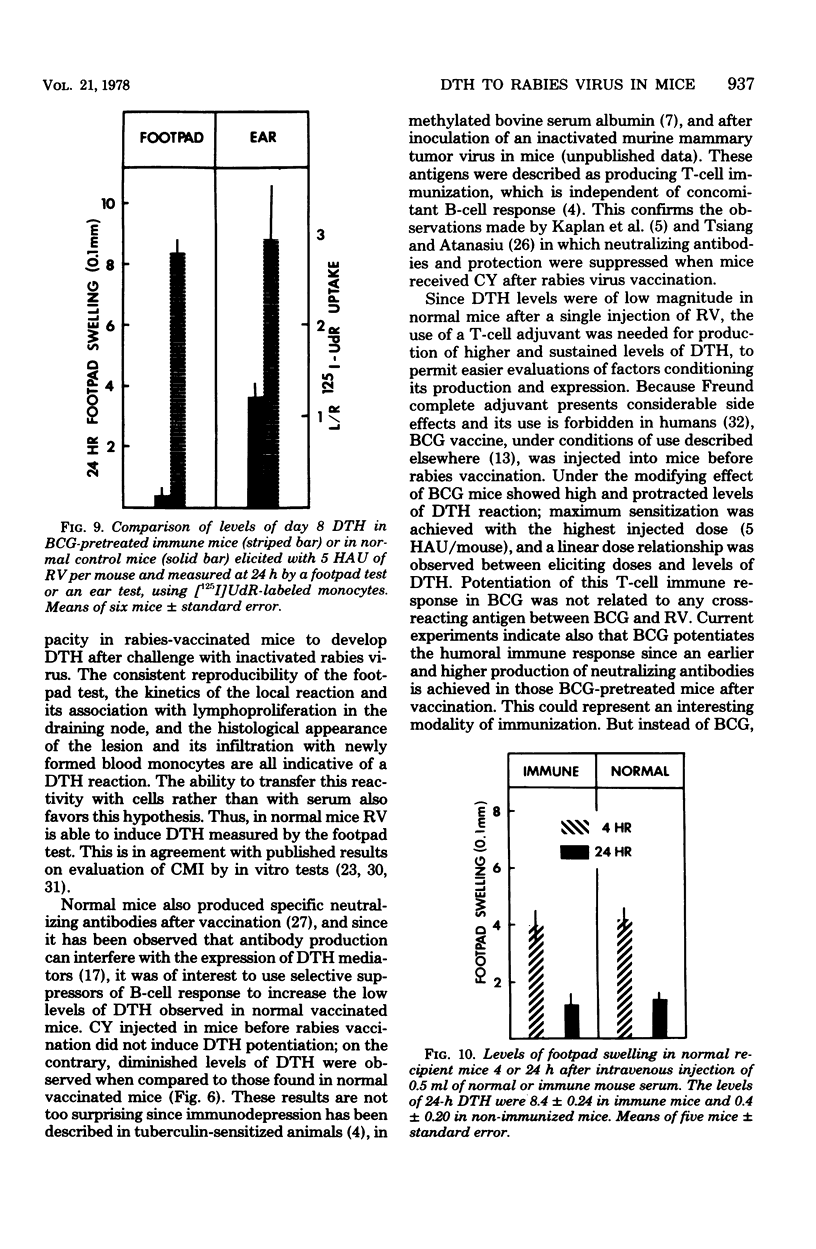
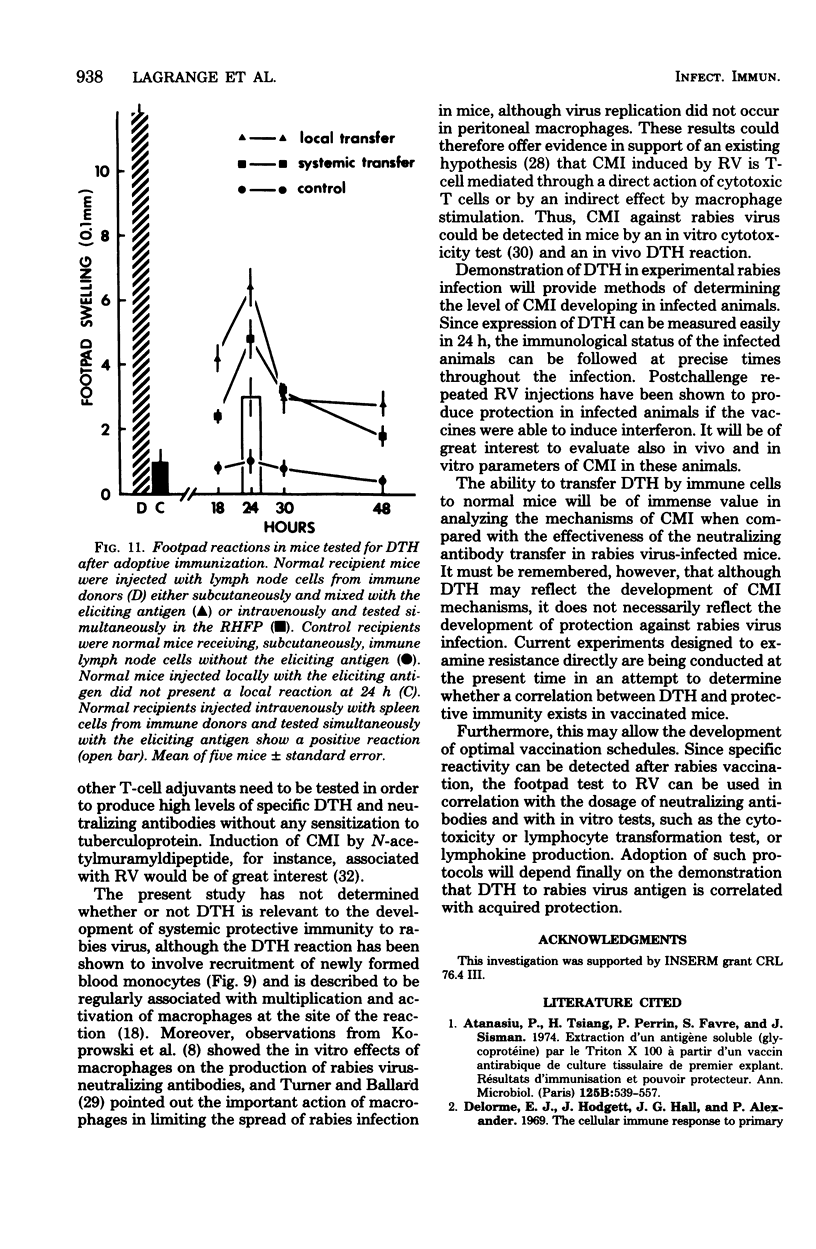
Abstract
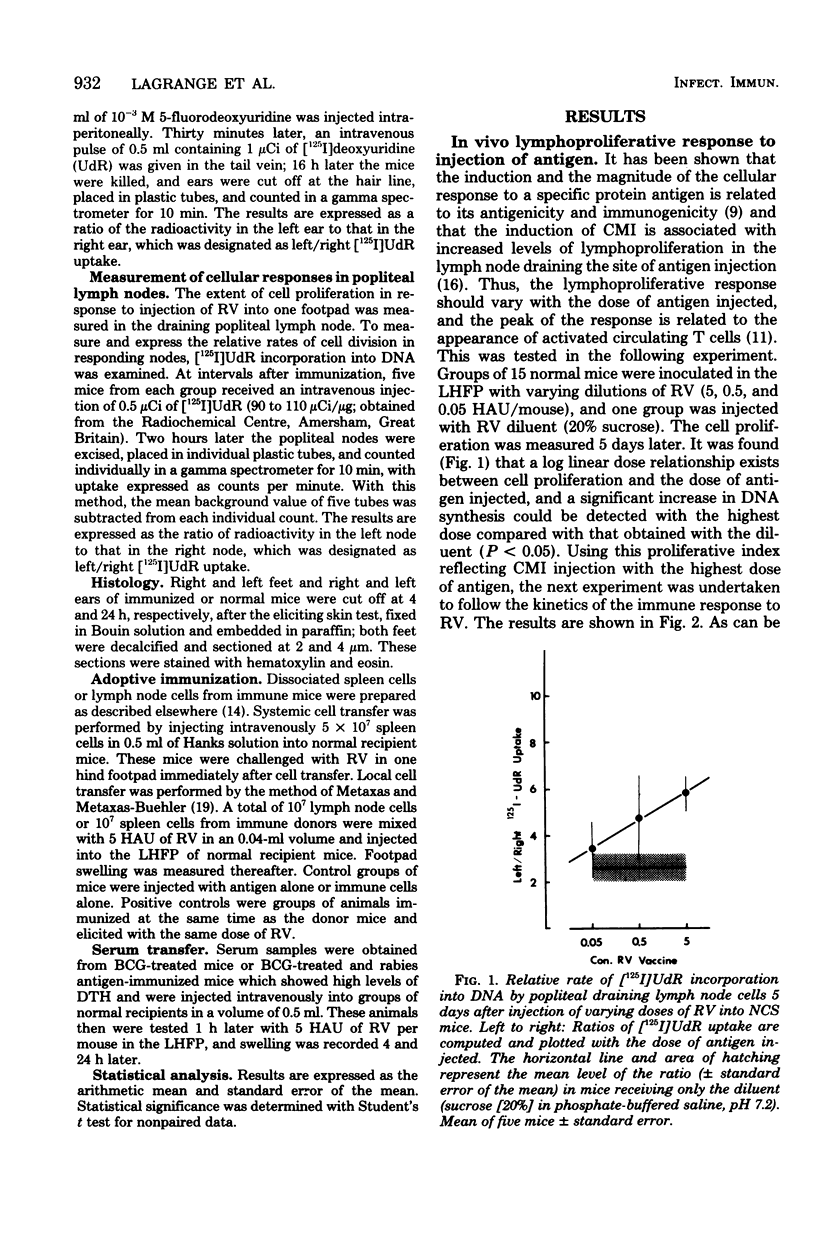
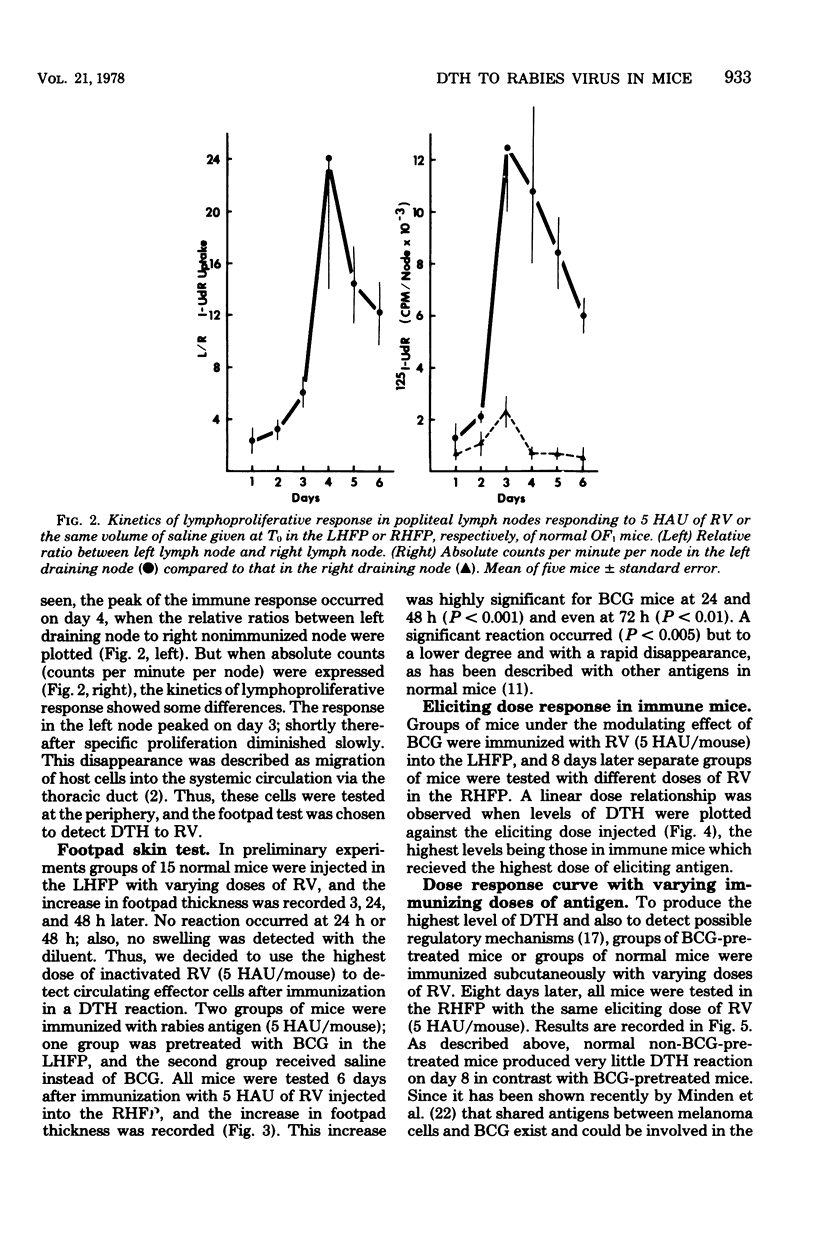
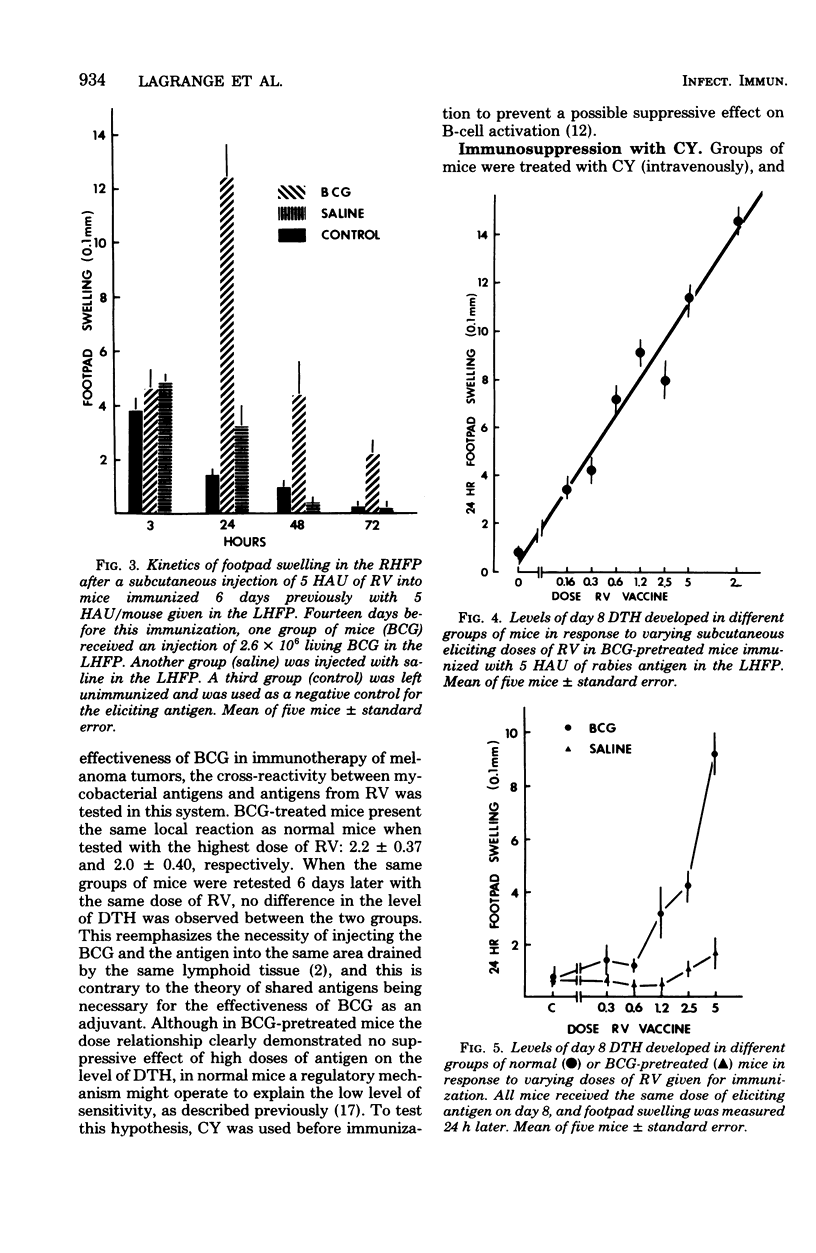
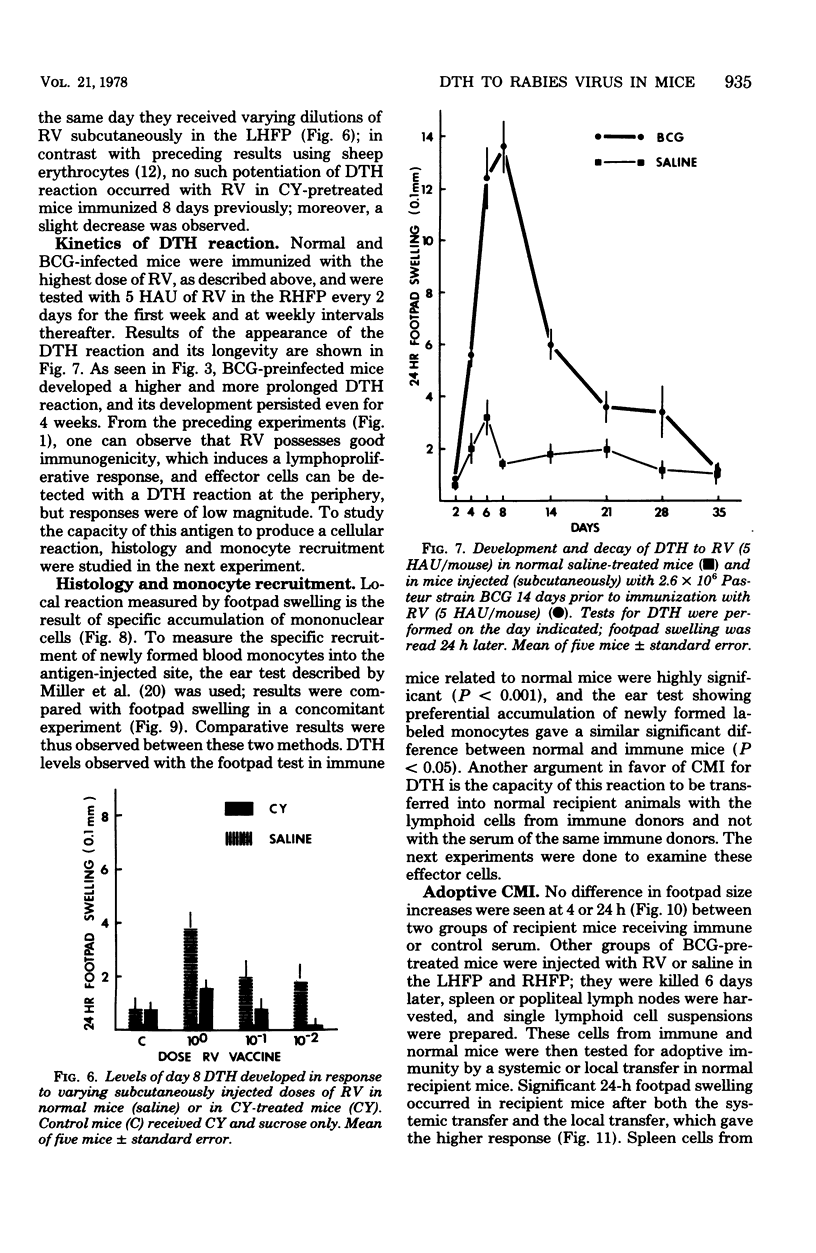
With a purified beta-propiolactone-inactivated rabies virus, a significant increase in footpad swelling was elicited in normal or in BCG-pretreated mice after immunization with varying doses of rabies vaccine. These footpad reactions were shown to peak at 24 h and to be associated with an infiltration of newly formed blood monocytes demonstrated by histology and [125I]deoxyuridine labeling. A relationship between the lymphoproliferation and the degree of sensitization is described, and the susceptibility to cyclophosphamide treatment is also examined. Adoptive transfer of specific reactivity to normal recipient mice with immune lymphoid cells, but not with immune serum, was demonstrated, and the results represent another argument for a cell-mediated immunological mechanism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atanasiu P., Tsiang H., Perrin P., Favre S., Sisman J. Extraction d'un antigène soluble (glycoprotéine) par le Triton X100. A partir d'un vaccin antirabique de culture tissulaire de premier explant. Résultats d'immunisation et pouvoir protecteur. Ann Microbiol (Paris) 1974 Dec;125(4):540–557. [PubMed] [Google Scholar]
- Fleer A., van der Hart M., Blok-Schut B. J., Schellekens P. T. Correlation of PPD and BCG-induced leukocyte migration inhibition, delayed cutaneous hypersensitivity, lymphocyte transformation in vitro and humoral antibodies to PPD in man. Eur J Immunol. 1976 Mar;6(3):163–167. doi: 10.1002/eji.1830060305. [DOI] [PubMed] [Google Scholar]
- Jokipii A. M., Jokipii L. Suppression of cell-mediated immunity by cyclophosphamide: its independence of concomitant B cell response. Cell Immunol. 1973 Dec;9(3):477–481. doi: 10.1016/0008-8749(73)90063-4. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Wiktor T. J., Koprowski H. Pathogenesis of rabies in immunodeficient mice. J Immunol. 1975 Jun;114(6):1761–1765. [PubMed] [Google Scholar]
- Kerckhaert J. A., Van den Berg G. J., Willers J. M. Influence of cyclophosphamide on the delayed hypersensitivity of the mouse. Ann Immunol (Paris) 1974 Mar-Apr;125(3):415–426. [PubMed] [Google Scholar]
- Kerckhaert J. A., van den Berg G. J., Hofhuis F. M. Influence of cyclophosphamide on the delayed hypersensitivity in the mouse after immunization with histocompatibility antigen. J Immunol. 1974 Dec;113(6):1801–1806. [PubMed] [Google Scholar]
- Koprowski H., Mocarelli P., Wiktor T. J. Antibody response in vitro to an animal virus: production of rabies virus neutralizing antibodies by mouse cells in culture. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2433–2436. doi: 10.1073/pnas.69.9.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger J., Gershon R. K. DNA synthetic response of thymocytes to a variety of antigens. J Immunol. 1972 Mar;108(3):581–585. [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B. A stable form of delayed-type hypersensitivity. J Exp Med. 1975 Jan 1;141(1):82–96. doi: 10.1084/jem.141.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B., Miller T. E. Influence of dose and route of antigen injection on the immunological induction of T cells. J Exp Med. 1974 Mar 1;139(3):528–542. doi: 10.1084/jem.139.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B., Miller T. E. Potentiation of T-cell-mediated immunity by selective suppression of antibody formation with cyclophosphamide. J Exp Med. 1974 Jun 1;139(6):1529–1539. doi: 10.1084/jem.139.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- METAXAS M. N., METAXAS-BUEHLER M. Studies on the cellular transfer of tuberculin sensitivity in the guinea pig. J Immunol. 1955 Nov;75(5):333–347. [PubMed] [Google Scholar]
- Mackaness G. B., Auclair D. J., Lagrange P. H. Immunopotentiation with BCG. I. Immune response to different strains and preparations. J Natl Cancer Inst. 1973 Nov;51(5):1655–1667. doi: 10.1093/jnci/51.5.1655. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B., Lagrange P. H., Miller T. E., Ishibashi T. Feedback inhibition of specifically sensitized lymphocytes. J Exp Med. 1974 Mar 1;139(3):543–559. doi: 10.1084/jem.139.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Vadas M. A., Whitelaw A., Gamble J. A radioisotopic method to measure delayed type hypersensitivity in the mouse. II. Cell transfer studies. Int Arch Allergy Appl Immunol. 1975;49(5):693–708. doi: 10.1159/000231450. [DOI] [PubMed] [Google Scholar]
- Miller T. E., Mackaness G. B., Lagrange P. H. Immunopotentiation with BCG. II. Modulation of the response to sheep red blood cells. J Natl Cancer Inst. 1973 Nov;51(5):1669–1676. doi: 10.1093/jnci/51.5.1669. [DOI] [PubMed] [Google Scholar]
- Minden P., Sharpton T. R., McClatchy J. K. Shared antigens between human malignant melanoma cells and Mycobacterium bovis (BCG). J Immunol. 1976 May;116(5):1407–1414. [PubMed] [Google Scholar]
- Nozaki J., Atanasiu P. Etude comparative in vitro des antigènes rabiques et des virus apparentés a la rage, par la technique des lymphocytes sensibilisés. Ann Microbiol (Paris) 1976 Apr;127(3):429–438. [PubMed] [Google Scholar]
- Peters R. L., Donahoe R. M., Kelloff G. J. Assay in the mouse for delayed-type hypersensitivity to murine leukemia virus. J Natl Cancer Inst. 1975 Nov;55(5):1089–1095. doi: 10.1093/jnci/55.5.1089. [DOI] [PubMed] [Google Scholar]
- Thursh D. R., Emeson E. E. Selective DNA synthesis by cells specifically localizing in response to xenogeneic erythrocytes. J Exp Med. 1973 Sep 1;138(3):659–671. doi: 10.1084/jem.138.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiang H., Atanasiu P. Effets de la cyclophosphamide sur l'infection rabique chez la Souris. Protection conférée par la sérothérapie. C R Acad Sci Hebd Seances Acad Sci D. 1975 Sep 29;281(13):957–960. [PubMed] [Google Scholar]
- Turner G. S., Ballard R. Interaction of mouse peritoneal macrophages with fixed rabies virus in vivo and in vitro. J Gen Virol. 1976 Feb;30(2):223–231. doi: 10.1099/0022-1317-30-2-223. [DOI] [PubMed] [Google Scholar]
- Turner G. S. Humoral and cellular immune responses of mice to rabies and smallpox vaccines. Nat New Biol. 1973 Jan 17;241(107):90–92. doi: 10.1038/newbio241090a0. [DOI] [PubMed] [Google Scholar]
- Turner G. S. Thymus dependence of rabies vaccine. J Gen Virol. 1976 Dec;33(3):535–538. doi: 10.1099/0022-1317-33-3-535. [DOI] [PubMed] [Google Scholar]
- Wiktor T. J., Doherty P. C., Koprowski H. In vitro evidence of cell-mediated immunity after exposure of mice to both live and inactivated rabies virus. Proc Natl Acad Sci U S A. 1977 Jan;74(1):334–338. doi: 10.1073/pnas.74.1.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., Kamo I., Koprowski H. In vitro stimulation of rabbit lymphocytes after immunization with live and inactivated rabies vaccines. J Immunol. 1974 Jun;112(6):2013–2019. [PubMed] [Google Scholar]



