Abstract
Type 1 diabetes is a disorder where slow destruction of pancreatic β-cells occurs through autoimmune mechanisms. The result is a progressive and ultimately complete lack of endogenous insulin. Due to β-cell lack, secondary abnormalities in glucagon and likely in incretins occur. These multiple hormonal abnormalities cause metabolic instability and extreme glycemic variability, which is the primary phenotype. As the disease progresses patients often develop hypoglycemia unawareness and defects in their counterregulatory defenses. Intensive insulin therapy may thus lead to 3-fold excess of severe hypoglycemia and severely hinder the effective and safe control of hyperglycemia. The main goal of the therapy for type 1 diabetes has long been physiological mimicry of normal insulin secretion based on monitoring which requires considerable effort and understanding of the underlying physiology. Attainment of this goal is challenged by the nature of the disease and our current lack of means to fully repair the abnormal endocrine pancreas interactive functions. As a result, various insulin preparations has been developed to partially compensate for the inability to deliver timely exogenous insulin directly to the portal/intrapancreatic circulation. It remains an ongoing task to identify the ideal routes and regimens of their delivery and potentially that of other hormones to restore the deficient and disordered hormonal environment of the pancreas to achieve a near normal metabolic state. Several recent technological advances help addressing these goals, including the rapid progress in insulin pumps, continuous glucose sensors, and ultimately the artificial pancreas closed-loop technology and the recent start of dual-hormone therapies.
Keywords: insulin, insulin delivery, type 1 diabetes, insulin delivery, hyperglycemia, pancreas, islets, hypoglycemia, counterregulation, diabetes mellitus, feedback, glucagon, hypoglycemia, pancreatic network, artificial pancreas
1. Introduction
Type 1 diabetes is an autoimmune disorder where slow destruction of pancreatic β-cells occurs through cell-mediated immunity mechanisms. The result is a progressive and ultimately complete lack of endogenous insulin. Due to insulin lack, secondary abnormalities in glucagon and likely in incretin physiology occur. These several hormonal abnormalities cause metabolic instability and extreme glycemic variability, which is the primary phenotype. In addition, as the disease progresses, patients develop hypoglycemia unawareness and partially or completely lose their counterregulatory defenses. Therefore, use of intensive insulin therapy may result in up to 3-fold excess of severe hypoglycemia, which is the primary obstacle to achieving optimal glycemic control. The main goal of therapy for type 1 diabetes has long been physiological mimicry of normal insulin secretion based on monitoring—a complex task that requires considerable effort and understanding of how the body works.
Achieving a near normal glycemic condition is made difficult by the severe insulin loss due to β-cell destruction and our current lack of means to fully repair the abnormal endocrine pancreas function without simply replacing normal islets. For example, the insulin preparations that have been developed since the discovery of insulin cannot successfully replicate all actions of endogenous insulin in health in large measure due to the replacement which is delivered to the peripheral venous circulation. Thus, maintaining glucose homeostasis does not restore normal intrapancreatic, portal vein, and hepatic actions of insulin and its balancing pancreatic co-hormone glucagon.
Given these limitations, it remains an ongoing task to identify the ideal routes and regimens of the delivery of insulin preparations and potentially that of other hormones such as glucagon, amylin or GLP-1 to restore the deficient and disordered hormonal environment of the pancreas and achieve a near normal metabolic state. One way to address these challenges would be to focus on diabetes technology to improve the existing insulin delivery devices and continuous glucose sensors, and ultimately to develop an artificial pancreas closed-loop platform that could accommodate a safe and efficient multi-hormone therapy for type 1 diabetes. It is our view that more attention to the disordered secretion and effects of glucagon may need to be a significant focus of the artificial pancreas design strategies.
2. Abbreviated history of insulin therapy in type 1 diabetes
Certain conceptual issues underlie the tasks of delivery and optimizing hormone therapy in diabetes. For type 1 diabetes mellitus, the primary focus has been on insulin replacement. Initially, after the first crude extracts from animal pancreas (primarily beef and pork) became available due to the work of Banting and Best and their colleagues, only regular (also called crystalline or soluble insulin) was used1, 2. Delivery was with glass syringes, large and uncomfortable needles for injection, which required boiling for sterilization. Frequent administration was needed due to the short duration of glucose lowering action (6–8 hours) of subcutaneously administered regular insulin preparations. Variable purity, variable absorption after injection, erratic peaks of action and duration perpetuated glycemic variability and did not permit safe achievement of near normoglycemia. There were local and sometimes systemic adverse side effects due to these dilute, impure preparations that were commercialized within a year (1922) after the discovery was originally made. The result was that patients with type 1 diabetes survived, but they did not thrive nor avoid chronic hyperglycemia related complications and commonly had problems with hypoglycemia.
Many forms of insulin emerged to enhance convenience and, recently, to enhance the mimicry of physiological delivery of insulin3. The first extended insulin was protamine zinc insulin (PZI) which lowered glucose up to 36 hours. Erratic absorption of such preparations however and their inconsistency in timing led to the development of neutral protamine Hagedorn (NPH) with a peak of glucose lowering action in mid to late afternoon after morning administration. Lente insulin, with similar timing as NPH was also used by altering zinc concentrations to generate several shorter and longer acting preparations, such as semilente and ultralente. Regular insulin however was necessary at the time to effectively cover insulin requirements at meals, albeit with a significant lag time (time from injection to eating) needed for optimal timing--a delay of 30–40 minutes after injection was needed to match insulin action to food absorption4.
Sanger’s discovery of the amino acid sequence for human insulin in the 1950s ultimately led to a movement away from animal insulin therapy. Thus, human insulin replaced animal insulin and the reduced antigenticity and better purity with removal of proinsulin and its fragments provided improved reproducibility of timing. The human insulins were absorbed more rapidly and had fewer skin reactions (e.g., lipodystrophy). Increased use of fixed ratio combination insulin such as 70/30 (human intermediate-acting [NPH] and short-acting [regular]) were provided to combine control at meals and between meals. Such premixed preparations offer convenience for glycemia control, but a trade off is an increased risk of hypoglycemia in patients with less consistent eating habits or when peak effects (maximum lowering of glucose) of NPH increased hypoglycemia risk with delayed meal appearance or overnight.
Two technological advances changed how insulin therapy was used in the 1980s. These were the development of self monitoring of blood glucose (SMBG) at home and the use of standard tests for non-enzymatically glycosylated hemoglobin A1c measurement5, 6. Compared to the urine glucose testing, SMBG more correctly showed the current state of glycemic control. Being able to test at any time of the day was invaluable for patients and allowed them to adjust their insulin therapy or eat to treat high or low blood glucose. The A1c (hemoglobin A1c) assay is a long term measure of average glycemic control over 3 months which could be related to risk of hyperglycemia exposure. Because of this test it was possible to accurately summarize glycemic risk while at the same time understand the immediate glucose values in most non-medical settings. Combined, the two methods substantially enhanced physician’s ability to gauge both long term and short term risk, and help patients to treat highs through use of supplemental insulin and lows with use of oral carbohydrate. These tools also made possible studies such as the Diabetes Control and Complications Trial (DCCT)7, the Kumamoto study8, the United Kingdom Prospective Diabetes Study (UKPDS)9 and others proving that near normal control glycemic control reduced long term complications in the eyes, kidneys and peripheral nerves. These studies using these two methods (hemoglobin A1c for long term and SMBG for immediate decisions) of monitoring of glycemia provided a basis for our modern therapy. These, and other studies as well, documented also the benefits of achieving nearly normal glycemia in regard to reducing microvascular diabetes complications and cardiovascular risk (DCCT, UKPDS)7, 9. However, those trying to accomplish better glycemic control through intensive insulin therapy face a 3-fold increased risk of severe hypoglycemia7, often without warning symptoms and potentially with severe consequences, especially to heart and brain10. Thus, reducing the risk of hypoglycemia and still maintaining optimal control, become a driving factor in the development of new insulin delivery strategies and devices and a significant effort was devoted to developing insulin therapies that closely mimic the activity of the pancreatic β-cell. Evidence of that is the development and use of insulin analogues capable of mimicking basal and meal bolus insulins better than NPH and regular insulin as injection therapy11 and the increasing implementation of insulin pumps which can provide even better protection against hypoglycemia12, 13.
A key insulin delivery tool that was developed and put into use during the 1980’s and which now has become the gold standard for physiological insulin delivery is the insulin pump. Initially, the insulin pump began as a prototype by Arnold Kadish in 1963 (colloquially referred to as the “blue brick”), which was a very large backpack-like machine, which injected both insulin and glucagon in order to supply both of the major hormones of the endocrine pancreas. Although this strategy was not particularly practical, development of a smaller more portable insulin pump began during the 1980’s came into more widespread use. In addition, even though abandoned for a while, the idea of dual hormonal therapy has more recently regained credence and is now being attempted in new ways by modern researchers14, 15, 16, 17.
3. Contemporary insulin analogs
Although animal insulin and human regular insulin gradually become more pure and associated with fewer skin reactions, they still had significant limitations in practical use, largely due to their timing of onset and peaks of actions to lower glucose. NPH, regular, Lente, Ultralente have a time course and variability of glucose lowering that is not in keeping with the physiological timing of insulin in non-diabetic individuals. A general principle that emerges in therapy with insulin is that the more severe the deficiency of insulin is (for type 1 and for some type 2 diabetes), the more important it is to have considerable mimicry of normal physiology to successfully lower glucose and do so with safety. Although not superior in overall glycemic lowering efficacy compared to human insulin, the analogs, insulins with altered amino acid structure (sometimes with side chain moieties) that are close analogs of human insulin but with different kinetics, have gained progressive popularity despite their increased cost. Today, analogs used as basal bolus therapy are considered the standard of care for patients who have type 1 diabetes mellitus and are increasingly used in type 2 diabetes.
3.1. Rapid acting insulin analogs
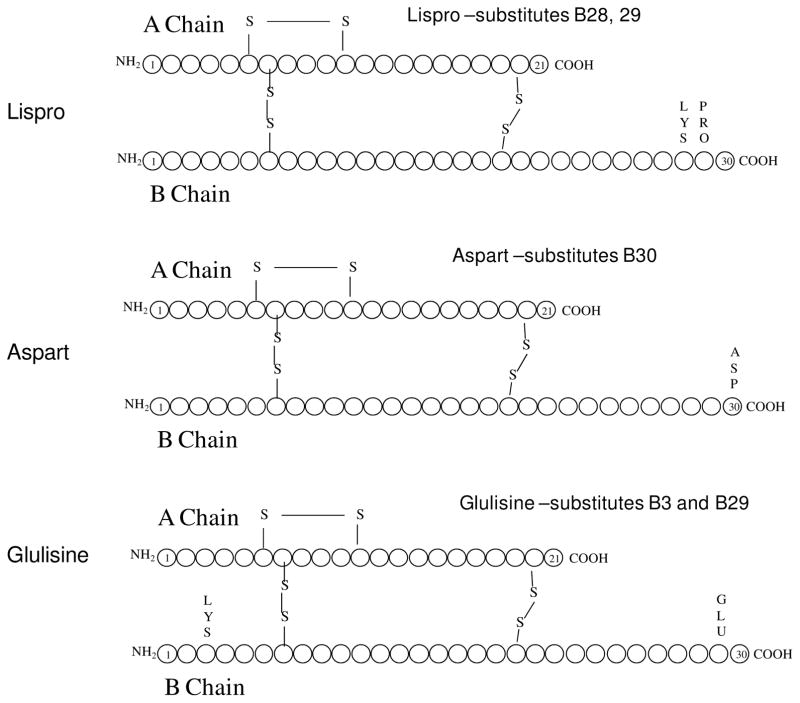
The first rapid analog available was lispro, which did not require administration 30–40 minutes in advance of eating, but could be taken within 5–15 minutes of meals (and sometimes shortly after eating), which is more practical for many patients, notably pediatric type 1 diabetes whose eating habits may be less reliable. As important, if not more important, is that the duration of action to lower glucose approximates 4 hours with a bolus injection. Most mixed meals that are not high in fat content are closely mimicked in this duration of action, as shown by Howey et al.18 Other rapid acting analogs with a similar time course have been developed as well. These include insulin aspart and glulisine insulin. Although there are some differences between them, in general these insulins serve a similar purpose and have similar time course of action: Figure 1. It should be noted that a rapid acting insulin analog may produce intense hypoglycemia when dosed with very low carbohydrate diets (as may happen with regular insulin).
Figure 1.
Rapid acting insulin analogs. Lispro’s name derives from the substitution of B28 and B29 amino acids with lysine and proline in the COOH terminus of the B chain. This substitution, as with the other two, led to less self aggregation into hexameric crystals and thereby more rapid absorption, higher peak effects and shorter duration of glucose lowering action than regular human insulin. Below lispro are depicted the two other amino acid substitutions in the B chain COOH terminus. For insulin aspart, it is aspartic acid at the B30 position. For glulisine there is a lysine substitution at B3 and a glutamic acid at B29
3.2. Slow acting (basal) insulin analogs
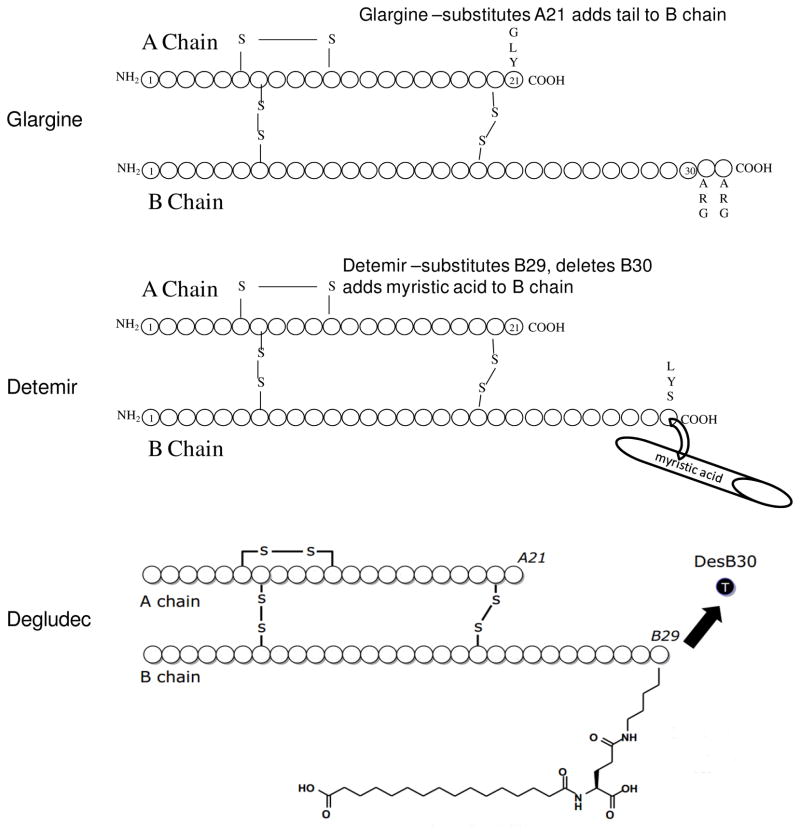
The first slow acting basal insulin analog was insulin glargine, so named due to its amino acid structural change from human insulin: Figure 2. Another currently available basal insulin is insulin detemir, which is modified by binding to a fatty acid moiety, myristic acid which influences protein binding in tissues and in the blood to prolong its action. The third basal insulin degludec (Figure 2, bottom), which has been recommended but not yet accepted for use by the US Food and Drug Administration (FDA), has the longest duration of glucose lowering action of the three and has in some studies been given less frequently than daily, which is perceived as a possible advantage19. In studies comparing different basal insulins (NPH, detemir, glargine) the basal insulin analogs all seem to have equal overall glycemic lowering efficacy as that of NPH insulin20, 21, but basal analogs appear to diminish the frequency of hypoglycemia in a number of studies20, 21. Of course this does not mean hypoglycemia does not occur, but some reduction in nocturnal hypoglycemia due to lesser peak effects of these basal analogs is likely to be important since hypoglycemia overnight is a known risk for hypoglycemia unawareness and the HAAF syndrome described by Cryer22, 23. Basal insulins of longer duration and less peak effect are an important part of modern type 1 diabetes management20, 21. They are the backbone of basal bolus therapy.
The minimum standard of care for type 1 diabetes treatment has become the basal-bolus therapy using basal and rapid acting insulin analogs.
Figure 2.
Structure of slow acting (basal) insulin analogs. Glargine (FDA-approved; top panel) has a substitution of glycine at A21 and two arginines added to the carboxy terminal of the B chain. In contrast, Detemir (FDA-approved; middle panel) has a myristic acid bound to lysine at B29. The bottom panel depicts Degludec (not approved by the agency), which has the B30 residue deleted, has no amino acid substitutions, and has conjugated hexadecanedioic acid to the lysine at B29.
4. How best to administer insulin?
In contrast to type 2 diabetes patients which usually start their insulin therapy with basal insulin alone, most patients with new onset type 1 diabetes start with basal plus bolus with about half being basal. When there is sufficient residual insulin the doses may be low and gradually increased and the regimen also gradually becomes more complex as insulin secretion wanes. Basal bolus therapy is as we argue a standard of care but it may be too restrictive for some patients, particularly those at a high risk of hypoglycemia. Therefore, it is desirable that the devices and the technology used to administer insulin in type 1 diabetic patients permit sufficient flexibility and allow patients to adapt the therapy to their individual needs.
4.1 Insulin pumps
Injectable analog insulins remain limited by their inflexible timing, especially long acting insulin—once given it cannot be taken away. Basal insulin needs may vary significantly throughout the day due to a diurnal rhythm and may require use of snacks to compensate for exercise effects to reduce hypoglycemia risk. Thus, the ability provided by modern insulin pumps to program basal rates that can be adapted to changing life circumstances gives the patient considerable flexibility. Insulin pump therapy can vary basal insulin rates at different times of the day and basal rates can be reduced during exercise temporarily and in the post exercise period to compensate for enhanced insulin sensitivity. Different weekend and weekday basal rates can be programmed with pumps as well.
Insulin pumps provide several advantages over the standard insulin delivery route. When properly inserted and changed with the needed frequency (every 2–3 days for most) they deliver insulin more consistently than injection therapy to meet basal needs and to match boluses with meals or snacks. Although pumps do not eliminate variability of blood sugar levels, their proper use markedly reduces this variability, which is one of the most important and frustrating features of type 1 diabetes. Reduction in variability of blood sugar levels makes it more likely that patients will safely achieve glycemic targets. Nonetheless, it should be understood that misuse of pumps can lead to extreme variability as with inappropriate proportions of basal and bolus insulin within regimens.
Use of increased temporary basal rates are particularly useful with acute illness and stressful situations and allow for more effective and consistent control than multiple extra boluses of insulin used alone in catch up (correction) dosing. Temporary basal rates are also very useful with reducing insulin to compensate for exercise with its immediate and delayed risks of hypoglycemia.
Insulin pumps also provide a much more precise dosing of insulin and patient who are quite sensitive to insulin and who therefore choose to use very low doses of insulin can give a more accurate delivery of the intended dose with accuracy of a 1/10th to 1/20th of a unit.
Finally, being able to deliver insulin boluses of both different duration and distribution (early vs. late) allows for more physiological mimicry of the way the body would deliver insulin when eating rich meals. Most pumps will have three types of bolus configurations. The first is a regular bolus which provides all of the insulin in one immediately administered bolus. A second type is an extended bolus, which comes in two forms: a simple extended bolus which gives the same dose evenly administered over a longer time period and a compound bolus with a regular bolus followed by extended bolus. The extended bolus may be from 30 minutes to 8 hours. Extended boluses are sometimes used for gastroparesis or prolonged snacking. The compound type of extended bolus is typically divided into early and late, often starting with 50% split between the two components, an initial regular bolus followed by an extended period of administration. Compound boluses are used often for large and rich meals with substantial amounts of fat and/or fiber which delay gastric emptying and the appearance of meal carbohydrates in the bloodstream. Meals such as pizza may need a prolonged compound bolus. Bolus calculators provided by most modern pumps help with accuracy in remembering and compensating for the insulin on board from prior boluses.
4.2. Alternative administration routes
A barrier to insulin use in many patients is that it is available only by injections. These patients resist multiple injection therapy as a burden and miss or skip some injections thereby limiting the ability to be as well controlled. To overcome these limitations, alternative insulin administration routs have been tested in studies, but none other than injections (and pump administration) is currently clinically available routinely.
One example is the insulin pen: Figure 3.
Figure 3.
Picture of different insulin pens (source: http://www.diabetesnet.com/about-diabetes/insulin/insulin-delivery/insulin-pens)
Insulin pens look like a pen with a cartridge. Some have replaceable cartridges of insulin while others are completely disposable. A short, fine needle is on the end of the pen. Users rotate a dial to select the amount of insulin and then press a plunger to deliver the insulin just under the skin. Insulin pens, like pumps, are a valuable tool for those who are on intensive (flexible) insulin therapy. They are easy to use and may reduce the variability of injection delivery. Insulin pens are more convenient than vial and syringe methods, and more accurate dosages can be delivered. It is expected that the technology with pens will continue to develop in areas such as use of smaller gauge needles and smart pens that can remember prior doses and keep track of their timing.
Another example is the jet injector, such as the Medijector, which has been used in the past to deliver insulin by a spray injector subcutaneously, but stopped being sold in 2009. Such devices could produce bruising and are not thus pain free.
Insulin has also been given by nasal inhalation24, and by powdered regular insulin inhaled and then absorbed through the lung’s surface25. Nasal insulin absorption proved too inefficient and too small a surface area for use without a surfactant, which produced some irritation. Early studies with nasal insulin certainly were promising and had favorable time course with a rapid onset and little or no lag time. Because only regular insulin was used, the duration of insulin action was for 6–8 hours which fitted some meals well, while other meals were less well controlled. In general one limitation of all alternative forms of insulin delivery relates to the inherently inefficient delivery which is typically one tenth as available biologically even when delivered to a large surface area such as the lung.
Pulmonary insulin administration to provide meal insulin needs was approved by the FDA and marketed for several years as Exubera by Pfizer. However, it was not commercially successful. The device did not use continuous dosing which meant that multiple inhalations were needed. Inhaled insulin competed with greater adoption of basal analog insulins and better analog meal insulin. Possible pulmonary function changes required lung monitoring which may have dimmed enthusiasm for this delivery route. Despite the lack of commercial success with the earlier product for pulmonary insulin, there is still a significant research presence and potential for pulmonary insulin. There has been also a continued push for approval of an insulin technosphere delivery (a form of pulmonary insulin formulation with regular insulin) which emanates from the MannKind Corporation. and has a very rapid onset which is as good as rapid analogs or better and generally quite good tolerance. Early in 2011 however, the FDA suggested the need for further studies, so the future is not clear as to the timing or its chance of future availability.
Intraperitoneal insulin delivery is another alternative treatment for patients with type 1 diabetes that has been available for more than two decades, but is not in use clinically. With this therapy, insulin is administered by an implantable pump and is primarily absorbed through the portal system which mimics more closely the action of insulin in health. It allows for a faster insulin action to control postprandial hyperglycemia and appearance profiles in the circulation that are more predictable as compared to subcutaneous delivery26, 27, 28. In addition, some studies suggest that intraperitoneal insulin may improve hepatic uptake, lower peripheral plasma insulin levels29, and may improve glucagon counterregulation and hepatic glucose production in response to hypoglycemia30. Comparisons between intraperitoneal and subcutaneous insulin delivery showed improved glycemic control with the intraperitoneal delivery (0.8% decrease in A1C and an 11% increase in the time spent in euglycemia), but no significant reduction in hypoglycemia31. The problem has been that long term intraperitoneal insulin pumps may create significant reaction to the material and the adjustments needed for control still remain problematic for many patients despite these promising experimental results.
5. Current diabetes treatment and advances in technology and their impact on insulin treatment strategies
Although lack of insulin is considered the primary defect defining T1DM in its pathophysiology and therapy, other abnormalities of the endocrine pancreas are more complex and have an important impact on efforts to control glycemia. As argued by Unger and colleagues32, 33, 34, defects in glucagon secretion are critical to the pathophysiology of the disease. Its inappropriately high levels contribute to the phenotype of poorly controlled type 1 diabetes, e.g., ketoacidotic patients. Moreover, deficient glucagon counterregulation (GCR), observed within the first few years of type 1 DM, impedes safe treatment of the disease with intensive insulin therapy35, 36. Therefore, strategies for insulin replacement should be viewed in the context of a general dysregulation of pancreatic endocrine secretion. In normal physiology, pancreatic insulin and glucagon traveling through the portal vein have reciprocal effects on the liver by suppressing or stimulating the hepatic glucose output (HGO). Consequently, the balance between these two hormones determines the net liver output. With destruction of the β-cells in T1DM this balance is heavily biased towards glucagon during euglycemia and hyperglycemia. On the other hand, hypoglycemia appears incapable of stimulating the GCR and thus fails to increase the HGO. It is possible that some compensatory effects may develop at the liver to counter the hormone concentration imbalance in the portal circulation. For example, some reports highlight the increase of the resistance of the liver to glucagon, which will likely impair additionally the counterregulation, whether the effects exists through affecting glycogenolysis37 or glucagon receptor action or other mechanisms. To effectively control glycemia, T1DM treatments should take into account these abnormalities and may require modification and extension of the standard insulin therapy (see Section 5.4)
Insulin replacement strategies also face problems associated with various delays including a delay in detecting changes in circulating glucose and a delay in insulin action caused by its non-physiological subcutaneous delivery. Such delays cause mistiming of the insulin bolus that could cause excessive glucose variability accompanied with hypo- and hyperglycemia. Trying to catch up with correction doses once hyperglycemia or treating hypoglycemia appear ineffective and often less than ideal. Anticipatory or proactive actions are required for best results, like giving the meal bolus at or before the time of the meal with keeping an appropriate timing. It is also necessary to take into account blood glucose dynamics rather than acting solely on a single blood glucose value. Such complex proactive actions are now possible in view of the latest technology advancements as is described below.
5.1. Continuous glucose monitoring sensors (CGM)
CGM is a new method of frequent monitoring particularly useful for people with type 1 diabetes using insulin pump therapy. It is available for use directly with a pump, but may also be used as a stand-alone device. Early studies suggest that sensor-augmented insulin pump therapy may be superior to standard home monitoring of fingerstick capillary blood glucose concentrations. The benefits in the JDRF study38 and the McGarraugh and Bergenstal study39 are lower hypoglycemia risk, especially in patients with a history of hypoglycemia, and improved glycemic control with reduced HbA1c levels. In general, more frequent monitoring (CGM or not) helps to reduce excessive glycemic variability and get better and safer glycemic control in type 1 diabetes40, 41. Despite the early evidence for benefits of CGM, there are nonetheless a number of concerns, and some potential misconceptions in the use of CGM. As a result the adoption of this technology in clinical practice has been slow with only 3% of CGM users of age ≤25 years and less than 14% in the 26–49 years age group42. The US Food and Drug Administration (FDA) has noted concern about the accuracy of CGM sensors. They also fail about 10% of the time. Thus when sensor values don’t agree well with blood glucose monitoring it is hard to make accurate clinical decisions. In addition, interstitial glucose, which is used by many CGM sensors as a surrogate for BG, differs in time course physiologically from blood glucose, particularly when blood glucose is changing rapidly. Such times when CGM may mislead include the responses to high glycemic load meals which lead to rapid BG excursions. Also, rapid decrements in blood glucose after accidental insulin overdosing during, or shortly after, exercise, as in those with HAAF may be associated with discrepancies between self monitoring and CGM values. CGM must be regularly calibrated (2–4 times daily seems best) with fingerstick monitoring of capillary blood glucose to facilitate accurate use and identification of failing sensors.
An important aspect of CGM use is that users and diabetes care providers can now pay more attention to the trends than absolute values of the CGM. It is critical, for example, to confirm hypoglycemia if there are suspicious symptoms and to treat even if the estimated glucose level on the monitor is not as low as is usually required for such actions. Some situations promote high glycemic variability where CGM can assist greatly. CGM can identify overnight hypoglycemia and is useful for helping patients to verify that their basal rates are correctly programmed. To do so requires patients to fast for 6–8 hour in the post absorptive state to perform such verification. CGM can be helpful in identifying patterns of sustained hyperglycemia or late hyperglycemia escape after very rich meals, typically foods such as pizza or macaroni and cheese. By doing so, a need for extended bolus or dual wave bolus treatment can be identified. Exercise is a common source of hypoglycemia risk, both during and for many hours after exercise, and the use of CGM may help to identify the need for prolonged temporary basal rate reduction. For example, high intensity exercise has a characteristic biphasic effects on glycemia, initially high due to stress hormones and later low, probably from restoration of muscle glycogen.
Most technology-savvy doctors and patients can readily learn to use CGM, but some patients find the learning curve steep and the amount of information so high that they lose sight of the forest for the trees. One issue with CGM is that there is no validated, specific pattern recognition that is used, like for example an ECG where we are pretty certain what is the problem—CGM interpretation is really part of the “art of diabetes care”. Moreover, there is no specific advice that is tied to an aberrant or dangerous glycemic configuration known generally to correct it. Most practitioners find that not everyone is going to be successful with CGM and that for most people, adequate training prior to using CGM for decision-making is critical. There is a surprisingly common tapering off of the use of CGM, especially if there are excessive alarms. It is certainly critical to the success of CGM in improving glycemic extremes for people to attend to it. CGM only works when it is worn (!) and the wearer needs to pay attention to the values and the trends it identifies - something that is not always done in clinical practice or even in the studies noted above. An expert panel met in 2012 to discuss recommendations for standardization of analysis and presentation of glucose monitoring data derived from CGM systems and identified the lack of standardization of glucose reporting among the various CGM devices as a major obstacle to overcoming the mentioned above underutilization of the CGM devices43.
One concern about interpreting clinical studies using CGM should be mentioned. It is a misconception that CGM itself makes the difference in terms of avoiding hypoglycemia or achieving better glycemic control. Instead, it is really what the patient and the diabetes specialist do with the CGM information that makes the difference. As examples, patient might need to avoid excessive basal insulin doses, minimize overcorrection of hyperglycemia or hypoglycemia. Controlling diabetes is both a matter of biology and behavior including the nature of the patient’s or the diabetes practitioner’s interaction with diabetes technology. The specifics of both are important to diabetes control and to safe use of insulin therapy, whether using pumps or basal bolus insulin treatment. Future studies may need to focus on what specific advice or remedies, such as some of the practical points mentioned above, should be applied in response to which specific patterns of glycemia. Those remedies may involve changing pump settings, but just as important is the need to clarify the role of safe behaviors, like adequate and prompt hypoglycemia treatment, some self discipline in adequate use of confirming fingerstick blood glucose tests, and regularly wearing of the sensors.
5.2 Pumps with CGM
Even insulin pumps used with CGM remain far from perfect although in the hands of a properly trained user it can be a big improvement. It may actually permit patients to change insulin delivery patterns (e.g., validation of basal rates) in response to observed trends or anticipated needs or to more clearly delineated patterns of glycemia related to behavioral factors, such as eating specific meals. The ability to compensate for erratic eating, the acute and long term effects of exercise, stresses that are related to acute illness, all may be improved. In this regard, the insulin pump/CGM combinations offer incredible flexibility and enormous advantage to those who realize for example that blood glucose of 100 mg/dl (5.5 mM) is treated completely differently when the trend is rapidly down vs. rapidly up.
Nonetheless, this technology is imperfect. Sensors continue to have accuracy problems with sensor drift when there are frequent rapid ups or downs in the glycemic patterns. Too little or too much calibration may affect the sensor performance. Also insufficient checking with fingerstick blood sugars may lead to inappropriate action by the patient. Insulin pumps likewise have many features, some of which many patients have not learned how to use. As an example, it is common practice on meeting an insulin pump user to ask what their insulin/carb ratios, their correction factor (often called insulin sensitivity) and pump settings are. Additionally, some of them do not even inquire about the advanced bolus features such as compound boluses for large meals and restaurant eating as well as use of temporary basal rates. Many patients do not know how to use these and thus do not take advantage of the flexibility that pumps offer. Again, it is clear that control of type 1 diabetes is a biological and behavioral issue and that adequate training, with an emphasis on gradual acquisition of a series of skills is critical for success with this powerful technology. Thus, there are significant risks of lows and highs despite the tremendous potential of the new technology, CGM with or without insulin pumps, to reduce the risk of variability when used properly.
How can one deal with the variability of type 1 diabetes in a way that is optimal? Proper selection of patients to use newer technologies appear to be critical, but even with well selected and trained patients, problems remain. Overall, average A1c values nationally for those with type 1 DM remain higher than would be recommended based on the DCCT trial, typically a point or more above the optimal range in half the patients from the US44. On the other hand, average control is not a useful indicator of problems with extremely variable patients45 and even A1c values within a desirable range are frequently found in individuals with substantially poor control, like children and adolescents who are often at a higher risk of hypoglycemia. As Frier has pointed out46, in these patients hypoglycemia remains an enormous problem and barrier to optimal control.
Preventing severe hypoglycemia requires also the therapeutical methods and the technology they use to be able to address other bio-behavioral issues as well. For example, Clarke et al47 early on pointed out that distinguishing behaviors that are helpful to avoid hypoglycemia are not reliably different in patients in which mild or more severe hypoglycemia occur. Thus, the authors of this work concluded: an educational intervention whose content stresses insulin, food, and exercise would be unlikely by itself to be sufficient to reduce the frequency of severe hypoglycemia episodes. One confusing bio-behavioral aspect of hypoglycemia occurrence is that there is a marked variability that accompanies the risk of severe hypoglycemia48. This variability exacerbation occurs both before and after severe hypoglycemia episodes. Thus, for 48 hours before and after profound hypoglycemia episodes there is a systemic instability in the glycemic pattern. This instability often confuses patients, leading them to uncertainty on which aspect (highs or lows) to focus their behavioral and medical interventions. In addition, severe swings in blood glucose levels themselves may alter cognitive efficiency and additionally exacerbate the problems in decision making.
How to properly use current diabetes technology to overcome the variability and the tendency to develop severe hypoglycemia is a very difficult task which cannot be the addressed by the technology alone due to the complex biological, behavioral, and psychological interplay47 that has been delineated above. However, there are some potential ways that might get around some of this complexity. The emerging artificial pancreas technology is one possibility which may solve several of the issues associated with the use of the current insulin pumps and CGMs as discussed below.
5.3. Artificial pancreas (AP)
The first AP-like machine, the Biostator, was developed in the early 1970s, and is still used for inpatient management of insulin dependent diabetes50. It has two iv lines, one for blood draw for glucose measurement (blood is discarded afterwards) and one for infusion of insulin controlled by a simple mathematical algorithm. In practice, the Biostator is quite large and unsuitable for outpatient use. In contrast, modern artificial pancreas (AP) closed-loop technology targets portability by integrating and using a subcutaneous continuous glucose monitor (CGM) and a subcutaneous insulin pump. The heart of the system is a computer-like device that communicates with the pump and the CGM sensor with an algorithm that dynamically adapts the pump rate of insulin delivery based on information provided by the CGM and possibly the user. This way, the AP technology, assists the patient in his attempt to reduce glucose variability by constantly monitoring the glucose levels and taking decisions for appropriate changes in insulin delivery.
There are several types of computer algorithms that have evolved all of which offering the potential to improve the safety and the efficacy of glycemic control in short term inpatient studies. The first AP studies employed a proportional-integral-derivative (PID) control algorithm51,52. Recently, model-predictive control based on extensively validated models of human glucose metabolism has been receiving considerable attention53,54,55,56 and it has been argued that they offer significant clinical and engineering advantages over PID control57. AP research has accelerated rapidly in recent years showing strong promise to significantly improve glycemic control and demonstrating ability to protect against hypoglycemia while also maximizing the time spent in tight control, including overnight52, 58, 59, 60. AP methodology is evolving rapidly and newer and updated models are being introduced, including dedicated safety supervision systems to avoid hypoglycemia58. Ultimately, there is a need to transfer this technology in real life in the field.
The AP technology overcomes many of the problems that CGM and different insulin pumps cannot address alone as part of an integrated insulin therapy. For example, the artificial pancreas may filter the CGM input information to enhance the sensor accuracy, it can predict more precisely than most patients the future needs for insulin adjustments and can compensate fairly effectively for exercise effects upon the risk of hypoglycemia60.
The AP can use in development, simulated (in silico) patients in place of both animal trials and early phase 1 and 2 trials to directly compare errors in information on glucose values, tactics and adjustments of insulin pump basal rate therapies in controlling patients within a target range61, 62. Therefore, there is a critical need for continued development of excellent physiological modeling originally designed to describe aspects of the glucose metabolism and the complex control of glycemia63, 64, 65, 66. These models assist the development, testing, and implementation of the AP technology67, 68.
One limitation of the current closed-loop therapy is the difficulty with management of meal related hyperglycemia which is either not very well controlled if done automatically or requires manual user intervention at meals similar to a standard meal/bolus therapy. Alterations in basal insulin and safety systems can help quite a bit, but meal hyperglycemia is probably the most important daily source of variability in management of type 1 diabetes. Typically insulin to carbohydrate ratios and estimation of the carbohydrate and fat content of foods are factors that are used with closed-loop therapy. Although superior to a sliding scale only approach and to the use of a fixed amount of meal insulin, an obvious issue with the use of insulin to carbohydrate ratios for determining the meal insulin is that the precision of most patients in identifying carbohydrates is limited and quite inexact Two additional limitations are the failure of all carbohydrates to cause equivalent hyperglycemia and the failure to recognize the importance of fat both on the overall need for insulin (temporary induction of insulin resistance) and the timing of the appearance of meal hyperglycemia which is variably delayed (largely due to different fat content).
5.4. Dual-hormone therapies
As already discussed, the autoimmune destruction of the β-cells in type 1 diabetes causes abnormalities of the endocrine pancreas that are more complex than a simple lack of insulin. These include an insulin/glucagon imbalance in the portal circulation and defects in glucagon counterregulation. In view of these defects typical for the β-cell deficient state, an insulin therapy alone is unlikely to achieve optimum stability and safety. A dual hormone strategy in which in addition to insulin a second hormone is used to manipulate the pancreatic-liver system and ultimately the HGO may be needed. How to achieve this is a matter of debate, but modulating the glucagon axes is a strategy that is worth exploring. Unger and colleagues have found that treatment of an insulin deficient animal model of uncontrolled T1DM with α-cell inhibition (leptin analog or leptin overexpression), reverses most of the glycemic instability even without provision of insulin34. Similarly, knockout of the glucagon receptors in an animal model also seems to stabilize insulin deficient diabetes33. Such studies indicate that suppression of glucagon secretion and/or action may be a critical intervention to stabilize insulin deficient diabetes although no human data is yet available. In contrast, El-Khatib and colleagues14, recognizing the at-times-deficient glucagon secretion in T1DM, have attempted to stabilize glucose control in closed-loop AP studies using mini-doses of glucagon in addition to insulin, which shows some signs of success.
Theoretically, these two seemingly discrepant approaches can both achieve improvement in the glucagon/insulin portal vein ratio and correct the impaired glucagon dynamics during low glucose levels. Notably, both strategies require a precise dynamic adaptation of glucagon (or its inhibitor) and insulin to changes in glycemia which may be achieved by utilization of the capabilities of the emerging AP technologies. There is an ongoing debate which strategy is more effective and as argued recently69 based on in silico70, 71 and in vivo72, 73 studies, inhibition of glucagon may provide several advantages over infusion of glucagon and is therefore the authors’ preference. The advantages of suppressing glucagon include69: (i) partial restoration of the abnormal balance between insulin and glucagon; (ii) preserving the liver glycogen stores; (iii) reducing the amount of insulin necessary to control the system; (iv) using endogenous rather than exogenous glucagon as hypoglycemia protection and potential repair of the defective glucagon counterregulation; (v) more robust control in the context of an artificial pancreas system. The most intriguing advantage from this list is the inferred possibility that certain glucagon suppressors can restore the defective glucagon counterregulation in type 1 diabetes. This concept is based on a series of experimental73, clinical72 and in silico70, 71 studies by the authors which suggest that glucagon counterregulation in response to hypoglycemia is feedback controlled. Its defects appear to be network related, linked to miscommunication between the different cell types in the pancreatic islets resulting from lack of β-cells in type 1 diabetes. The defects can potentially be repaired to enhance glucagon counterregulation by using α-cell inhibitors either by reduction of basal hyperglucagonemia or by suppression of glucagon under normal conditions accompanied by release of this suppression during hypoglycemia to trigger a rebound glucagon response69.
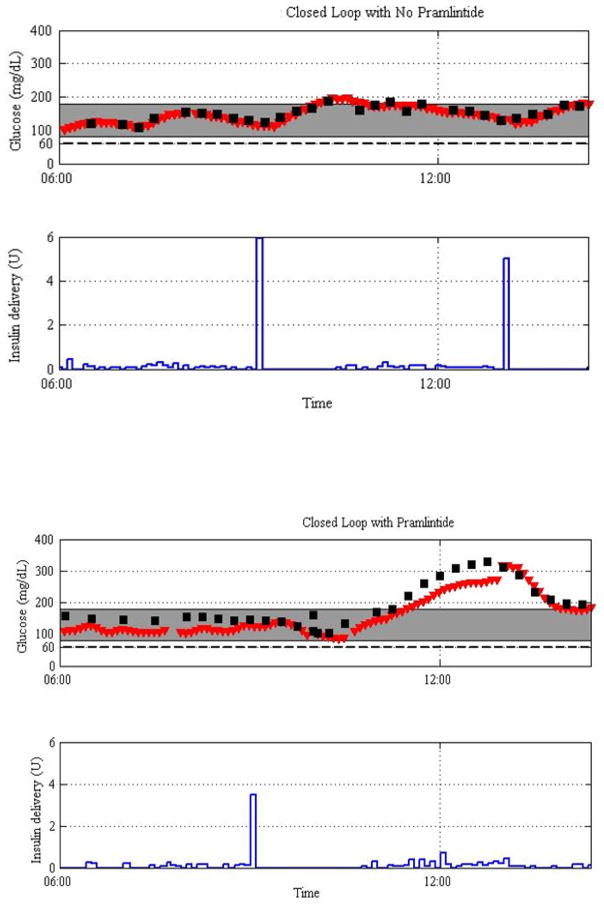
Currently, there are several FDA-approved drugs in the US that could be, at least in theory, used to manipulate the glucagon axis in type 1 diabetes. Those include various amylin analogs, GLP-1 analogs, and DPP-4 inhibitors. These drugs have been successful in maintaining more normal blood glucose concentrations by significantly improving the postprandial glucose excursions (which are exaggerated partly to due inappropriate hyperglucagonemia) in patients with type 2 diabetes when added to their mealtime insulin. However, in type 1 there are two key problems that should be addressed when using these drugs to complement the traditional insulin therapy. First, if α-cell inhibitors are constantly delivered (not only at meal time) a modification (reduction) of the basal insulin delivery strategy will be needed to account for the reduced HGO. And second, most of the glucagon-suppressing drugs delay the gastric emptying which in turn requires a modification of the timing and pattern of delivery of meal insulin to avoid hypoglycemia occurring before the carbohydrates from the meal even begin to appear in the bloodstream. These issues are illustrated by data from one subject collected during an artificial pancreas closed-loop study recently performed by the authors: Figure 4. The experiment included the use of the amylin analog pramlintide (top) or saline (bottom) given together with an insulin bolus at the time of the meal. What one can observe from these graphic depictions of glycemia is that in the absence of pramlintide a usual insulin bolus is not enough to control post meal hyperglycemia effectively. With the addition of pramlintide, it is obvious that a different set of problems are encountered. First, although there is as expected a marked prevention of and some reduction of early post meal hyperglycemia, there is an increased risk of hypoglycemia after the meal. This is a known potential limitation. The second issue is that as a result of the need to reduce the dose of insulin to minimize early post meal hypoglycemia, the other consequences of the combined pramlintide strategy is that later, after the meal, marked post meal hyperglycemia emerges when using a regular albeit somewhat reduced bolus amount. The cause of this set of challenges is clearly related to the temporary delay in the appearance of meal hyperglycemia presumably mostly due to delayed gastric emptying. Moreover, the precise timing of this delay appears highly individual, so that altering the timing of the bolus or simply extending the bolus is likely not sufficient to rectify these limitations of α-cell inhibiting medications. We have posited that what is needed is a flexible adaptive meal insulin strategy that will permit both safe early post meal hyperglycemia control and also sufficient targeted control of late post meal hyperglycemia. This is going to be an important challenge of use of α-cell inhibition strategies in the artificial pancreas setting.
Figure 4.
Glucose and insulin dynamics during a closed loop glucose control study in one subject with type 1 diabetes. The graphs show the glucose dynamics and insulin pump rates without (top two panels) and with standard pramlintide therapy (bottom two panels).
6. Challenges faced by insulin delivery technologies
While recent advances in diabetes technology show great promise, there are many challenges to be addressed before these advances become more universally accepted by patients and medical care professionals. For example, with the current stage of the technology not all type 1 patients are suitable candidates for a pump and/or a CGM therapy. Patients should be motivated and committed since the use of the technology will require more rather than less work. They should be capable of learning carbohydrate counting and being able to demonstrate that mastery prior to embarking on use of these therapeutic tools. They must be willing to keep good records and do frequent monitoring or CGM calibration. It is not appropriate to “coast” or go on “autopilot” (i.e., insufficient monitoring and dose adjustment) with a pump because it can be dangerous. It is therefore unwise to “sell” a patient on an insulin pump of a glucose sensor if they are uncomfortable with the idea or don’t themselves see it as an advantage. Patients also need good dexterity, visual acuity, and their hearing should be acute enough to hear the device alarms. The use of pumps and sensors requires knowledge and technical skills and most patients will need to attend classes and individual instruction on how to use these devices. Therefore, people with physical, intellectual or other limitations may not be good candidates. There is also a significant financial commitment for pump or CGM use and a need to be comfortable with the technology of such machines. Another challenge is the “body image” issue - many people are concerned about an external device of considerable size attached to the body and find that it makes them uncomfortable even to contemplate participating in the therapy. Finally, and in some ways most importantly, to be able to take full advantage of this technology the patient must commit to communicate information about blood sugar levels and the factors that influence them in an honest and open fashion to the diabetes educators and doctors they work with.
7. Conclusion
Since the discovery of insulin the primary objective of the therapies of type 1 diabetes has been to use exogenous insulin to maintain almost normal glycemic levels without marked hyperglycemia or severe hypoglycemia. Traditionally, this objective has been targeted by the development of various insulin preparations and devices which either measure blood glucose levels or deliver insulin in an attempt to replicate the function of the healthy pancreas. This has been an ongoing task for many years and the ideal routes and regimens of the delivery of the insulin preparations have yet to be discovered.
Contemporary insulin therapies for type 1 diabetes face difficulties in mimicking key properties of the healthy pancreas - its ability to detect almost immediately changes in circulating glucose and to react instantaneously by (reciprocal) changes in insulin and glucagon. Many different insulin preparations have been developed to address these challenges. As a result, basal bolus therapy with modern analog insulins is far superior in safety and more convenient for most patients than earlier insulin therapies such as NPH and regular insulin. Basal bolus therapy is however still limited for the physically active person with type 1 diabetes due to the risk of hypoglycemia particularly from basal insulin and the sometimes multiple meal insulin adjustments that are needed. Moreover, the standard strategies for dosing and timing insulin boluses whether with basal bolus therapy by use of analog injections or even with insulin pumps still remain limited in determining how best to give meal insulin. There is more flexibility with insulin pumps but many people with type 1 diabetes struggle with the meal and exercise induced variability. However, even with the many advances in insulin formulations, glucose measuring techniques and insulin delivery devices patients with type 1 diabetes still face significant challenges, one of which is the inability of most of the patients to react with precision and to control their pump dynamically in concert with the CGM readings. The sophistication and dedication needed to succeed with this never ending task should lead us to admire those who manage to thrive despite the difficulties. It should also lead us to go back to the drawing board to find ways to help those who continue the struggle to prevent diabetes complications with further advances in technology including the artificial pancreas in its many emerging forms.
The most recent insulin therapies based on AP technology not only take full advantage of the advances in CGM and insulin pump devices, but also add a new component that uses the information from the CGM sensor to automatically control the pump. Thereby, the AP technology attempts to overcome one of the key limitations of the traditional insulin therapy - the impossibility to constantly monitor the glucose levels, interpret the information, and act by precisely adjusting the pump settings.
Even with the new emerging promising AP technology, insulin alone may be insufficient to achieve optimum stability and safety in type 1 patients due to many unaddressed abnormalities of the endocrine pancreas, like for example the imbalance between insulin and glucagon in the portal circulation and the defects in counterregulation. A dual hormone strategy in which in addition to insulin a second hormone is used to manipulate the hepatic glucose output may be needed. Given the many already existing and approved glucagon suppressing drugs, modulating the glucagon axes is a strategy worth exploring. However, successful implementation of the glucagon inhibiting drugs as adjunct to the insulin therapy requires addressing the issues related to the side effects of these drugs like the delay in gastric emptying that necessitates changes in the meal bolus insulin delivery. Resolution of these problems could lead to the development of an artificial pancreas technology that accommodates a multi-hormone therapy for type 1 diabetes that will be safer and more efficient than the mono-hormone strategy.
Acknowledgments
Funding: NIH/NIDDK grant R01 DK082805
Footnotes
Conflicts of interest: none
References
- 1.Bliss M. The Discovery of Insulin. Chicago, IL: University of Chicago Press; 1982. [Google Scholar]
- 2.Tibaldi JM. Evolution of insulin development: focus on key parameters. Adv Ther. 2012;29(7):590–619. doi: 10.1007/s12325-012-0034-8. [DOI] [PubMed] [Google Scholar]
- 3.Gualandi-Signorini AM, Giorgi G. Insulin formulations – a review. Eur Rev Med Pharmacol Sci. 2001;5:73–83. [PubMed] [Google Scholar]
- 4.Dimitriadis G, Gerich J. Importance of timing of preprandial subcutaneous insulin administration in the management of diabetes mellitus. Diabetes Care. 1983;6:374–7. doi: 10.2337/diacare.6.4.374. [DOI] [PubMed] [Google Scholar]
- 5.Little RR, Rohlfing CL. The long and winding road to optimal HbA1c measurement. Clin Chim Acta. 2013;418C:63–71. doi: 10.1016/j.cca.2012.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins T. HbA(1c)--an analyte of increasing importance. Clin Biochem. 2012;45(13–14):1038–45. doi: 10.1016/j.clinbiochem.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 7.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 8.Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–17. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 9.United Kingdom Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 10.McCall AL. Insulin therapy and hypoglycemia. Endocrinol Metab Clin North Am. 2012;41(1):57–87. doi: 10.1016/j.ecl.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirsch IB. Insulin analogues. N Engl J Med. 2005;352(2):174–83. doi: 10.1056/NEJMra040832. [DOI] [PubMed] [Google Scholar]
- 12.Didangelos T, Iliadis F. Insulin pump therapy in adults. Diabetes Res Clin Pract. 2011;93(Suppl 1):S109–13. doi: 10.1016/S0168-8227(11)70025-0. [DOI] [PubMed] [Google Scholar]
- 13.Shetty G, Wolpert H. Insulin pump use in adults with type 1 diabetes--practical issues. Diabetes Technol Ther. 2010;12(Suppl 1):S11–6. doi: 10.1089/dia.2010.0002. [DOI] [PubMed] [Google Scholar]
- 14.El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med. 2010;2(27):27ra27. doi: 10.1126/scitranslmed.3000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kowalski A, Lum JW. Juvenile Diabetes Research Foundation Artificial Pancreas Consortium Update. J Diab Sci Tech. 2009;3(5):1224–6. doi: 10.1177/193229680900300531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haidar A, Legault L, Dallaire M, Alkhateeb A, Coriati A, Messier V, et al. Glucose-responsive insulin and glucagon delivery (dual-hormone artificial pancreas) in adults with type 1 diabetes: a randomized crossover controlled trial. CMAJ. 2013 doi: 10.1503/cmaj.121265. Available from: http://www.cmaj.ca/content/early/2013/01/28/cmaj.121265.long. [DOI] [PMC free article] [PubMed]
- 17.Bequette BW. Challenges and Recent Progress in the Development of a Closed-loop Artificial Pancreas. Annu Rev Control. 2012;36(2):255–66. doi: 10.1016/j.arcontrol.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howey DC, Bowsher RR, Brunelle RL, Woodworth JR. [Lys(B28), Pro(B29)]-human insulin. A rapidly absorbed analogue of human insulin. Diabetes. 1994;43(3):396–402. doi: 10.2337/diab.43.3.396. [DOI] [PubMed] [Google Scholar]
- 19.Birkeland KI, Home PD, Wendisch U, Ratner RE, Johansen T, Endahl LA, et al. Insulin degludec in type 1 diabetes: a randomized controlled trial of a new-generation ultra-long-acting insulin compared with insulin glargine. Diabetes Care. 2011;34 (3):661–5. doi: 10.2337/dc10-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riddle MC, Rosenstock J, Gerich J. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080–6. doi: 10.2337/diacare.26.11.3080. [DOI] [PubMed] [Google Scholar]
- 21.Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care. 2006;29(6):1269–74. doi: 10.2337/dc05-1365. [DOI] [PubMed] [Google Scholar]
- 22.Segel SA, Paramore DS, Cryer PE. Hypoglycemia-associated autonomic failure in advanced type 2 diabetes. Diabetes. 2002;51:724–33. doi: 10.2337/diabetes.51.3.724. [DOI] [PubMed] [Google Scholar]
- 23.Dagogo-Jack SE, Craft S, Cryer PE. Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. J Clin Invest. 1993;91:819–28. doi: 10.1172/JCI116302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salzman R, Manson JE, Griffing GT, Kimmerle R, Ruderman N, McCall AL, et al. Intranasal aerosolized insulin. Mixed-meal studies and long-term use in type I diabetes. N Engl J Med. 1985;312(17):1078–84. doi: 10.1056/NEJM198504253121702. [DOI] [PubMed] [Google Scholar]
- 25.Royle P, Waugh N, Deakin M, Philip S. WITHDRAWN: Inhaled insulin in diabetes mellitus. Cochrane Database Syst Rev. 2009;1:CD003890. doi: 10.1002/14651858.CD003890.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wredling R, Liu D, Lins PE, Adamson U. Variation of insulin absorption during subcutaneous and peritoneal infusion in insulin dependent diabetic patients with unsatisfactory long-term glycaemic response to continuous subcutaneous insulin infusion. Diabetes Metab. 1991;17:456–9. [PubMed] [Google Scholar]
- 27.Micossi P, Cristallo M, Librenti MC, Petrella G, Galimberti G, Melandri M, et al. Free-insulin profiles after intraperitoneal intramuscular, subcutaneous insulin administration. Diabetes Care. 1986;9:575–8. doi: 10.2337/diacare.9.6.575. [DOI] [PubMed] [Google Scholar]
- 28.Nathan DM, Dunn FL, Bruch J, McKitrick C, Larkin M, Haggan C, et al. Postprandial insulin profiles with implantable pump therapy may explain decreased frequency of severe hypoglycaemia, compared with intensive subcutaneous regimens, in insulin-dependent diabetes mellitus patients. Am J Med. 1996;100:412–7. doi: 10.1016/S0002-9343(97)89516-2. [DOI] [PubMed] [Google Scholar]
- 29.Giacca A, Caumo A, Galimberti G, Petrella G, Librenti MC, Scavini M, et al. Peritoneal and subcutaneous absorption of insulin in type I diabetic subjects. J Clin Endocrinol Metab. 1993;77:738–42. doi: 10.1210/jcem.77.3.8370695. [DOI] [PubMed] [Google Scholar]
- 30.Wan CK, Giacca A, Matsuhisa M, El-Bahrani B, Lam L, Rodgers C, et al. Increased responses of glucagon and glucose production to hypoglycemia with intraperitoneal versus subcutaneous insulin treatment. Metabolism. 2000;49:984–9. doi: 10.1053/meta.2000.7727. [DOI] [PubMed] [Google Scholar]
- 31.Logtenberg SJ, Kleefstra N, Houweling ST, Groenier KH, Gans RO, van Ballegooie E, et al. Improved glycemic control with intraperitoneal versus subcutaneous insulin in type 1 diabetes: a randomized controlled trial. Diabetes Care. 2009;32(8):1372–7. doi: 10.2337/dc08-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Unger RH, Orci L. Paracrinology of islets and the paracrinopathy of diabetes. Proc Natl Acad Sci USA. 2010;107(37):16009–12. doi: 10.1073/pnas.1006639107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee Y, Wang MY, Du XQ, Charron MJ, Unger RH. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes. 2011;60(2):391–397. doi: 10.2337/db10-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang MY, Chen L, Clark GO, Lee Y, Stevens RD, Ilkayeva OR, Wenner BR, Bain JR, Charron MJ, Newgard CB, Unger RH. Leptin therapy in insulin-deficient type I diabetes. Proc Natl Acad Sci USA. 2010;107(11):4813–9. doi: 10.1073/pnas.0909422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cryer PE. Hypoglycemia is the limiting factor in the management of diabetes. Diabetes Metab Res Rev. 1999;15:42–6. doi: 10.1002/(sici)1520-7560(199901/02)15:1<42::aid-dmrr1>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 36.Cryer PE. Hypoglycemia the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia. 2002;45:937–48. doi: 10.1007/s00125-002-0822-9. [DOI] [PubMed] [Google Scholar]
- 37.Ramnanan CJ, Edgerton DS, Kraft G, Cherrington AD. Physiologic action of glucagon on liver glucose metabolism. Diabetes Obes Metab. 2011;13(Suppl 1):118–25. doi: 10.1111/j.1463-1326.2011.01454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruedy KJ, Tamborlane WV Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. The landmark JDRF continuous glucose monitoring randomized trials: a look back at the accumulated evidence. J Cardiovasc Transl Res. 2012;5(4):380–7. doi: 10.1007/s12265-012-9364-9. [DOI] [PubMed] [Google Scholar]
- 39.McGarraugh G, Bergenstal R. Detection of hypoglycemia with continuous interstitial and traditional blood glucose monitoring using the FreeStyle Navigator Continuous Glucose Monitoring System. Diabetes Technol Ther. 2009;11(3):145–50. doi: 10.1089/dia.2008.0047. [DOI] [PubMed] [Google Scholar]
- 40.Miller KM, Beck RW, Bergenstal RM, Goland RS, Haller MJ, McGill JB, et al. Evidence of a Strong Association Between Frequency of Self-Monitoring of Blood Glucose and Hemoglobin A1C Levels in T1D Exchange Clinic Registry Participants. Diabetes Care. 2013 doi: 10.2337/dc12-1770. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vazeou A. Continuous blood glucose monitoring in diabetes treatment. Diabetes Research and Clinical Practice. 2011;93(Suppl 1):S125–30. doi: 10.1016/S0168-8227(11)70028-6. [DOI] [PubMed] [Google Scholar]
- 42.Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, Dubose SN, Hall CA. T1D Exchange Clinic Network: The T1D Exchange Clinic Registry. J Clin Endocrinol Metab. 2012;97:4383–9. doi: 10.1210/jc.2012-1561. [DOI] [PubMed] [Google Scholar]
- 43.Bergenstal RM, Ahmann AJ, Bailey T, Beck RW, Bissen J, Buckingham B, et al. Recommendations for Standardizing Glucose Reporting and Analysis to Optimize Clinical Decision Making in Diabetes: The Ambulatory Glucose Pro le (AGP) Diabetes Technol Ther. 2013;15(3) doi: 10.1089/dia.2013.0051. [DOI] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention. Mean/Median A1c Among Adults with Diagnosed Diabetes, United States, 1988–1994 to 1999–2006. Available from: http://www.cdc.gov/diabetes/statistics/a1c/A1c_Mean_Median.htm.
- 45.McCall AL, Kovatchev BP. The median is not the only message: a clinician’s perspective on mathematical analysis of glycemic variability and modeling in diabetes mellitus. J Diabetes Sci Technol. 2009;3(1):3–11. doi: 10.1177/193229680900300102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frier BM. How hypoglycaemia can affect the life of a person with diabetes. Diabetes Metab Res Rev. 2007;24:87–92. doi: 10.1002/dmrr.796. [DOI] [PubMed] [Google Scholar]
- 47.Clarke WL, Cox DJ, Gonder-Frederick L, Julian D, Kovatchev B, Young-Hyman D. Biopsychobehavioral model of risk of severe hypoglycemia. Self-management behaviors. Diabetes Care. 1999;22(4):580–4. doi: 10.2337/diacare.22.4.580. [DOI] [PubMed] [Google Scholar]
- 48.Kovatchev BP, Cox DJ, Farhy LS, Straume M, Gonder-Frederick L, Clarke WL. Episodes of severe hypoglycemia in type 1 diabetes are preceded and followed within 48 hours by measurable disturbances in blood glucose. J Clin Endocrinol Metab. 2000;85(11):4287–92. doi: 10.1210/jcem.85.11.6999. [DOI] [PubMed] [Google Scholar]
- 49.Cox D, Gonder-Frederick L, McCall A, Kovatchev B, Clarke W. The effects of glucose fluctuation on cognitive function and QOL: the functional costs of hypoglycaemia and hyperglycaemia among adults with type 1 or type 2 diabetes. Int J Clin Pract Suppl. 2002;129:20–6. [PubMed] [Google Scholar]
- 50.Clemens AH, Chang PH, Myers RW. The development of Biostator, a Glucose Controlled Insulin Infusion System (GCIIS) Horm Metab Res. 1977;(Suppl 7):23–33. [PubMed] [Google Scholar]
- 51.Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes. 2006;55(12):3344–50. doi: 10.2337/db06-0419. [DOI] [PubMed] [Google Scholar]
- 52.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31(5):934–9. doi: 10.2337/dc07-1967. [DOI] [PubMed] [Google Scholar]
- 53.Parker RS, Doyle FJ, 3rd, Peppas NA. A model-based algorithm for blood glucose control in Type I diabetic patients. IEEE Trans Biomed Eng. 1999;48(2):148–57. doi: 10.1109/10.740877. [DOI] [PubMed] [Google Scholar]
- 54.Hovorka R, Canonico V, Chassin LJ, Haueter U, Massi-Benedetti M, Orsini Federici M, et al. Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol Meas. 2004;25(4):905–20. doi: 10.1088/0967-3334/25/4/010. [DOI] [PubMed] [Google Scholar]
- 55.Dua P, Doyle FJ, 3rd, Pistikopoulos EN. Model-based blood glucose control for type 1 diabetes via parametric programming. IEEE Trans Biomed Eng. 2006;53(8):1478–91. doi: 10.1109/TBME.2006.878075. [DOI] [PubMed] [Google Scholar]
- 56.Magni L, Raimondo F, Bossi L, Dalla Man C, De Nicolao G, Kovatchev BP, et al. Model predictive control of type 1 diabetes: an in silico trial. J Diabetes Sci Technol. 2007;1(6):804–12. doi: 10.1177/193229680700100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kovatchev B, Patek S, Dassau E, Doyle FJ, 3rd, Magni L, De Nicolao G, et al. Juvenile Diabetes Research Foundation Artificial Pancreas Consortium. Control to range for diabetes: functionality and modular architecture. J Diabetes Sci Technol. 2009;3(5):1058–65. doi: 10.1177/193229680900300509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Renard EM, Farret A, Place J, Cobelli C, Kovatchev BP, Breton MD. Closed-loop insulin delivery using subcutaneous infusion and glucose sensing, and equipped with a dedicated safety supervision algorithm, improves safety of glucose control in type 1 diabetes. Proc. 46th EASD Annual Mtg; Stockholm. 2010. [Google Scholar]
- 59.Buckingham B, et al. Prevention of Nocturnal Hypoglycemia Using Predictive Alarm Algorithms and Insulin Pump Suspension. Diabetes Care. doi: 10.2337/dc09-2303. published ahead of print March 3, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Breton M, Farret A, Bruttomesso D, Anderson S, Magni L, Patek S, et al. Fully integrated artificial pancreas in type 1 diabetes: modular closed-loop glucose control maintains near normoglycemia. Diabetes. 2012;61(9):2230–7. doi: 10.2337/db11-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kovatchev BP, Breton MD, Dalla Man C, Cobelli C. In Silico Preclinical Trials: A Proof of Concept in Closed-Loop Control of Type 1 Diabetes. J Diabetes Sci Technol. 2009;3:44–55. doi: 10.1177/193229680900300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patek SD, Bequette BW, Breton MD, Buckingham BA, Dassau E, Doyle FJ, 3rd, et al. In Silico Preclinical Trials: Methodology and Engineering Guide to Closed-Loop Control in Type 1 Diabetes Mellitus. J Diabetes Sci Technol. 2009;3(2):269–82. doi: 10.1177/193229680900300207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bergman RN, Ider YZ, Boeden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol. 1979;236:E667–77. doi: 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- 64.Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C. Minimal model estimation of glucose absorption and insulin sensitivity from oral test: validation with a tracer method. Am J Physiol Endocrinol Metab. 2004;287:E637–43. doi: 10.1152/ajpendo.00319.2003. [DOI] [PubMed] [Google Scholar]
- 65.Breton MD, Kovatchev BP. Impact of blood glucose self-monitoring errors on glucose variability, risk for hypoglycemia, and average glucose control in type 1 diabetes: an in silico study. J Diabetes Sci Technol. 2010;4(3):562–570. doi: 10.1177/193229681000400309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Breton MD, Clarke WL, Farhy LS, Kovatchev BP. A model of self-treatment behavior, glucose variability, and hypoglycemia-associated autonomic failure in type 1 diabetes. J Diabetes Sci Technol. 2007;1(3):331–7. doi: 10.1901/jaba.2007.1-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harvey RA, Wang Y, Grosman B, Percival MW, Bevier W, Finan DA, et al. Quest for the artificial pancreas: combining technology with treatment. IEEE Eng Med Biol Mag. 2010;29(2):53–62. doi: 10.1109/MEMB.2009.935711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klonoff D. The need for a glycemia modeling comparison workshop to facilitate development of an artificial pancreas. J Diabetes Sci Technol. 2010;4(1):1–3. doi: 10.1177/193229681000400101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Farhy LS, McCall AL. Optimizing reduction in basal hyperglucagonaemia to repair defective glucagon counterregulation in insulin deficiency. Diabetes Obes Metab. 2011;13(Suppl 1):133–43. doi: 10.1111/j.1463-1326.2011.01455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Farhy LS, McCall AL. Models of Glucagon Secretion, Their Application to the Analysis of the Defects in Glucagon Counterregulation and Potential Extension to Approximate Glucagon Action. J Diabetes Sci Technol. 2010;4(6):1345–56. doi: 10.1177/193229681000400608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Farhy LS, McCall AL. System-Level Control to Optimize Glucagon Counterregulation by Switch-Off of α-Cell Suppressing Signals in β-Cell Deficiency. J Diabetes Sci Technol. 2009;3(1):21–33. doi: 10.1177/193229680900300104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Farhy LS, Chan A, Breton MD, Anderson SM, Kovatchev BP, McCall AL. Association of Basal Hyperglucagonemia with Impaired Glucagon Counterregulation in Type 1 Diabetes. Front Physiol. 2012;3:40. doi: 10.3389/fphys.2012.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Farhy LS, Du Z, Zeng Q, Veldhuis PP, Johnson ML, Brayman KL, et al. Amplification of pulsatile glucagon counterregulation by switch-off of α-cell-suppressing signals in streptozotocin-treated rats. Am J Physiol Endocrinol Metab. 2008;295(3):E575–85. doi: 10.1152/ajpendo.90372.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]