Abstract
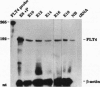
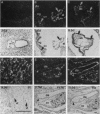
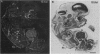
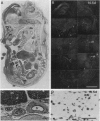
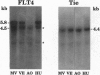
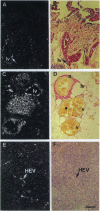
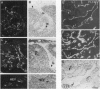
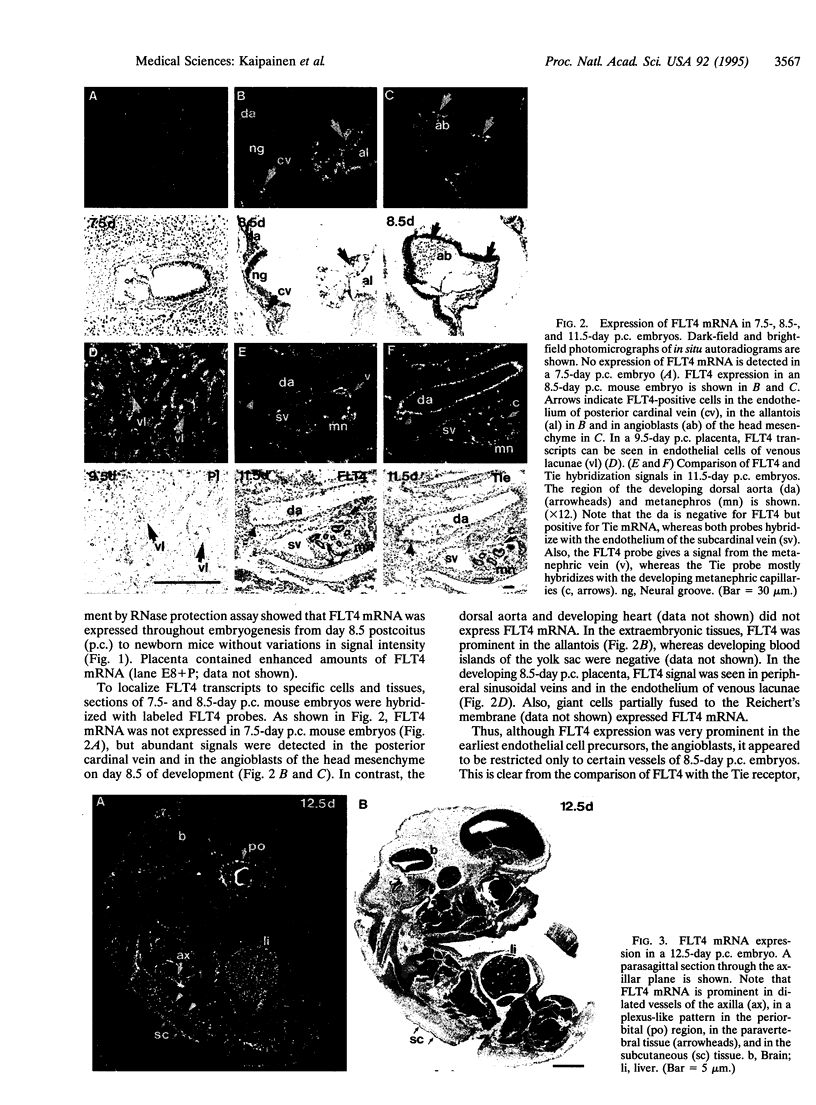
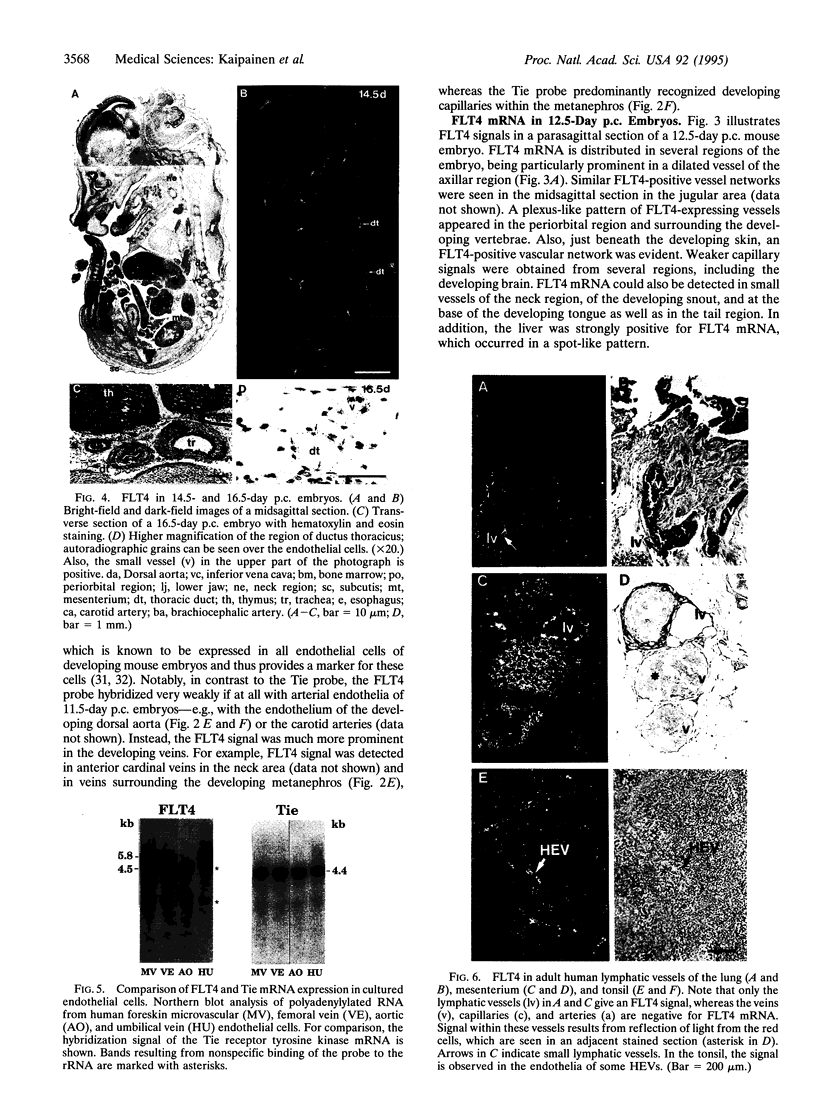
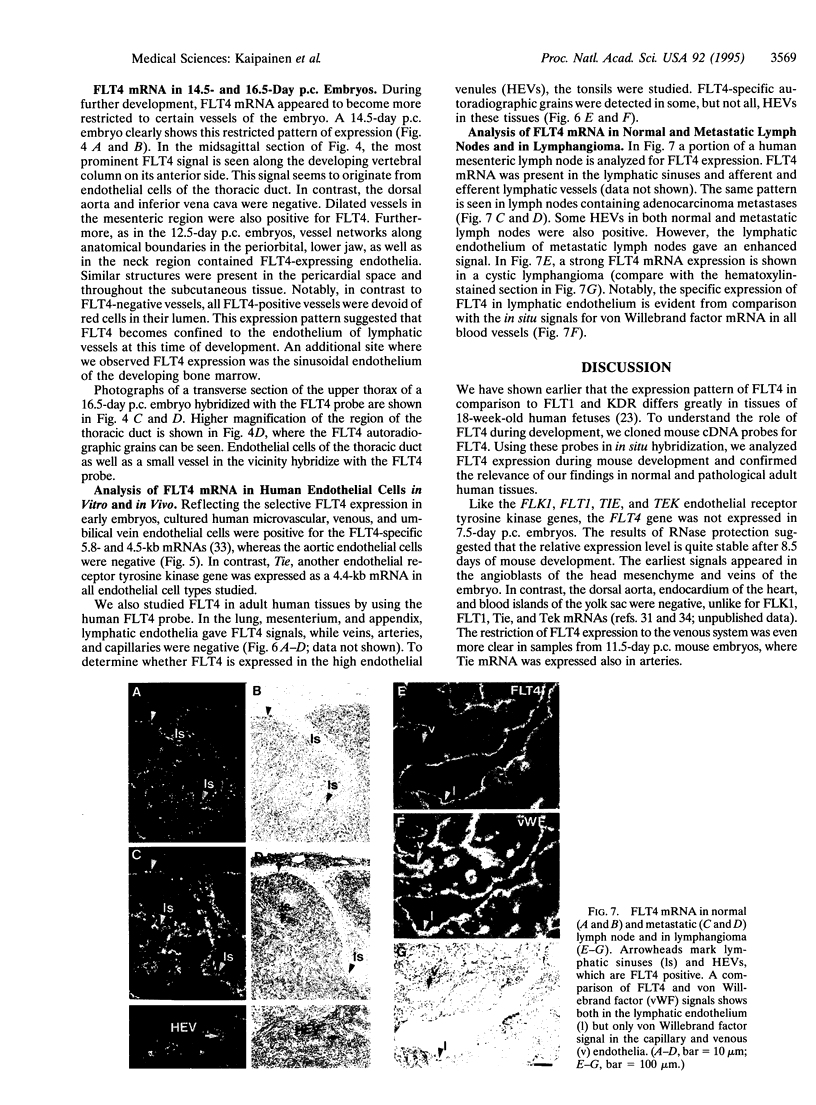
We have recently cloned the human fms-like tyrosine kinase 4 gene FLT4, whose protein product is related to two vascular endothelial growth factor receptors FLT1 and KDR/FLK1. Here the expression of FLT4 has been analyzed by in situ hybridization during mouse embryogenesis and in adult human tissues. The FLT4 mRNA signals first became detectable in the angioblasts of head mesenchyme, the cardinal vein, and extraembryonally in the allantois of 8.5-day postcoitus (p.c.) embryos. In 12.5-day p.c. embryos, the FLT4 signal decorated developing venous and presumptive lymphatic endothelia, but arterial endothelia were negative. During later stages of development, FLT4 mRNA became restricted to vascular plexuses devoid of red cells, representing developing lymphatic vessels. Only the lymphatic endothelia and some high endothelial venules expressed FLT4 mRNA in adult human tissues. Increased expression occurred in lymphatic sinuses in metastatic lymph nodes and in lymphangioma. Our results suggest that FLT4 is a marker for lymphatic vessels and some high endothelial venules in human adult tissues. They also support the theory on the venous origin of lymphatic vessels.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonthron D., Orr E. C., Mitsock L. M., Ginsburg D., Handin R. I., Orkin S. H. Nucleotide sequence of pre-pro-von Willebrand factor cDNA. Nucleic Acids Res. 1986 Sep 11;14(17):7125–7127. doi: 10.1093/nar/14.17.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattoretti G., Becker M. H., Key G., Duchrow M., Schlüter C., Galle J., Gerdes J. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol. 1992 Dec;168(4):357–363. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- Coffin J. D., Poole T. J. Embryonic vascular development: immunohistochemical identification of the origin and subsequent morphogenesis of the major vessel primordia in quail embryos. Development. 1988 Apr;102(4):735–748. doi: 10.1242/dev.102.4.735. [DOI] [PubMed] [Google Scholar]
- Finnerty H., Kelleher K., Morris G. E., Bean K., Merberg D. M., Kriz R., Morris J. C., Sookdeo H., Turner K. J., Wood C. R. Molecular cloning of murine FLT and FLT4. Oncogene. 1993 Aug;8(8):2293–2298. [PubMed] [Google Scholar]
- Flamme I., Risau W. Induction of vasculogenesis and hematopoiesis in vitro. Development. 1992 Oct;116(2):435–439. doi: 10.1242/dev.116.2.435. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Folkman J., Shing Y. Angiogenesis. J Biol Chem. 1992 Jun 5;267(16):10931–10934. [PubMed] [Google Scholar]
- Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol. 1992 Apr;3(2):65–71. [PubMed] [Google Scholar]
- Gonzalez-Crussi F. Vasculogenesis in the chick embryo. An ultrastructural study. Am J Anat. 1971 Apr;130(4):441–460. doi: 10.1002/aja.1001300406. [DOI] [PubMed] [Google Scholar]
- Hobson B., Denekamp J. Endothelial proliferation in tumours and normal tissues: continuous labelling studies. Br J Cancer. 1984 Apr;49(4):405–413. doi: 10.1038/bjc.1984.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaipainen A., Korhonen J., Pajusola K., Aprelikova O., Persico M. G., Terman B. I., Alitalo K. The related FLT4, FLT1, and KDR receptor tyrosine kinases show distinct expression patterns in human fetal endothelial cells. J Exp Med. 1993 Dec 1;178(6):2077–2088. doi: 10.1084/jem.178.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klagsbrun M., D'Amore P. A. Regulators of angiogenesis. Annu Rev Physiol. 1991;53:217–239. doi: 10.1146/annurev.ph.53.030191.001245. [DOI] [PubMed] [Google Scholar]
- Korhonen J., Partanen J., Armstrong E., Vaahtokari A., Elenius K., Jalkanen M., Alitalo K. Enhanced expression of the tie receptor tyrosine kinase in endothelial cells during neovascularization. Blood. 1992 Nov 15;80(10):2548–2555. [PubMed] [Google Scholar]
- Korhonen J., Polvi A., Partanen J., Alitalo K. The mouse tie receptor tyrosine kinase gene: expression during embryonic angiogenesis. Oncogene. 1994 Feb;9(2):395–403. [PubMed] [Google Scholar]
- Millauer B., Wizigmann-Voos S., Schnürch H., Martinez R., Møller N. P., Risau W., Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993 Mar 26;72(6):835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- Noden D. M. Embryonic origins and assembly of blood vessels. Am Rev Respir Dis. 1989 Oct;140(4):1097–1103. doi: 10.1164/ajrccm/140.4.1097. [DOI] [PubMed] [Google Scholar]
- Pajusola K., Aprelikova O., Armstrong E., Morris S., Alitalo K. Two human FLT4 receptor tyrosine kinase isoforms with distinct carboxy terminal tails are produced by alternative processing of primary transcripts. Oncogene. 1993 Nov;8(11):2931–2937. [PubMed] [Google Scholar]
- Pajusola K., Aprelikova O., Korhonen J., Kaipainen A., Pertovaara L., Alitalo R., Alitalo K. FLT4 receptor tyrosine kinase contains seven immunoglobulin-like loops and is expressed in multiple human tissues and cell lines. Cancer Res. 1992 Oct 15;52(20):5738–5743. [PubMed] [Google Scholar]
- Pajusola K., Aprelikova O., Pelicci G., Weich H., Claesson-Welsh L., Alitalo K. Signalling properties of FLT4, a proteolytically processed receptor tyrosine kinase related to two VEGF receptors. Oncogene. 1994 Dec;9(12):3545–3555. [PubMed] [Google Scholar]
- Pardanaud L., Altmann C., Kitos P., Dieterlen-Lievre F., Buck C. A. Vasculogenesis in the early quail blastodisc as studied with a monoclonal antibody recognizing endothelial cells. Development. 1987 Jun;100(2):339–349. doi: 10.1242/dev.100.2.339. [DOI] [PubMed] [Google Scholar]
- Partanen J., Armstrong E., Mäkelä T. P., Korhonen J., Sandberg M., Renkonen R., Knuutila S., Huebner K., Alitalo K. A novel endothelial cell surface receptor tyrosine kinase with extracellular epidermal growth factor homology domains. Mol Cell Biol. 1992 Apr;12(4):1698–1707. doi: 10.1128/mcb.12.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters K., Ornitz D., Werner S., Williams L. Unique expression pattern of the FGF receptor 3 gene during mouse organogenesis. Dev Biol. 1993 Feb;155(2):423–430. doi: 10.1006/dbio.1993.1040. [DOI] [PubMed] [Google Scholar]
- Sato T. N., Qin Y., Kozak C. A., Audus K. L. Tie-1 and tie-2 define another class of putative receptor tyrosine kinase genes expressed in early embryonic vascular system. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9355–9358. doi: 10.1073/pnas.90.20.9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi E., Kan M., Xu J. M., McKeehan W. L. 16-kilodalton heparin binding (fibroblast) growth factor type one appears in a stable 40-kilodalton complex after receptor-dependent internalization. J Biol Chem. 1991 Mar 25;266(9):5774–5779. [PubMed] [Google Scholar]
- Terman B. I., Dougher-Vermazen M., Carrion M. E., Dimitrov D., Armellino D. C., Gospodarowicz D., Böhlen P. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1579–1586. doi: 10.1016/0006-291x(92)90483-2. [DOI] [PubMed] [Google Scholar]
- Tokunaga K., Taniguchi H., Yoda K., Shimizu M., Sakiyama S. Nucleotide sequence of a full-length cDNA for mouse cytoskeletal beta-actin mRNA. Nucleic Acids Res. 1986 Mar 25;14(6):2829–2829. doi: 10.1093/nar/14.6.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hinsbergh V. W., Sprengers E. D., Kooistra T. Effect of thrombin on the production of plasminogen activators and PA inhibitor-1 by human foreskin microvascular endothelial cells. Thromb Haemost. 1987 Apr 7;57(2):148–153. [PubMed] [Google Scholar]
- Zadvinskis D. P., Benson M. T., Kerr H. H., Mancuso A. A., Cacciarelli A. A., Madrazo B. L., Mafee M. F., Dalen K. Congenital malformations of the cervicothoracic lymphatic system: embryology and pathogenesis. Radiographics. 1992 Nov;12(6):1175–1189. doi: 10.1148/radiographics.12.6.1439020. [DOI] [PubMed] [Google Scholar]
- de Vries C., Escobedo J. A., Ueno H., Houck K., Ferrara N., Williams L. T. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992 Feb 21;255(5047):989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- van Hinsbergh V. W., Binnema D., Scheffer M. A., Sprengers E. D., Kooistra T., Rijken D. C. Production of plasminogen activators and inhibitor by serially propagated endothelial cells from adult human blood vessels. Arteriosclerosis. 1987 Jul-Aug;7(4):389–400. doi: 10.1161/01.atv.7.4.389. [DOI] [PubMed] [Google Scholar]
- van der Putte S. C. The development of the lymphatic system in man. Adv Anat Embryol Cell Biol. 1975;51(1):3–60. [PubMed] [Google Scholar]