarise resting cells. 27 In contrast to SACK, SACNS are distinctly sensitive to a peptide, isolated by Sachs et al. from Chilean tarantula venom: GsMTx-4 (Grammostola spatulata Mechano-Toxin 4 41 ). The use of GsMTx-4 has allowed researchers to extend the evidence on whole-cell ISAC,NS towards identification of SACNS effects at the tissue and whole organ levels. At the same time, no SACNS single-channel recordings from freshly-isolated adult ventricular cardiomyocytes have been reported. This has led to the suggestion that SACNS may be localised in membrane regions that are difficult to access in patch clamp studies, such as transverse tubules (T-tubules 42 ), caveolae (which, themselves, form a mechanosensitive structural domain that may be integrated into the surface sarcolemma by excess stretch 43 ), or at intercalated discs. 44 The main molecular candidates for cardiac SACNS, TRP channels 45 and the recently discovered Piezo1 protein, 46 will be discussed in more detail.
TRP channels
TRP proteins form a family of widely expressed cation channels, responsible for a variety of cellular functions. Polymodal regulation is a distinct feature of TRP (http://www.ncbi.nlm.nih.gov/gene/724608). Known activators of TRP channels include chemical stimuli, temperature elevation, and mechanical interventions ranging from local patch deformation to membrane stretch and shear strain. 47 In particular, the so-called ‘canonical’ TRP channels TRPC1 (http://www.ncbi.nlm.nih.gov/gene/7220) and TRPC6 (http://www.ncbi.nlm.nih.gov/gene/7225) have been implicated as candidates for cardiac SACNS.
TRPC1: Analysis of mRNA expression suggested that TRPC1 is present in the human heart. 48 Using immuno-histochemical labelling and confocal imaging, TRPC1 protein was found to colocalise with phalloidin stain in rat ventricular myocytes. 42 This suggests that TRPC1 may be located in T-tubules and is consistent with the hypothesised spatial distribution of endogenous SACNS in adult ventricular cardiomyocytes.
Mechanosensitivity of TRPC1 was first noted by Maroto et al. 49 in Xenopus oocytes. In their experiments, ISAC,NS was measured after membrane protein fractionation and reconstitution of individual proteins in liposomes. A particularly mechanosensitive fraction was found to contain an 80-kDa protein which was immunoreactive to TRPC1 antibody, indicating the presence of a TRPC1 homologue. Further expression of the human TRPC1 (hTRPC1) isoform in Xenopus oocytes and Chinese hamster ovary (CHO) K1 cells increased ISAC,NS tenfold, whereas microinjection of antisense hTRPC1 RNA greatly reduced ISAC,NS in both cell types.
Since publication, these findings have been challenged by several studies, including one by some of the authors of the original report. They found that transfection of hTRPC1 into COS cells (a fibroblast-related cell line, originally derived from kidney tissue of monkey) had no discernible effect, while transfection of a different putative SAC (the SACK TREK-1; see below), induced an increase by three orders of magnitude in mechanosensitive whole-cell currents. This result puts into question the significance of the less pronounced (ten-fold) increase seen in the earlier experiments. 50 The authors of the later study found limited ion channel expression at the sarcolemma, which is in agreement with a more recent report showing predominantly intracellular expression of transfected TRPC1 in a skeletal muscle cell line, unless co-expressed with Cav3 (http://www.ncbi.nlm.nih.gov/gene/859). 51 Thus, even if TRPC1 is successfully transfected, it may require associated molecular machinery for a correct subcellular localization and/or proper function. In addition, TRPC1 may require other TRPC isoforms to form a functional heteromeric channel. 52
The conflicting results reported above highlight problems that can be associated with the use of heterologous expression systems to study cardiac ion channels. Clearly, the intracellular environment of stable cell lines differs significantly from that of cardiomyocytes, while additional transfection with exogenous ion channels can alter the structure and function of recipient cells. 50 Given the dependence of SAC gating properties on micro-mechanical and structural properties of a cell, it is difficult to establish suitable control protocols, 50 or to arrive at definitive conclusions from these experiments.
Interestingly, mice in whom TRPC1 has been knocked out (TRPC1− / − ) exhibit no significant difference in ventricular slow force response (SFR, initially characterised in cat papillary muscle, 53 is a gradual, calcium-related increase in muscle contractility upon exposure to maintained stretch), compared to wild-type controls. 54 This suggests that TRPC1 may not be an obligatory and/or exclusive component of the SFR (similar findings were reported for TRPC3 (http://www.ncbi.nlm.nih.gov/gene/7222) 55 ). However, as with all knockout experiments, there is always the possibility of compensatory changes in expression of other genes. One way of assessing this would be to use acute knockdown experiments, ideally involving tissue-specific drivers of protein expression. It would also be instructive to explore acute MEF responses that would be expected to precede the SFR in cardiac myocytes or tissue preparations of TRPC1− / − mice.
TRPC6: Mammalian TRPC6 was initially identified as a mechanosensitive ion channel by Spassova et al., 56 who found that overexpression of TRPC6 in human embryonic kidney cell line 293 (HEK293) cells induced ISAC,NS. However, a subsequent study by Gottlieb et al. 50 found that TRPC6 overexpression in CHO and COS cells had no significant effect. More recently, it has been suggested that TRPC6 is not mechanosensitive, unless co-expressed with the angiotensin II type 1 (AT1) receptor. 45,47 Data, more directly relevant for cardiac mechanosensitivity, came from Dyachenko et al., 58 who used mouse ventricular myocytes, as opposed to heterologous expression systems. Their whole-cell patch clamp experiments identified a robust ISAC,NS in response to shear stimuli, which was inhibited by pore-blocking TRPC6 antibodies. TRPC6 knockout blunts the SFR in wild-type murine models, while its genetic down-regulation or pharmacological block returns ‘hyper-responsive’ murine models of Duchenne muscular dystrophy back to normal SFR levels, 55 highlighting the potential clinical relevance of targeted TRPC6 manipulation.
TRPC6 is among a small number of SAC candidates that is highly expressed in human heart homogenates. 48 In murine heart, TRPC6 appears to be localised to T-tubules. 58 In agreement with this observation, detubulation inhibits ISAC,NS in murine cardiomyocytes. 58 Interestingly, a recent paper has suggested that the localization of TRPC6 shows marked plasticity in response to sympathetic stimulation via α1A receptors, and that these channels can translocate from T-tubules to the sarcolemma. 59 Whether this occurs physiologically is unclear; however, pre-treatment with α1A-agonists might serve as a useful experimental intervention to facilitate single-channel recordings of TRPC6, and potentially other channels localised in T-tubules, in adult ventricular myocytes.
Other TRP channels: Several other members of the TRP family are mechanosensitive and are expressed in the heart. The TRPC3 protein has been identified in rat ventricular myocytes, also located in T-tubules. 60 Mechanical stretch of neonatal cardiomyocytes in a mouse model displays TRPC3 overexpression induced ROS production, similar to TRPC3-activation by 1-oleoyl-2-acetyl-sn-glycerol (OAG). However, OAG is a fairly unspecific activator of TRPC channels, so effects cannot be attributed with confidence to TRPC3. 60 The vanilloid TRP channel 2 (TRPV2 (http://www.ncbi.nlm.nih.gov/gene/51393)) has been reported to be mechanosensitive (studied using cell-volume changes and patch pipette suction 61 ) and is expressed in the mouse heart. 44,62 Using the TRPV2 agonist probenicid in wild-type and TRPV2− / − constitutive knockout mice, it was shown that this channel appears to contribute to baseline cardiac function, participating in the calcium-handling machinery of heart cells. 62
TRPV4 (http://www.ncbi.nlm.nih.gov/gene/59341) mRNA is weakly expressed in cardiac muscle, 63 and TRPV4 protein was located in cultured neonatal rat ventricular myocytes only in the nucleus. 64 However, caution is warranted here, regarding mRNA or protein expression in tissue. Firstly, mRNA and protein expression levels do not necessarily correlate 65 and secondly, while high expression levels are confirmatory of a significant presence and indicative of functional relevance, low expression in whole tissue homogenates should be interpreted with care. If a protein is present in minority cell populations of the heart (such as Purkinje fibres), it could be of immense functional relevance, even if it was only detected at trace levels. In addition, some SAC, such as TREK, may be expressed at the membrane, but can be strongly inhibited in resting conditions, 66 making the assessment of availability of functional channels even more difficult.
Finally, the melastatin TRP channel 4 (TRPM4) is expressed in cardiomyocytes, 45 and has been implicated in stretch-activated responses of vasculature smooth muscle cells. 67 Overexpression of TRPM4 may be involved in an inheritable form of progressive familial heart block type I, 68 and the identification of a possible stretch-activated component of this disease – mediated by TRPM4 – would be of pronounced clinical relevance. Thus, in addition to TRPC1 and TRPC6, the ion channels TRPC3, TRPV2, TRPV4 and TRPM4 form translationally-relevant targets for further basic and applied research.
Piezo1
The discovery of Piezo1 and Piezo2 (http://www.ncbi.nlm.nih.gov/gene/63895) by Patapoutian's group in 2010 46 represents one of the most important breakthroughs in the field of mechanotransduction in recent years. Piezo1 was initially identified in the neuro-2a neuronal cell line by siRNA knockdown of the expression of membrane proteins with unknown function. Knockdown of the FAM38A (http://www.ncbi.nlm.nih.gov/gene/415849) gene inhibited ISAC,NS and the gene product was named Piezo1. Mechanosensitivity was confirmed by heterologous expression of Piezo1 in HEK cells, which induced a robust ISAC,NS. Piezo1 is huge, containing approximately 2,500 amino acids, arranged in 24–32 transmembrane domains that assemble as a tetramer. 69 Intuitively, this massive structure, and the associated large surface area, could be well-adapted for sensing changes in bilayer curvature and/or stretch. In line with this hypothesis, it has been shown that Piezo1 gating is associated with dimensional changes. 70
Currently, no data has been published directly addressing Piezo1 mechanosensitivity in the heart. However, Piezo1 channel electrophysiological properties are similar to that of endogenous cardiac SACNS, including weak voltage dependency, comparable single channel conductance, inactivation, and sensitivity to GsMTx-4. 71–73 Furthermore, Piezo1 mRNA is expressed in the murine heart 46,74 albeit at low levels (see comment on whole-tissue expression levels, above). Piezo1 is involved in erythrocyte volume homeostasis. Morpholino-mediated knockdown of Piezo1 results in swelling and lysis of red blood cells and consequent anemia. 75 Interestingly, this function is close to that of bacterial mechanosensitive channels of large and small conductance (MscL and MscS). 76
Undoubtedly this is an exciting and dynamic area of development. Basic science questions concerning structure, protein partners, and regulation of Piezo1 need to be addressed, 77 as does the question of whether Piezo1 is present in, and relevant for, the human heart.
SACK
Whole-cell SACK currents (ISAC,K) were first described by Kim et al. 35 in rat atrial myocytes. In contrast to SACNS, SACK, are outwardly rectifying, and as such, allow potassium ions to move more easily out of the cell than into it. Compared to SACNS, SACK tend to have larger single channel conductances. They also inactivate in a time-dependent manner and are generally insensitive to GsMTx-4. 78 Being potassium-selective, their reversal potential lies negative to the resting membrane potential of cardiac cells, so activation of SACK will generally cause membrane repolarisation or hyperpolarisation. 27 To date, single-channel recordings of ISAC,K in adult mammalian cardiac myocytes have been obtained from atrial 35 and ventricular myocytes, 36,79,80 suggesting that their subcellular compartmentalization differs from SACNS. Primary molecular candidates for cardiac SACK are TREK-1, BKCa and KATP.
TREK-1
TREK-1 is a member of the two-pore domain potassium channel family, which is associated with a ‘leak’ potassium ion conductance in cardiomyocytes. 81 TREK-1, however, displays more complex permeation and gating properties than a simple ‘leak’ channel, and is regulated by a number of factors including pH, temperature, second messenger systems, and membrane deformation/stretch. 82 Mechanosensitivity was attributed to TREK-1 by Patel et al. 39 based on single-channel patch clamp recordings from transfected COS cells. Subsequently, Terrenoire et al. 83 demonstrated that ISAC,K (endogenous to rat atrial myocytes) displays a number of properties that bear striking similarities to recombinant TREK-1 channels. This includes their single-channel conductance, lack of voltage dependency, ‘burst mode’ activity, and sensitivity to pharmacological interventions, and, in particular, a unique sensitivity to volatile anaesthetics not shared by other cardiac potassium ion channels. 81
A number of studies have identified TREK-1 mRNA expression in rat atria, as well as in left, right, and septal ventricular myocytes. 83–86 The protein appears to be arranged in longitudinal stripes at the surface of cardiomyocytes: a pattern that might support directional stretch sensing. 80 At the tissue level, TREK-1 expression is distinctly heterogeneous, with a gradient of mRNA expression that increases, transmurally, from epicardial to endocardial cells. 86 This heterogeneity appears to correlate with transmural changes in MEF sensitivity, where stretch causes the most pronounced action potential shortening in the sub-endocardium. 87 To our knowledge, mRNA analysis thus far has failed to identify TREK-1 expression in the human heart. 88,89 It has been suggested that the TWIK-related arachidonic acid-activated potassium channel (TRAAK (http://www.ncbi.nlm.nih.gov/gene/50801)) or TWIK-related acid-sensitive potassium channel (TASK-1 (http://www.ncbi.nlm.nih.gov/gene/3777)) of the K2P family, which appear to be expressed in the human heart, 90 may act as TREK-1 homologues. Indeed, when TRAAK is expressed in heterologous model systems, it forms a channel with very similar properties to TREK-1. 91 Characterisation of both the presence and functional relevance of these ion channels in human requires further elucidation.
BKCa
BKCa channels have large conductances, and they respond to voltage changes and alterations in intracellular calcium ion concentration in a manner that allows them to contribute to repolarisation. 92 Functionally, BKCa channels have been suggested to control heart rate and to offer cardioprotection during ischaemia. 93 Kawakubo et al. 94 identified mechanosensitivity of BKCa channels in membrane patches excised from cultured embryonic chick ventricular myocytes. Attempts to measure single-channel activity in post-hatch chick cardiomyocytes have been unsuccessful, although Iribe et al. 95 characterised a whole-cell ISAC,K which was sensitive to iberiotoxin (a BKCa inhibitor). Interestingly, this ISAC,K was also sensitive to extracellular sodium. The authors suggest that BKCa activation could occur secondary to mechanical modulation of sodium ion influx (e.g. via SACNS), and consequentially shift sodium-calcium exchanger activity towards preservation of intracellular calcium. If this is the case, BKCa might not be directly stretch sensitive.
Whether or not BKCa channels are responsible for ISAC,K in embryonic chick cardiomyocytes, there is little evidence to suggest that BKCa channels form cardiac SACK in other species. In the human heart, BKCa channels are sparsely expressed, 92 and they may be confined to cardiac fibroblasts (where BKCa was detected using Western blot 96 ). However, functional fibroblast-myocyte electrical coupling can occur (at least in some areas of the heart 97,98 ) so it is possible that stretch-activation of BKCa channels in cardiac fibroblasts could still affect myocyte function via a heterocellular coupling pathway.
KATP
Although classically considered to be ligand-gated, Van Wagoner et al. 99 obtained single-channel inside-out patch clamp recordings from neonatal rat atrial myocytes which revealed that patch pipette negative pressure increased KATP channel ATP-sensitivity. 99 Synergistically, ATP-reduction potentiated stretch sensitivity. 99 Ischaemia, simulated in adult guinea pig ventricular myocytes by application of a metabolic uncoupler, also uncovered KATP mechanosensitivity that was absent in control conditions, 27 an observation that was more recently confirmed in rat cardiomyocytes. 100 The synergism for KATP channel activation by metabolic and mechanical stress might explain differences in quantitative aspects of ATP reduction needed to activate KATP in isolated cells, compared to the organ level (where KATP open at less depleted ATP levels). A reason for this difference could be the fact that isolated cells are not normally subjected to mechanical co-activation, while at the organ level ischaemia is usually associated with decreased shortening, or even stretch, of the tissue affected by reduced availability of ATP.
In keeping with this notion, ‘stretch-preconditioning’ has been reported to reduce ischaemia- reperfusion injury, an effect that disappears when KATP channels are blocked. 68 Moreover, cardiac fibroblasts progressively express functional KATP channels in scar and border zone tissue following infarction, suggesting that we must consider the effect of cells other than cardiomyocytes in pre-/post- conditioning of the heart, and the role of SAC in these processes. 101–103
Although there is little evidence to suggest that KATP are responsible for mechanosensitivity of the heart in normal beat-by-beat physiology (in healthy cells and tissue, diastolic mechanical stimulation depolarizes cardiomyocytes), the potential role of these ion channels in ischaemic or other disease conditions warrants further research.
SAC modulators
Several pharmacological compounds have been identified to modulate SAC activity (Figure 3), 104,105 and their potential role as pharmacological tools for heart rhythm management has been previously reviewed by White. 106
Most of the known SAC-modulators are non-specific inhibitors, such as gadolinium ions, amiloride and cationic antibiotics (streptomycin, penicillin, kanamycin). Among the very few specific SAC inhibitors 107 reported so far is the peptide GsMTx-4. It inhibits TRPC5 when activated by hypo-osmotic and receptor stimulation, 108,109 as well as TRPC6, 56 and Piezo1 channels when applied to the external face of the membrane. 72,73 GsMTx-4 is active both in its D and L enantiomers, showing the mechanism of action is not stereospecfic or chiral. 110 Instead, the mode of action of GsMTx-4 is thought to involve insertion into the outer membrane leaflet in the proximity of the channel, relieving lipid stress and favouring the closed state of SAC. 110 Counterintuitively, GsMTx-4 sensitizes the bacterial channels, MscS and MscL, to tension, 111 while it has no effect on TREK-1 channels, 72 so that overall the mode of action of GsMTx-4 still requires further elucidation.
TREK-1 is poorly responsive to classic potassium channel blockers, 112 but its functions are modified by a variety of pharmacologic agents such as volatile anaesthetics, 112 riluzole, 113 fluoxetine 114 and spadin. 115,116 Recently, new modulators of TREK-1 were identified by Bagriantsev et al. 117 They characterized inhibitors and, importantly, activators (which are very rare for SAC channels). Known openers for SAC are amphipathic substances which insert selectively into one membrane leaflet only, locally inducing either concave or convex curvature, which may induce SAC-activating tension. 118
Other useful substances include probenicid, which is a TRP agonist that is fairly specific to TRPV2, 119 and 9-phenanthrol, which blocks TRPM4. 120
It is important to note, though, that not all SAC blockers that work at the level of isolated or cultured cells are equally efficient in native tissue. Streptomycin, for example, may not be able to easily access SAC in whole cardiac tissue, 121 even though it is an efficient SACNS blocker in single cardiomyocytes (an important consideration for cell-culture based work, which often employs media containing streptomycin by default). This will be one of the reasons for which antibiotics, such as streptomycin, can be prescribed to patients without instantaneous side effects on stretch-sensing. Another compound, Gd3+, is used clinically in a chelated formulation, which explains the lack of pronounced immediate SAC-effects in radio-contrast studies. As an aside, Gd3+ precipitates in physiologically buffered solutions. 122 Clearly caution is called for when assessing (potentially false-) negative results on Gd3+ effects in physiological solutions, or on streptomycin effects in vivo.
Discussion
The heart is a superbly mechanosensitive organ. SAC are thought to provide one of the mechanisms underlying cardiac MEF, 20,123,124 the process by which changes in the mechanical environment of the heart lead to altered cardiac electrical activity. Identification of molecular substrates for cardiac SAC will not only provide novel insight into processes that underlie MEF, but also support the long-term aim of developing SAC-specific drugs for the treatment of mechanically induced cardiac pathologies. 106
In terms of physiological beat-by-beat effects, activation of SAC has been shown to underlie the stretch-induced increase in spontaneous sino-atrial node (SAN) cell pacemaking rate. 16 Block of SAC using GsMTx-4 terminates this positive chronotropic response in SAN tissue 121 which, in its guise of a respiratory sinus arrhythmia, persists at whole body level – even in heart transplant recipients after additional pharmacological denervation. 125
At the same time, sustained pressure and/or volume overload favour arrhythmogenesis. 25,124,126,127 Application of SAC-blockers such as GsMTx-4 has been shown to reversibly reduce the preload dependent increase in both incidence and duration of burst-pacing induced atrial fibrillation in isolated heart experiments. 28 In patients, it can be difficult to distinguish stretch-induced changes in electrophysiology from other chronically occurring aspects of structural and functional remodelling. However, an impressive illustration of acute effects of ventricular loading has been provided by Waxman et al., 128 who showed that performing the Valsalva manoeuvre may terminate ventricular tachycardia by temporary reduction of ventricular filling. The Valsalva manoeuvre, an attempt to forcefully exhale against the closed glottis, increases intrathoracic pressure, favouring a net reduction of intravascular volume in the chest (i.e. impeding venous return and favouring arterial drainage to other parts of the body). In this study, the reduction in cardiac dimensions was confirmed radiographically. Cessation of ventricular tachycardia coincided with removal of ventricular strain, while arrhythmia resumption occurred upon refilling after the end of the manoeuvre. Since this type of response can be seen not only in neurologically intact, but also in pharmacologically 128 or surgically 7 denervated patients (transplant recipients), it is not attributable to a nervous reflex. This highlights how removal of strain may unmask the presence of stretch-induced arrhythmias, even in a chronic setting.
Various SAC have been implicated in the heart's (patho-)physiological responses to mechanical stimuli, but in the absence of firm identification of molecular substrates for cardiac SAC, successful mechanistic exploration of cardiac mechanosensitivity is a challenging task.
Conceptually, it is pragmatic to subdivide SAC into two categories, SACNS and SACK. For both, there are several candidate proteins. SACNS were initially thought to be formed by TRP proteins and, most convincingly, TRPC6 antibodies inhibit whole-cell ISAC,NS in mouse ventricular myocytes. 58 However, subsequent heterologous expression studies yielded conflicting results. 50,56 More recently, attention has turned towards the newly discovered Piezo1 channels. 46 Although there is no published data yet on specific electrophysiological effects of Piezo1 in cardiomyocytes, comparative kinetic analysis suggests that these proteins may function as cardiac SACNS. In as far as cardiac SACK are concerned, recombinant TREK-1 has remarkably similar properties to endogenous SACK, 78 but the protein has yet to be identified in human heart. 88 BKCa channels have also received considerable interest, but their stretch-sensitivity, if present, 95 may be limited to immature 129 and/or non-mammalian cardiomyocytes, 94 or to cardiac connective tissue. 96 Finally, KATP channels display metabolic and mechanical co-activation, 99 which may help explain some of the differences between experimental and clinical findings on the extent of ATP-reduction needed to activate them. In addition, this insight could shed light on hitherto ill-explored links between ischaemic and mechanical preconditioning.
As will be apparent from the above, the currently available information on the molecular substrates of cardiac SAC poses more questions than it answers. A number of reasons contribute to this. It is notoriously difficult to control and/or quantify the extent and quality of local mechanical stimuli that an individual ion channel is exposed to. 130 Tools to apply strain at whole-cell, tissue, and organ levels exist (including the application of shear stress, axial stretch, or cell volume changes), but there is no commonly implemented ‘gold standard’ for the stimulation of SAC. 27 Furthermore, these techniques have been used with a wide variety of cellular models from different species and developmental stages, making cross comparison of results challenging. In addition, it is difficult to interrelate macroscopic interventions and observations at cell and tissue levels with molecular substrates: in part because there is no ‘zero-strain’ reference even in patch clamp studies. Attempts to explore causal links from low-level mechanism to integrated response, and back, include changes in gene expression, 87 pharmacological interventions, 131 and computational modelling. 132–135
Further challenges arise from the possibility that ventricular SAC may be localised in T-tubules, caveolae, or intercalated discs. This is thought to explain why patch clamping of single SAC is so rare in freshly isolated ventricular cardiomyocytes from adult mammals. 130 One possible way around this problem may be to use pre-exposure to α1A receptor stimulation, to aid SAC translocation from T-tubules to the sarcolemma. 59 Another would be pre-stretching of the cardiac tissue prior to cell isolation, as this can cause surface membrane incorporation of caveolae. 43 Thirdly, one could isolate the T-tubules using sequential centrifugation of homogenised cardiomyocytes followed by purification of T-tubule membranes by vesicle immuno-isolation and reconstitution into a continuous membrane. 136 It might then be possible to directly patch clamp SAC on the isolated T-tubule membrane. Less invasively, scanning ion conductance microscopy, which generates a three-dimensional topographical map of the cell surface prior to patch clamping, has been suggested as a means to directly target the T-tubule ostium where SAC are more likely to be present. 137 On the other hand, there is evidence to suggest that SAC may activate indirectly via second messenger signalling cascades. 138 Therefore patch clamping single ion channels (where SAC activation probably occurs as a direct result of bilayer deformation 130 ) may provide only a partial picture of patho-physiological function.
In addition to SAC in the outer cell membrane, there may be non-sarcolemmal SAC in the sarcoplasmic reticulum 12 or mitochondria. 139,140 Cardiac non-myocytes are also mechanosensitive and exhibit electrophysiological properties modulated by mechanical stimuli. 18,141–143 Channels, such as Nav1.5 and TRPM7, that were initially identified as stretch-modulated in non-cardiac cell types, 144,145 have now been found in cardiac fibroblasts. 146,147
Finally, there is a growing body of evidence to suggest that many cardiac ion channels, even those that are not classically considered as SAC (e.g. voltage- or ligand-gated channels), are sensitive to mechanical modulation of their gating behaviour. 148 Future research should therefore focus on characterising the mechanical stimuli experienced by cardiomyocytes in vivo, so that they can be more closely replicated in vitro. This can be aided greatly by high-resolution imaging of the beating heart, 149 followed by whole heart histological reconstruction 150,151 and subsequent computational re-integration of tissue deformation 152,153 with a granularity that allows identification of local stress-strain dynamics 154 and prediction of microstructural effects on electrophysiology. 155 Direct measurement, and validation of modelling predictions, currently suffers from a number of technical limitations, in particular the inability to measure locally acting forces in situ. The recent development of Förster Resonance Energy Transfer (FRET)-based force sensors that can be genetically inserted into intracellular proteins, 156 may open up a treasure chest of novel insight if they can be applied to heart research. These force sensors are based on the energy transfer between two compatible fluorophores. The efficacy of the energy transfer is inversely proportional to the distance between the donor and the acceptor, multiplied by 106, making the FRET signal very sensitive to small distance changes. Meng and Sachs 157 have calibrated their probe using DNA to be able to quantify forces from fluorescent signal changes. These sensors constitute a very powerful tool for the assessment of the mechanical state in components of single cells or tissues. Until now little is known about forces within the cell/cytoskeleton, both when cells are at rest, or while mechanically stimulated. In addition, intracellular force reporters would be very useful to improve our understanding of the interplay of SAC with other mechanosensors, like integrins and the cytoskeleton.
Armed with a more thorough understanding of physiological mechanical stimuli, and novel techniques, we expect to improve our understanding of the molecular substrate of cardiac SAC, and to better predict their pathophysiological roles for the regulation of heart rate and rhythm in the mechanosensitive heart (Figure 4).
Figure 4.
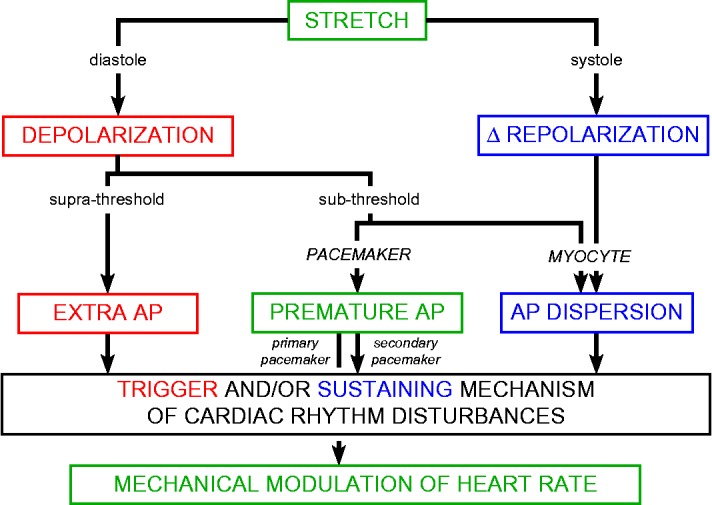
Timing-, amplitude-, and target-dependent stretch effects on heart rhythm. AP: action potential, Δ: change in. Adapted from Kohl et al., 1998, with permission.
Conclusion
The heart is an integrated electro-mechanical system (Figure 1). SAC (Figure 2) are key contributors to cardiac mechanosensitivity. SACK generally cause re- (or even hyper-) polarisation, while SACNS depolarise resting tissue and have differential effects on activated cells (speeding up early, delaying late, repolarisation; Figure 3). Systemic effects of SAC activation therefore depend on timing (relative to the cardiac cycle), intensity (e.g. relative to excitation threshold), and location (relative to the different components of cardiac tissue; Figure 4). Thus, diastolic stretch may accelerate heart rate, or even trigger extra beats, depending on whether pacemaker cells or working myocardium drive the response. This is believed to underlie mechanical pacing by pre-cordial percussion, and the occasionally observed termination of re-entrant arrhythmias upon application of pre-cordial thump. 160–162 Systolic stretch will alter repolarisation dynamics which, in particular in the context of spatially heterogeneous mechanical stimuli and/or regionally varying expression of SAC, may act as an arrhythmia-sustaining mechanism for mechanically-induced rhythm disturbances.
We are on the brink of obtaining an improved understanding of the molecular substrates of cardiac SAC, and of the way in which macroscopic mechanical interventions translate into stimuli at subcellular levels. This is hoped to lead to improved insight into the physiological relevance of SAC, their involvement in acute and chronic diseases, and to guide the development of novel means to targeting cardiac SAC for therapeutic benefit.
Funding
This work has been supported by the UK Biotechnology and Biological Sciences Research Council, the European Research Council, and the Magdi Yacoub Institute at Harefield. RP is holder of an Imperial College Junior Research Fellowship; PK is a Senior Fellow of the British Heart Foundation.
Competing interests
None.
List of Abbreviations
BKCa: Large-conductance calcium-activated potassium channel
CHO: Chinese hamster ovary cell line
GsMTx-4: Grammostola spatulata Mechano-Toxin 4
HEK: Human embryonic kidney cell line
ISAC,K: Potassium-selective stretch-activated current
ISAC,NS: Cation non-selective stretch-activated current
KATP: ATP-sensitive potassium channel
MEF: Mechano-electric feedback
MSC: Mechanosensitive ion channels
OAG: 1-oleoyl-2-acetyl-sn-glycerol
SAC: Stretch-activated channels
SACK: Potassium-selective SAC
SACNS: Cation non-selective SAC
SFR: Slow force response
TASK: TWIK-related acid-sensitive potassium channel
TRAAK: TWIK-related arachidonic acid-activated potassium channel
TREK: TWIK-related potassium channel
TRP: Transient receptor potential
TRPC: Canonical TRP channel
TRPM: Melastatin TRP channel
TRPV: Vanilloid TRP channel
TWIK: Tandem of two-pore K+ domains in a weak inwardly rectifying K+ channel
VAC: Volume-activated ion channels
References
- 1.Frank O. Die Grundform des arteriellen Pulses. Zeitschrift für Biologie. 1899;37:483–526. doi: 10.1016/0022-2828(90)91459-k. [DOI] [PubMed] [Google Scholar]
- 2.Starling E. The law of the heart (Linacre Lecture, given at Cambridge, 1915) London: Longmans, Green and Co.; 1918. [Google Scholar]
- 3.Bainbridge FA. The influence of venous filling upon the rate of the heart. The Journal of Physiology. 1915;50:65–84. doi: 10.1113/jphysiol.1915.sp001736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franz MR, Cima R, Wang D, Profitt D, Kurz R. Electrophysiological effects of myocardial stretch and mechanical determinants of stretch-activated arrhythmias. Circulation. 1992;86:968–978. doi: 10.1161/01.cir.86.3.968. [DOI] [PubMed] [Google Scholar]
- 5.Blinks JR. Positive chronotropic effect of increasing right atrial pressure in the isolated mammalian heart. American Journal of Physiology. 1956;186:299–303. doi: 10.1152/ajplegacy.1956.186.2.299. [DOI] [PubMed] [Google Scholar]
- 6.Keatinge WR. The effect of increased filling pressure on rhythmicity and atrioventricular conduction in isolated hearts. The Journal of Physiology. 1959;149:193–208. doi: 10.1113/jphysiol.1959.sp006334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambrosi P, Habib G, Kreitmann B, Faugere G, Metras D. Valsalva manoeuvre for supraventricular tachycardia in transplanted heart recipient. Lancet. 1995;346:713. doi: 10.1016/s0140-6736(95)92331-4. [DOI] [PubMed] [Google Scholar]
- 8.Bernardi L, Salvucci F, Suardi R, Solda PL, Calciati A, Perlini S, Falcone C, Ricciardi L. Evidence for an intrinsic mechanism regulating heart rate variability in the transplanted and the intact heart during submaximal dynamic exercise? Cardiovascular Research. 1990;24:969–981. doi: 10.1093/cvr/24.12.969. [DOI] [PubMed] [Google Scholar]
- 9.Bernardi L, Keller F, Sanders M, Reddy PS, Griffith B, Meno F, Pinsky MR. Respiratory sinus arrhythmia in the denervated human heart. Journal of Applied Physiology. 1989;67:1447–1455. doi: 10.1152/jappl.1989.67.4.1447. [DOI] [PubMed] [Google Scholar]
- 10.Markhasin VS, Solovyova O, Katsnelson LB, Protsenko Y, Kohl P, Noble D. Mechano-electric interactions in heterogeneous myocardium: development of fundamental experimental and theoretical models. Progress in Biophysics and Molecular Biology. 2003;82:207–220. doi: 10.1016/s0079-6107(03)00017-8. [DOI] [PubMed] [Google Scholar]
- 11.ter Keurs HE, Shinozaki T, Zhang YM, Zhang ML, Wakayama Y, Sugai Y, Kagaya Y, Miura M, Boyden PA, Stuyvers BD, Landesberg A. Sarcomere mechanics in uniform and non-uniform cardiac muscle: a link between pump function and arrhythmias. Progress in Biophysics and Molecular Biology. 2008;97:312–331. doi: 10.1016/j.pbiomolbio.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Iribe G, Ward CW, Camelliti P, Bollensdorff C, Mason F, Burton RA, Garny A, Morphew MK, Hoenger A, Lederer WJ, Kohl P. Axial stretch of rat single ventricular cardiomyocytes causes an acute and transient increase in Ca2+ spark rate. Circulation Research. 2009;104:787–795. doi: 10.1161/CIRCRESAHA.108.193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gannier F, White E, Lacampagne A, Garnier D, Le Guennec JY. Streptomycin reverses a large stretch induced increases in [Ca2+]i in isolated guinea pig ventricular myocytes. Cardiovascular Research. 1994;28:1193–1198. doi: 10.1093/cvr/28.8.1193. [DOI] [PubMed] [Google Scholar]
- 14.Craelius W. Stretch-activation of rat cardiac myocytes. Experimental Physiology. 1993;78:411–423. doi: 10.1113/expphysiol.1993.sp003695. [DOI] [PubMed] [Google Scholar]
- 15.Iribe G, Helmes M, Kohl P. Force-length relations in isolated intact cardiomyocytes subjected to dynamic changes in mechanical load. American Journal of Physiology Heart and Circulatory Physiology. 2007;292:H1487–H1497. doi: 10.1152/ajpheart.00909.2006. [DOI] [PubMed] [Google Scholar]
- 16.Cooper PJ, Lei M, Cheng LX, Kohl P. Selected contribution: axial stretch increases spontaneous pacemaker activity in rabbit isolated sinoatrial node cells. Journal of Applied Physiology. 2000;89:2099–2104. doi: 10.1152/jappl.2000.89.5.2099. [DOI] [PubMed] [Google Scholar]
- 17.Kohl P, Kamkin AG, Kiseleva IS, Streubel T. Mechanosensitive cells in the atrium of frog heart. Experimental Physiology. 1992;77:213–216. doi: 10.1113/expphysiol.1992.sp003576. [DOI] [PubMed] [Google Scholar]
- 18.Kiseleva I, Kamkin A, Kohl P, Lab MJ. Calcium and Mechanically induced potentials in fibroblasts of rat atrium. Cardiovascular Research. 1996;32:98–111. [PubMed] [Google Scholar]
- 19.Kamkin A, Kirischuk S, Kiseleva I. Single mechano-gated channels activated by mechanical deformation of acutely isolated cardiac fibroblasts from rats. Acta Physiologica. 2010;199:277–292. doi: 10.1111/j.1748-1716.2010.02086.x. [DOI] [PubMed] [Google Scholar]
- 20.Kohl P, Ravens U. Cardiac mechano-electric feedback: past, present, and prospect. Progress in Biophysics and Molecular Biology. 2003;82:3–9. doi: 10.1016/s0079-6107(03)00022-1. [DOI] [PubMed] [Google Scholar]
- 21.Kohl P, Hunter P, Noble D. Stretch-induced changes in heart rate and rhythm: clinical observations, experiments and mathematical models. Progress in Biophysics and Molecular Biology. 1999;71:91–138. doi: 10.1016/s0079-6107(98)00038-8. [DOI] [PubMed] [Google Scholar]
- 22.Link MS, Wang PJ, Pandian NG, Bharati S, Udelson JE, Lee MY, Vecchiotti MA, VanderBrink BA, Mirra G, Maron BJ, Estes NA. An experimental model of sudden death due to low-energy chest-wall impact (commotio cordis) The New England Journal of Medicine. 1998;338:1805–1811. doi: 10.1056/NEJM199806183382504. [DOI] [PubMed] [Google Scholar]
- 23.Nesbitt AD, Cooper PJ, Kohl P. Rediscovering commotio cordis. Lancet. 2001;357:1195–1197. doi: 10.1016/S0140-6736(00)04338-5. [DOI] [PubMed] [Google Scholar]
- 24.Kohl P, Nesbitt AD, Cooper PJ, Lei M. Sudden cardiac death by Commotio cordis: role of mechano-electric feedback. Cardiovascular Research. 2001;50:280–289. doi: 10.1016/s0008-6363(01)00194-8. [DOI] [PubMed] [Google Scholar]
- 25.Taggart P, Lab M. Cardiac mechano-electric feedback and electrical restitution in humans. Progress in Biophysics and Molecular Biology. 2008;97:452–460. doi: 10.1016/j.pbiomolbio.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 26.Kohl P, Sachs F, Franz MR. Cardiac Mechano-Electric Coupling and Arrhythmias. 2 ed. Oxford: Oxford University Press; 2011. [Google Scholar]
- 27.Kohl P, Bollensdorff C, Garny A. Effects of mechanosensitive ion channels on ventricular electrophysiology: experimental and theoretical models. Experimental Physiology. 2006;91:307–321. doi: 10.1113/expphysiol.2005.031062. [DOI] [PubMed] [Google Scholar]
- 28.Bode F, Sachs F, Franz MR. Tarantula peptide inhibits atrial fibrillation. Nature. 2001;409:35–36. doi: 10.1038/35051165. [DOI] [PubMed] [Google Scholar]
- 29.Hansen DE, Borganelli M, Stacy GP, Jr, Taylor LK. Dose-dependent inhibition of stretch- induced arrhythmias by gadolinium in isolated canine ventricles. Evidence for a unique mode of antiarrhythmic action. Circulation Research. 1991;69:820–831. doi: 10.1161/01.res.69.3.820. [DOI] [PubMed] [Google Scholar]
- 30.Baumgarten CM, Clemo HF. Swelling-activated chloride channels in cardiac physiology and pathophysiology. Progress in Biophysics and Molecular Biology. 2003;82:25–42. doi: 10.1016/s0079-6107(03)00003-8. [DOI] [PubMed] [Google Scholar]
- 31.Guharay F, Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. The Journal of Physiology. 1984;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnadottir J, Chalfie M. Eukaryotic mechanosensitive channels. Annual Review of Biophysics. 2010;39:111–137. doi: 10.1146/annurev.biophys.37.032807.125836. [DOI] [PubMed] [Google Scholar]
- 33.Martinac B. Bacterial mechanosensitive channels as a paradigm for mechanosensory transduction. Cellular Physiology and Biochemistry. 2011;28:1051–1060. doi: 10.1159/000335842. [DOI] [PubMed] [Google Scholar]
- 34.Craelius W, Chen V, el-Sherif N. Stretch activated ion channels in ventricular myocytes. Bioscience Reports. 1988;8:407–414. doi: 10.1007/BF01121637. [DOI] [PubMed] [Google Scholar]
- 35.Kim D. A mechanosensitive K+ channel in heart cells. Activation by arachidonic acid. Journal of General Physiology. 1992;100:1021–1040. doi: 10.1085/jgp.100.6.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W, Zhang M, Li P, Yuan H, Feng N, Peng Y, Wang L, Wang X. An increased TREK-1-like potassium current in ventricular myocytes during rat cardiac hypertrophy. Journal of Cardiovascular Pharmacology. 2013;61:302–310. doi: 10.1097/FJC.0b013e318280c5a9. [DOI] [PubMed] [Google Scholar]
- 37.Levina N, Totemeyer S, Stokes NR, Louis P, Jones MA, Booth IR. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 1999;18:1730–1737. doi: 10.1093/emboj/18.7.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sukharev SI, Martinac B, Blount P, Kung C. Functional reconstitution as an assay for biochemical isolation of channel proteins: application to the molecular identification of a bacterial mechanosensitive channel. Methods. 1994;6:51–59. [Google Scholar]
- 39.Patel AJ, Honore E, Maingret F, Lesage F, Fink M, Duprat F, Lazdunski M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 1998;17:4283–4290. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng T, Bett GC, Sachs F. Stretch-activated whole cell currents in adult rat cardiac myocytes. American Journal of Physiology Heart and Circulatory Physiology. 2000;278:H548–H557. doi: 10.1152/ajpheart.2000.278.2.H548. [DOI] [PubMed] [Google Scholar]
- 41.Bowman CL, Gottlieb PA, Suchyna TM, Murphy YK, Sachs F. Mechanosensitive ion channels and the peptide inhibitor GsMTx-4: history, properties, mechanisms and pharmacology. Toxicon: Official Journal of the International Society on Toxinology. 2007;49:249–270. doi: 10.1016/j.toxicon.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang H, Wang W, Liu P, Jiang Y, Zhao Y, Wei H, Niu W. TRPC1 expression and distribution in rat hearts. European Journal of Histochemistry: EJH. 2009;53:e26. doi: 10.4081/ejh.2009.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kohl P, Cooper PJ, Holloway H. Effects of acute ventricular volume manipulation on in situ cardiomyocyte cell membrane configuration. Progress in Biophysics and Molecular Biology. 2003;82:221–227. doi: 10.1016/s0079-6107(03)00024-5. [DOI] [PubMed] [Google Scholar]
- 44.Iwata Y, Katanosaka Y, Arai Y, Komamura K, Miyatake K, Shigekawa M. A novel mechanism of myocyte degeneration involving the Ca2+-permeable growth factor-regulated channel. The Journal of Cell Biology. 2003;161:957–967. doi: 10.1083/jcb.200301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vennekens R. Emerging concepts for the role of TRP channels in the cardiovascular system. The Journal of Physiology. 2011;589:1527–1534. doi: 10.1113/jphysiol.2010.202077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inoue R, Jian Z, Kawarabayashi Y, Mechanosensitive TRP. channels in cardiovascular pathophysiology. Pharmacology & Therapeutics. 2009;123:371–385. doi: 10.1016/j.pharmthera.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 48.Riccio A, Medhurst AD, Mattei C, Kelsell RE, Calver AR, Randall AD, Benham CD, Pangalos MN. mRNA distribution analysis of human TRPC family in CNS and peripheral tissues. Brain Research Molecular Brain Research. 2002;109:95–104. doi: 10.1016/s0169-328x(02)00527-2. [DOI] [PubMed] [Google Scholar]
- 49.Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nature Cell Biology. 2005;7:179–185. doi: 10.1038/ncb1218. [DOI] [PubMed] [Google Scholar]
- 50.Gottlieb P, Folgering J, Maroto R, Raso A, Wood TG, Kurosky A, Bowman C, Bichet D, Patel A, Sachs F, Martinac B, Hamill OP, Honoré E. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflugers Archiv: European Journal of Physiology. 2008;455:1097–1103. doi: 10.1007/s00424-007-0359-3. [DOI] [PubMed] [Google Scholar]
- 51.Allen DG, Ward ML. Roles of cardiac SAC beyond mechano-electric coupling: stretch-enhanced force generation and muscular dystrophy. In: Kohl P, Sachs F, Franz MR, editors. Cardiac Mechano-Electric Coupling and Arrhythmias. 2 ed. Oxford: Oxford University Press; 2011. pp. 435–441. [Google Scholar]
- 52.Wu X, Eder P, Chang BJ, Molkentin JD. TRPC channels are necessary mediators of pathologic cardiac hypertrophy. Proc Natl Acad Sci USA. 2010;107:7000–7005. doi: 10.1073/pnas.1001825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parmley WW, Chuck L. Length-dependent changes in myocardial contractile state. American Journal of Physiology. 1973;224:1195–1199. doi: 10.1152/ajplegacy.1973.224.5.1195. [DOI] [PubMed] [Google Scholar]
- 54.Ward ML, Williams IA, Chu Y, Cooper PJ, Ju YK, Allen DG. Stretch-activated channels in the heart: Contributions to length-dependence and to cardiomyopathy. Prog Biophys Mol Bio. 2008;97:232–249. doi: 10.1016/j.pbiomolbio.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 55.Seo K, Rainer PP, Lee DI, Hao S, Bedja D, Birnbaumer L, Cingolani OH, Kass DA. Hyperactive adverse mechanical stress responses in dystrophic heart are coupled to transient receptor potential canonical 6 and blocked by cGMP-protein kinase G modulation. Circulation Research. 2014;114:823–832. doi: 10.1161/CIRCRESAHA.114.302614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci USA. 2006;103:16586–16891. doi: 10.1073/pnas.0606894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schnitzler MMY, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M, Gudermann T. G(q)-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J. 2008;27:3092–3103. doi: 10.1038/emboj.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dyachenko V, Husse B, Rueckschloss U, Isenberg G. Mechanical deformation of ventricular myocytes modulates both TRPC6 and Kir2.3 channels. Cell Calcium. 2009;45:38–54. doi: 10.1016/j.ceca.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 59.Mohl MC, Iismaa SE, Xiao XH, Friedrich O, Wagner S, Nikolova-Krstevski V, Wu J, Yu ZY, Feneley M, Fatkin D, Allen DG, Graham RM. Regulation of murine cardiac contractility by activation of alpha(1A)-adrenergic receptor-operated Ca2+ entry. Cardiovascular Research. 2011;91:310–319. doi: 10.1093/cvr/cvr081. [DOI] [PubMed] [Google Scholar]
- 60.Friedrich O, Wagner S, Battle AR, Schurmann S, Martinac B. Mechano-regulation of the beating heart at the cellular level - Mechanosensitive channels in normal and diseased heart. Prog Biophys Mol Bio. 2012;110:226–238. doi: 10.1016/j.pbiomolbio.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 61.Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circulation Research. 2003;93:829–838. doi: 10.1161/01.RES.0000097263.10220.0C. [DOI] [PubMed] [Google Scholar]
- 62.Rubinstein J, Lasko VM, Koch SE, Singh VP, Carreira V, Robbins N, Patel AR, Jiang M, Bidwell P, Kranias EG, Jones WK, Lorenz JN. Novel Role of Transient Receptor Potential Vanilloid 2 in the Regulation of Cardiac Performance. American Journal of Physiology Heart and Circulatory Physiology. 2014;306:H574–H584. doi: 10.1152/ajpheart.00854.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kunert-Keil C, Bisping F, Kruger J, Brinkmeier H. Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genomics. 2006;7:159. doi: 10.1186/1471-2164-7-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao Y, Huang H, Jiang Y, Wei H, Liu P, Wang W, Niu W. Unusual localization and translocation of TRPV4 protein in cultured ventricular myocytes of the neonatal rat. European Journal of Histochemistry: EJH. 2012;56:e32. doi: 10.4081/ejh.2012.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2012;13:227–232. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dedman A, Sharif-Naeini R, Folgering JH, Duprat F, Patel A, Honore E. The mechano-gated K2P channel TREK-1. Eur Biophys J. 2009;38:293–303. doi: 10.1007/s00249-008-0318-8. [DOI] [PubMed] [Google Scholar]
- 67.Morita H, Honda A, Inoue R, Ito Y, Abe K, Nelson MT, Brayden JE. Membrane stretch-induced activation of a TRPM4-like nonselective cation channel in cerebral artery myocytes. J Pharmacol Sci. 2007;103:417–426. doi: 10.1254/jphs.fp0061332. [DOI] [PubMed] [Google Scholar]
- 68.Takahashi K, Kakimoto Y, Toda K, Naruse K. Mechanobiology in cardiac physiology and diseases. J Cell Mol Med. 2013;17:225–232. doi: 10.1111/jcmm.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coste B, Xiao BL, Santos JS, Syeda R, Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE, Montal M, Patapoutian A. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483:176–U72. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bae C, Gottlieb PA, Sachs F. Human PIEZO1: removing inactivation. Biophys J. 2013;105:880–886. doi: 10.1016/j.bpj.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gottlieb PA, Bae C, Sachs F. Gating the mechanical channel Piezo1. A comparison between whole-cell and patch recording. Channels. 2012;6:282–289. doi: 10.4161/chan.21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bae C, Sachs F, Gottlieb PA. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry. 2011;50:6295–6300. doi: 10.1021/bi200770q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peyronnet R, Martins JR, Duprat F, Demolombe S, Arhatte M, Jodar M, Tauc M, Duranton C, Paulais M, Teulon J, Honoré E, Patel A. Piezo1-dependent stretch-activated channels are inhibited by Polycystin-2 in renal tubular epithelial cells. EMBO Reports. 2013;14:1143–1148. doi: 10.1038/embor.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Satoh K, Hata M, Takahara S, Tsuzaki H, Yokota H, Akatsu H, Yamamoto T, Kosaka K, Yamada T. A novel membrane protein, encoded by the gene covering KIAA0233, is transcriptionally induced in senile plaque-associated astrocytes. Brain Res. 2006;1108:19–27. doi: 10.1016/j.brainres.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 75.Faucherre A, Kissa K, Nargeot J, Mangoni ME, Jopling C. Piezo1 plays a role in erythrocyte volume homeostasis. Haematologica. 2014;99:70–75. doi: 10.3324/haematol.2013.086090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levina N, Totemeyer S, Stokes NR, Louis P, Jones MA, Booth IR. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 1999;18:1730–1737. doi: 10.1093/emboj/18.7.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nilius B, Honore E. Sensing pressure with ion channels. Trends Neurosci. 2012;35:477–486. doi: 10.1016/j.tins.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 78.Honoré E, Patel A. The mechano–gated K2p channel TREK-1 in the cardiovascular system. In: Kohl P, Sachs F, Franz MR, editors. Cardiac Mechano-Electric Coupling and Arrhythmias. 2 ed. Oxford: Oxford University Press; 2011. pp. 19–26. [Google Scholar]
- 79.Tan JH, Liu W, Saint DA. TREK-like potassium channels in rat cardiac ventricular myocytes are activated by intracellular ATP. Journal of Membrane Biology. 2002;185:201–207. doi: 10.1007/s00232-001-0123-0. [DOI] [PubMed] [Google Scholar]
- 80.Xian Tao L, Dyachenko V, Zuzarte M, Putzke C, Preisig-Muller R, Isenberg G, Daut J. The stretch-activated potassium channel TREK-1 in rat cardiac ventricular muscle. Cardiovascular Research. 2006;69:86–97. doi: 10.1016/j.cardiores.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 81.Goonetilleke L, Quayle J. TREK-1 K+ channels in the cardiovascular system: their significance and potential as a therapeutic target. Cardiovasc Ther. 2012;30:e23–e9. doi: 10.1111/j.1755-5922.2010.00227.x. [DOI] [PubMed] [Google Scholar]
- 82.Patel AJ, Honore E. Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci. 2001;24:339–346. doi: 10.1016/s0166-2236(00)01810-5. [DOI] [PubMed] [Google Scholar]
- 83.Terrenoire C, Lauritzen I, Lesage F, Romey G, Lazdunski M. A TREK-1-like potassium channel in atrial cells inhibited by beta-adrenergic stimulation and activated by volatile anesthetics. Circulation Research. 2001;89:336–342. doi: 10.1161/hh1601.094979. [DOI] [PubMed] [Google Scholar]
- 84.Liu W, Saint DA. Heterogeneous expression of tandem-pore K+ channel genes in adult and embryonic rat heart quantified by real-time polymerase chain reaction. Clin Exp Pharmacol P. 2004;31:174–178. doi: 10.1111/j.1440-1681.2004.03964.x. [DOI] [PubMed] [Google Scholar]
- 85.Aimond F, Rauzier JM, Bony C, Vassort G. Simultaneous activation of p38 MAPK and p42/44 MAPK by ATP stimulates the K+ current ITREK in cardiomyocytes. Journal of Biological Chemistry. 2000;275:39110–39116. doi: 10.1074/jbc.M008192200. [DOI] [PubMed] [Google Scholar]
- 86.Tan JH, Liu W, Saint DA. Differential expression of the mechanosensitive potassium channel TREK-1 in epicardial and endocardial myocytes in rat ventricle. Experimental Physiology. 2004;89:237–242. doi: 10.1113/expphysiol.2003.027052. [DOI] [PubMed] [Google Scholar]
- 87.Kelly D, Mackenzie L, Hunter P, Smaill B, Saint DA. Gene expression of stretch-activated channels and mechanoelectric feedback in the heart. Clin Exp Pharmacol P. 2006;33:642–648. doi: 10.1111/j.1440-1681.2006.04392.x. [DOI] [PubMed] [Google Scholar]
- 88.Medhurst AD, Rennie G, Chapman CG, Meadows H, Duckworth MD, Kelsell RE, Gloger II, Pangalos MN. Distribution analysis of human two pore domain potassium channels in tissues of the central nervous system and periphery. Mol Brain Res. 2001;86:101–114. doi: 10.1016/s0169-328x(00)00263-1. [DOI] [PubMed] [Google Scholar]
- 89.Gurney A, Manoury B. Two-pore potassium channels in the cardiovascular system. Eur Biophys J. 2009;38:305–318. doi: 10.1007/s00249-008-0326-8. [DOI] [PubMed] [Google Scholar]
- 90.Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO Journal. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ozaita A, Vega-Saenz de Miera E. Cloning of two transcripts, HKT4.1a and HKT4.1b, from the human two-pore K+ channel gene KCNK4 - Chromosomal localization, tissue distribution and functional expression. Mol Brain Res. 2002;102:18–27. doi: 10.1016/s0169-328x(02)00157-2. [DOI] [PubMed] [Google Scholar]
- 92.Takahashi K, Naruse K. Stretch-activated BK channel and heart function. Prog Biophys Mol Bio. 2012;110:239–244. doi: 10.1016/j.pbiomolbio.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 93.Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, O'Rourke B. Cytoprotective role of Ca2+- activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298:1029–1033. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- 94.Kawakubo T, Naruse K, Matsubara T, Hotta N, Sokabe M. Characterization of a newly found stretch-activated K-Ca,K-ATP channel in cultured chick ventricular myocytes. Am J Physiol-Heart C. 1999;276:H1827–H38. doi: 10.1152/ajpheart.1999.276.6.H1827. [DOI] [PubMed] [Google Scholar]
- 95.Iribe G, Jin H, Kaihara K, Naruse K. Effects of axial stretch on sarcolemmal BKCa channels in post-hatch chick ventricular myocytes. Experimental Physiology. 2010;95:699–711. doi: 10.1113/expphysiol.2009.051896. [DOI] [PubMed] [Google Scholar]
- 96.Wang YJ, Sung RJ, Lin MW, Wu SN. Contribution of BKCa-channel activity in human cardiac fibroblasts to electrical coupling of cardiomyocytes-fibroblasts. J Membrane Biol. 2006;213:175–185. doi: 10.1007/s00232-007-0027-8. [DOI] [PubMed] [Google Scholar]
- 97.Camelliti P, Green CR, LeGrice I, Kohl P. Fibroblast network in rabbit sinoatrial node - Structural and functional identification of homogeneous and heterogeneous cell coupling. Circulation Research. 2004;94:828–835. doi: 10.1161/01.RES.0000122382.19400.14. [DOI] [PubMed] [Google Scholar]
- 98.Kohl P, Gourdie RG. Fibroblast-myocyte electrotonic coupling: does it occur in native cardiac tissue? J Mol Cell Cardiol. 2014;70:37–46. doi: 10.1016/j.yjmcc.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Van Wagoner DR, Lamorgese M. Ischemia potentiates the mechanosensitive modulation of atrial ATP-sensitive potassium channels. Annals of the New York Academy of Sciences. 1994;723:392–395. [PubMed] [Google Scholar]
- 100.Huang H, Liang L, Liu P, Wei H, Sachs F, Niu W, Wang W. Mechanical Effects on KATP Channel Gating in Rat Ventricular Myocytes. PloS One. 2013;8:e63337. doi: 10.1371/journal.pone.0063337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Benamer N, Vasquez C, Mahoney VM, Steinhardt MJ, Coetzee WA, Morley GE. Fibroblast KATP currents modulate myocyte electrophysiology in infarcted hearts. American Journal of Physiology Heart and Circulatory Physiology. 2013;304:H1231–H1239. doi: 10.1152/ajpheart.00878.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bell RM, Yellon DM. Conditioning the whole heart–not just the cardiomyocyte. Journal of Molecular and Cellular Cardiology. 2012;53:24–32. doi: 10.1016/j.yjmcc.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 103.Abrial M, Da Silva CC, Pillot B, Augeul L, Ivanes F, Teixeira G, Cartier R, Angoulvant D, Ovize M, Ferrera R. Cardiac fibroblasts protect cardiomyocytes against lethal ischemia-reperfusion injury. J Mol Cell Cardiol. 2014;68:56–65. doi: 10.1016/j.yjmcc.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 104.Sachs F, Morris CE. Mechanosensitive ion channels in nonspecialized cells. Reviews of Physiology, Biochemistry and Pharmacology. 1998;132:1–77. doi: 10.1007/BFb0004985. [DOI] [PubMed] [Google Scholar]
- 105.Hamill OP, McBride DW., Jr The pharmacology of mechanogated membrane ion channels. Pharmacological Reviews. 1996;48:231–252. [PubMed] [Google Scholar]
- 106.White E. Mechanosensitive channels: therapeutic targets in the myocardium? Current Pharmaceutical Design. 2006;12:3645–3663. doi: 10.2174/138161206778522083. [DOI] [PubMed] [Google Scholar]
- 107.Suchyna TM, Johnson JH, Hamer K, Leykam JF, Gage DA, Clemo HF, Baumgarten CM, Sachs F. Identification of a peptide toxin from Grammostola spatulata spider venom that blocks cation-selective stretch-activated channels. The Journal of General Physiology. 2000;115:583–598. doi: 10.1085/jgp.115.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gomis A, Soriano S, Belmonte C, Viana F. Hypoosmotic- and pressure-induced membrane stretch activate TRPC5 channels. The Journal of Physiology. 2008;586:5633–5649. doi: 10.1113/jphysiol.2008.161257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lembrechts R, Brouns I, Schnorbusch K, Pintelon I, Timmermans JP, Adriaensen D. Neuroepithelial bodies as mechanotransducers in the intrapulmonary airway epithelium: involvement of TRPC5. American Journal of Respiratory Cell and Molecular Biology. 2012;47:315–323. doi: 10.1165/rcmb.2012-0068OC. [DOI] [PubMed] [Google Scholar]
- 110.Suchyna TM, Tape SE, Koeppe RE, 2nd, Andersen OS, Sachs F, Gottlieb PA. Bilayer- dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature. 2004;430:235–240. doi: 10.1038/nature02743. [DOI] [PubMed] [Google Scholar]
- 111.Kamaraju K, Gottlieb PA, Sachs F, Sukharev S. Effects of GsMTx4 on bacterial mechanosensitive channels in inside-out patches from giant spheroplasts. Biophysical Journal. 2010;99:2870–2878. doi: 10.1016/j.bpj.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lotshaw DP. Biophysical, pharmacological, functional characteristics of cloned and native mammalian two-pore domain K+ channels. Cell Biochem Biophys. 2007;47:209–256. doi: 10.1007/s12013-007-0007-8. [DOI] [PubMed] [Google Scholar]
- 113.Duprat F, Lesage F, Patel AJ, Fink M, Romey G, Lazdunski M. The neuroprotective agent riluzole activates the two P domain K+ channels TREK-1 and TRAAK. Mol Pharmacol. 2000;57:906–912. [PubMed] [Google Scholar]
- 114.Kennard LE, Chumbley JR, Ranatunga KM, Armstrong SJ, Veale EL, Mathie A. Inhibition of the human two-pore domain potassium channel, TREK-1, by fluoxetine and its metabolite norfluoxetine. Brit J Pharmacol. 2005;144:821–829. doi: 10.1038/sj.bjp.0706068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moha ou Maati H, Peyronnet R, Devader C, Veyssiere J, Labbal F, Gandin C, Mazella J, Heurteaux C, Borsotto M. A human TREK-1/HEK cell line: a highly efficient screening tool for drug development in neurological diseases. PLoS One. 2011;6:e25602. doi: 10.1371/journal.pone.0025602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mazella J, Petrault O, Lucas G, Deval E, Beraud-Dufour S, Gandin C, El-Yacoubi M, Widmann C, Guyon A, Chevet E, Taouji S, Conductier G, Corinus A, Coppola T, Gobbi G, Nahon JL, Heurteauxet C, Borsotto M. Spadin, a sortilin-derived peptide, targeting rodent TREK-1 channels: A new concept in the antidepressant drug design. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bagriantsev SN, Ang KH, Gallardo-Godoy A, Clark KA, Arkin MR, Renslo AR, Minor DL. A high- throughput functional screen identifies small molecule regulators of temperature- and mechano- sensitive K2P channels. ACS Chemical Biology. 2013;8:1841–1851. doi: 10.1021/cb400289x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sheetz MP, Singer SJ. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc Natl Acad Sci USA. 1974;71:4457–4461. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Koch SE, Gao X, Haar L, Jiang M, Lasko VM, Robbins N, Cai W, Brokamp C, Varma P, Tranter M, Liu Y, Ren X, Lorenz JN, Wang HS, Jones WK, Rubinstein J. Probenecid: novel use as a non-injurious positive inotrope acting via cardiac TRPV2 stimulation. Journal of Molecular and Cellular Cardiology. 2012;53:134–144. doi: 10.1016/j.yjmcc.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guinamard R, Hof T, Del Negro CA. The TRPM4 channel inhibitor 9-phenanthrol. Brit J Pharmacol. 2014;171:1600–1613. doi: 10.1111/bph.12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cooper PJ, Kohl P. Species- and preparation-dependence of stretch effects on sino-atrial node pacemaking. Annals of the New York Academy of Sciences. 2005;1047:324–335. doi: 10.1196/annals.1341.029. [DOI] [PubMed] [Google Scholar]
- 122.Caldwell RA, Clemo HF, Baumgarten CM. Using gadolinium to identify stretch-activated channels: technical considerations. American Journal of Physiology. 1998;275:C619–C621. doi: 10.1152/ajpcell.1998.275.2.C619. [DOI] [PubMed] [Google Scholar]
- 123.Kohl P, Sachs F. Mechanoelectric feedback in cardiac cells. Philosophical Transactions of the Royal Society of London Series A: 2001;359:1173–1185. [Google Scholar]
- 124.Lab MJ. Mechanosensitive-mediated interaction, integration, and cardiac control. Annals of the New York Academy of Sciences. 2006;1080:282–300. doi: 10.1196/annals.1380.022. [DOI] [PubMed] [Google Scholar]
- 125.Slovut DP, Wenstrom JC, Moeckel RB, Wilson RF, Osborn JW, Abrams JH. Respiratory sinus dysrhythmia persists in transplanted human hearts following autonomic blockade. Clinical and Experimental Pharmacology & Physiology. 1998;25:322–330. doi: 10.1111/j.1440-1681.1998.tb02358.x. [DOI] [PubMed] [Google Scholar]
- 126.Franz MR, Bode F. Mechano-electrical feedback underlying arrhythmias: the atrial fibrillation case. Progress in Biophysics and Molecular Biology. 2003;82:163–174. doi: 10.1016/s0079-6107(03)00013-0. [DOI] [PubMed] [Google Scholar]
- 127.Levine JH, Guarnieri T, Kadish AH, White RI, Calkins H, Kan JS. Changes in myocardial repolarisation in patients undergoing balloon valvuloplasty for congenital pulmonary stenosis: evidence for contraction-excitation feedback in humans. Circulation. 1988;77:70–77. doi: 10.1161/01.cir.77.1.70. [DOI] [PubMed] [Google Scholar]
- 128.Waxman MB, Wald RW, Finley JP, Bonet JF, Downar E, Sharma AD. Valsalva termination of ventricular tachycardia. Circulation. 1980;62:843–851. doi: 10.1161/01.cir.62.4.843. [DOI] [PubMed] [Google Scholar]
- 129.Iribe G, Jin H, Naruse K. Role of sarcolemmal BKCa channels in stretch-induced extrasystoles in isolated chick hearts. Circulation Journal: Official Journal of the Japanese Circulation Society. 2011;75:2552–2558. doi: 10.1253/circj.cj-11-0486. [DOI] [PubMed] [Google Scholar]
- 130.Sachs F. Stretch-activated channels in the heart. In: Kohl P, Sachs F, Franz MR, editors. Cardiac Mechano-Electric Coupling and Arrhythmias. 2 ed. Oxford: Oxford University Press; 2011. pp. 11–18. [Google Scholar]
- 131.Iribe G, Kohl P. Axial stretch enhances sarcoplasmic reticulum Ca2+ leak and cellular Ca2+ reuptake in guinea pig ventricular myocytes: experiments and models. Progress in Biophysics and Molecular Biology. 2008;97:298–311. doi: 10.1016/j.pbiomolbio.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 132.Trayanova NA, Constantino J, Gurev V. Models of stretch-activated ventricular arrhythmias. Journal of Electrocardiology. 2010;43:479–485. doi: 10.1016/j.jelectrocard.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li W, Kohl P, Trayanova N. Induction of ventricular arrhythmias following mechanical impact: a simulation study in 3D. Journal of Molecular Histology. 2004;35:679–686. doi: 10.1007/s10735-004-2666-8. [DOI] [PubMed] [Google Scholar]
- 134.Healy SN, McCulloch AD. An ionic model of stretch-activated and stretch-modulated currents in rabbit ventricular myocytes. Europace. 2005;7:128–134. doi: 10.1016/j.eupc.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 135.Kohl P, Day K, Noble D. Cellular mechanisms of cardiac mechano-electric feedback in a mathematical model. The Canadian Journal of Cardiology. 1998;14:111–119. [PubMed] [Google Scholar]
- 136.Zorzano A, Camps M. Isolation of T-tubules from skeletal muscle. Current Protocols in Cell Biology. 2006 doi: 10.1002/0471143030.cb0324s31. Chapter 3:Unit 3 24. [DOI] [PubMed] [Google Scholar]
- 137.Lab MJ, Bhargava A, Wright PT, Gorelik J. The scanning ion conductance microscope for cellular physiology. American Journal of Physiology Heart and Circulatory Physiology. 2013;304:H1–H11. doi: 10.1152/ajpheart.00499.2012. [DOI] [PubMed] [Google Scholar]
- 138.Balligand J-L, Dessy C. Stretch effects on second messengers. In: Kohl P, Sachs F, Franz MR, editors. Cardiac Mechano-Electric Coupling and Arrhythmias. 2 ed. Oxford: Oxford University Press; 2011. pp. 81–86. [Google Scholar]
- 139.Iribe G, Kohl P. Non-sarcolemmal stretch–activated channels. In: Kohl P, Sachs F, Franz MR, editors. Cardiac Mechano-Electric Coupling and Arrhythmias. 2 ed. Oxford: Oxford University Press; 2011. pp. 35–41. [Google Scholar]
- 140.Belmonte S, Morad M. Shear fluid-induced Ca2+ release and the role of mitochondria in rat cardiac myocytes. Annals of the New York Academy of Sciences. 2008;1123:58–63. doi: 10.1196/annals.1420.007. [DOI] [PubMed] [Google Scholar]
- 141.Kiseleva I, Kamkin A, Pylaev A, Kondratjev D, Leiterer KP, Theres H, Wagner KD, Persson PB, Gunther J. Electrophysiological properties of mechanosensitive atrial fibroblasts from chronic infarcted rat heart. Journal of Molecular and Cellular Cardiology. 1998;30:1083–1093. doi: 10.1006/jmcc.1998.0673. [DOI] [PubMed] [Google Scholar]
- 142.Kamkin A, Kiseleva I, Isenberg G. Activation and inactivation of a non-selective cation conductance by local mechanical deformation of acutely isolated cardiac fibroblasts. Cardiovascular Research. 2003;57:793–803. doi: 10.1016/s0008-6363(02)00775-7. [DOI] [PubMed] [Google Scholar]
- 143.Kohl P, Kamkin A, Kiseleva I, Noble D. Mechanosensitive fibroblasts in the sino-atrial node region of rat heart: interaction with cardiomyocytes and possible role. Experimental Physiology. 1994;79:943–956. doi: 10.1113/expphysiol.1994.sp003819. [DOI] [PubMed] [Google Scholar]
- 144.Beyder A, Rae JL, Bernard C, Strege PR, Sachs F, Farrugia G. Mechanosensitivity of Nav1.5, a voltage-sensitive sodium channel. The Journal of Physiology. 2010;588:4969–4985. doi: 10.1113/jphysiol.2010.199034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Numata T, Shimizu T, Okada Y. Direct mechano-stress sensitivity of TRPM7 channel. Cellular Physiology and Biochemistry. 2007;19:1–8. doi: 10.1159/000099187. [DOI] [PubMed] [Google Scholar]
- 146.Du J, Xie J, Zhang Z, Tsujikawa H, Fusco D, Silverman D, Liang B, Yue L. TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circulation Research. 2010;106:992–1003. doi: 10.1161/CIRCRESAHA.109.206771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chatelier A, Mercier A, Tremblier B, Theriault O, Moubarak M, Benamer N, Corbi P, Bois P, Chahine M, Faivre JF. A distinct de novo expression of Nav1.5 sodium channels in human atrial fibroblasts differentiated into myofibroblasts. The Journal of Physiology. 2012;590:4307–4319. doi: 10.1113/jphysiol.2012.233593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Morris CE. Voltage-gated channel mechanosensitivity: fact or fiction? Frontiers in Physiology. 2011;2:25. doi: 10.3389/fphys.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hales PW, Schneider JE, Burton RA, Wright BJ, Bollensdorff C, Kohl P. Histo-anatomical structure of the living isolated rat heart in two contraction states assessed by diffusion tensor MRI. Progress in Biophysics and Molecular Biology. 2012;110:319–330. doi: 10.1016/j.pbiomolbio.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Burton RA, Plank G, Schneider JE, Grau V, Ahammer H, Keeling SL, Lee J, Smith NP, Gavaghan D, Trayanova N, Kohl P. Three-dimensional models of individual cardiac histoanatomy: tools and challenges. Annals of the New York Academy of Sciences. 2006;1080:301–319. doi: 10.1196/annals.1380.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bishop MJ, Plank G, Burton RA, Schneider JE, Gavaghan DJ, Grau V, Kohl P. Development of an anatomically detailed MRI-derived rabbit ventricular model and assessment of its impact on simulations of electrophysiological function. American Journal of Physiology Heart and Circulatory Physiology. 2010;298:H699–H718. doi: 10.1152/ajpheart.00606.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vadakkumpadan F, Arevalo H, Prassl AJ, Chen J, Kickinger F, Kohl P, Plank G, Trayanova N. Image-based models of cardiac structure in health and disease. Wiley Interdisciplinary Reviews Systems Biology and Medicine. 2010;2:489–506. doi: 10.1002/wsbm.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Austin TM, Hooks DA, Hunter PJ, Nickerson DP, Pullan AJ, Sands GB, Smaill BH, Trew ML. Modeling cardiac electrical activity at the cell and tissue levels. Annals of the New York Academy of Sciences. 2006;1080:334–347. doi: 10.1196/annals.1380.025. [DOI] [PubMed] [Google Scholar]
- 154.Hunter P. Mathematical models of cardiac structure and function: mechanistic insights from models of heart failure. In: Kohl P, Sachs F, Franz MR, editors. Cardiac Mechano-Electric Coupling and Arrhythmias. 2 ed. Oxford: Oxford University Press; 2011. pp. 241–250. [Google Scholar]
- 155.Smaill BH, Zhao J, Trew ML. Three-dimensional impulse propagation in myocardium: arrhythmogenic mechanisms at the tissue level. Circulation Research. 2013;112:834–848. doi: 10.1161/CIRCRESAHA.111.300157. [DOI] [PubMed] [Google Scholar]
- 156.Meng F, Suchyna TM, Sachs F. A fluorescence energy transfer-based mechanical stress sensor for specific proteins in situ. FEBS Journal. 2008;275:3072–3087. doi: 10.1111/j.1742-4658.2008.06461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Meng F, Sachs F. Visualizing dynamic cytoplasmic forces with a compliance-matched FRET sensor. Journal of Cell Science. 2011;124:261–269. doi: 10.1242/jcs.071928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Guinamard R, Demion M, Magaud C, Potreau D, Bois P. Functional expression of the TRPM4 cationic current in ventricular cardiomyocytes from spontaneously hypertensive rats. Hypertension. 2006;48:587–594. doi: 10.1161/01.HYP.0000237864.65019.a5. [DOI] [PubMed] [Google Scholar]
- 159.Van Wagoner DR. Mechanosensitive gating of atrial ATP-sensitive potassium channels. Circulation Research. 1993;72:973–983. doi: 10.1161/01.res.72.5.973. [DOI] [PubMed] [Google Scholar]
- 160.Eich C, Bleckmann A, Schwarz SK. Percussion pacing - an almost forgotten procedure for haemodynamically unstable bradycardias? A report of three case studies and review of the literature. Br J Anaesth. 2007;98:429–433. doi: 10.1093/bja/aem007. [DOI] [PubMed] [Google Scholar]
- 161.Pellis T, Kette F, Lovisa D, Franceschino E, Magagnin L, Mercante WP, Kohl P. Utility of pre-cordial thump for treatment of out of hospital cardiac arrest: a prospective study. Resuscitation. 2009;80:17–23. doi: 10.1016/j.resuscitation.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 162.Monteleone PP, Alibertis K, Brady WJ. Emergent precordial percussion revisitedpacing the heart in asystole. Am J Emerg Med. 2011;29:563–565. doi: 10.1016/j.ajem.2010.01.030. [DOI] [PubMed] [Google Scholar]