Abstract
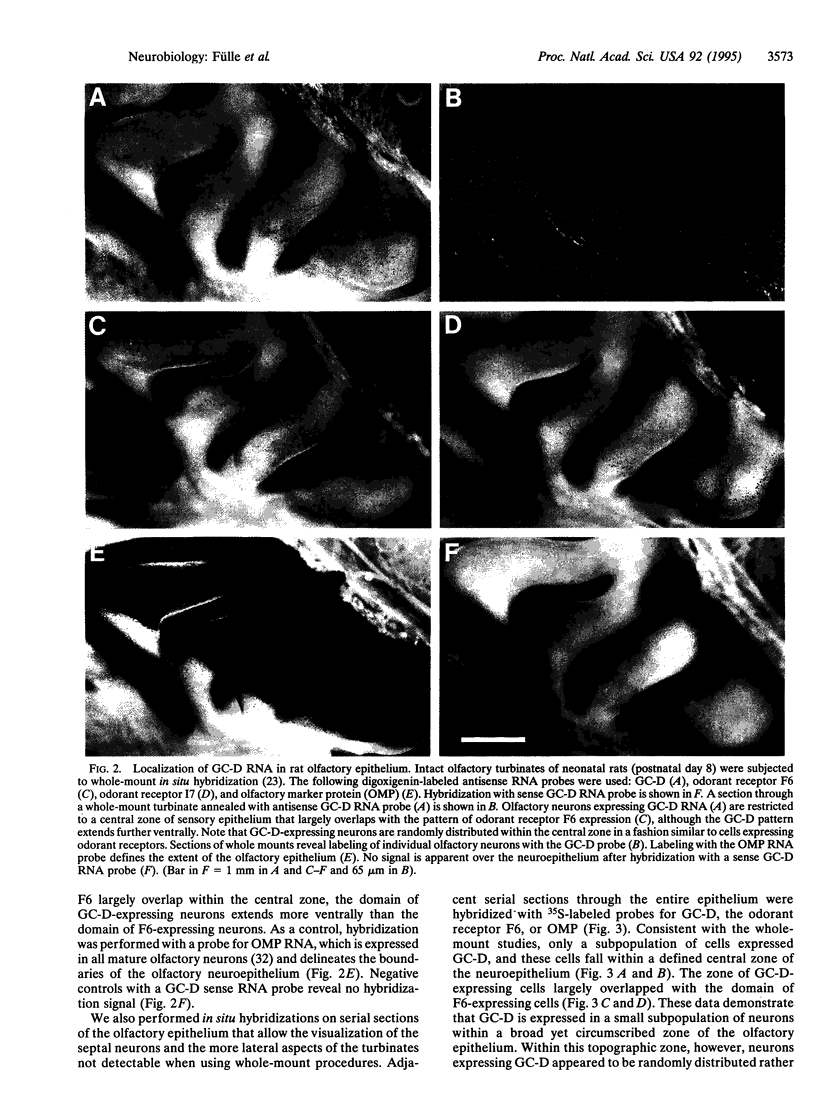
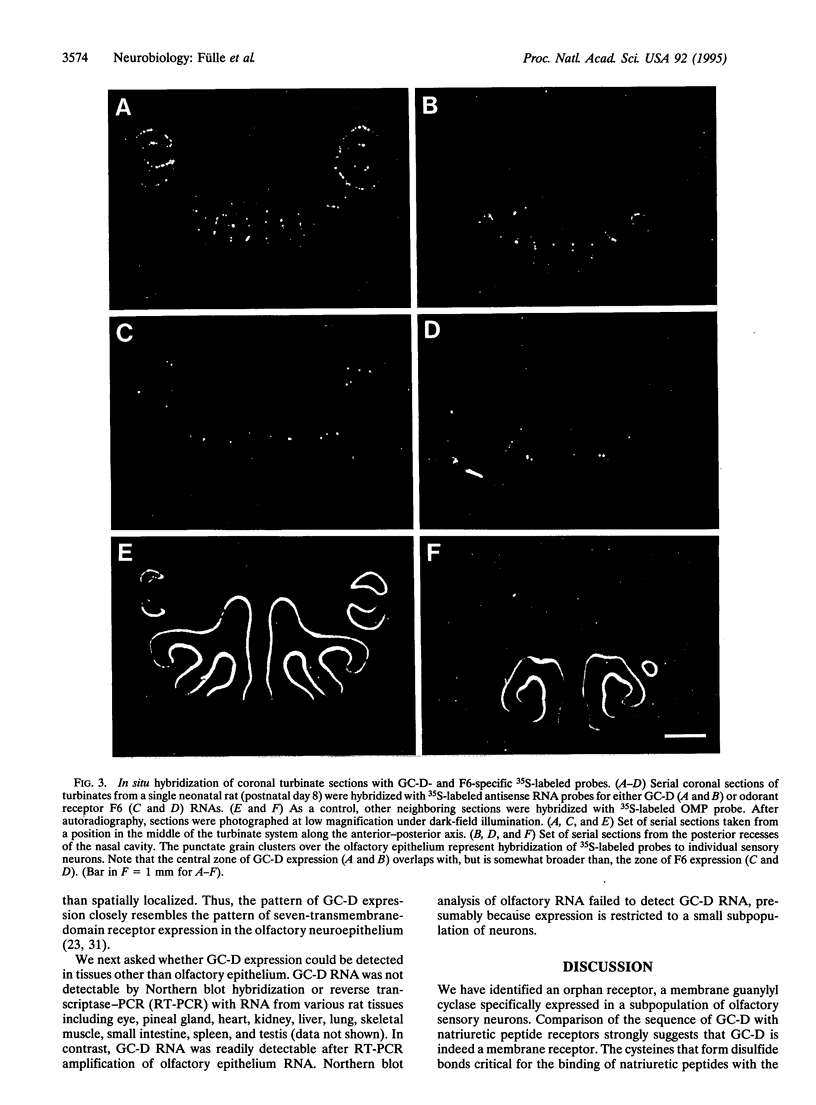
We have cloned an additional member (GC-D) of the membrane receptor guanylyl cyclase [GTP pyrophosphate-lyase (cyclizing), EC 4.6.1.2] family that is specifically expressed in a subpopulation of olfactory sensory neurons. The extracellular, putative ligand-binding domain of the olfactory cyclase is similar in primary structure to two guanylyl cyclases expressed in the retina but diverges considerably from other known guanylyl cyclases. The expression of GC-D RNA is restricted to a small, randomly dispersed population of neurons that is within a single topographic zone in the olfactory neuroepithelium and resembles the pattern of the more diverse seven-transmembrane-domain odorant receptors. These observations suggest that GC-D may function directly in odor recognition or in modulating the sensitivity of a subpopulation of sensory neurons to specific odors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson S., Davis D. L., Dahlbäck H., Jörnvall H., Russell D. W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989 May 15;264(14):8222–8229. [PubMed] [Google Scholar]
- Boekhoff I., Tareilus E., Strotmann J., Breer H. Rapid activation of alternative second messenger pathways in olfactory cilia from rats by different odorants. EMBO J. 1990 Aug;9(8):2453–2458. doi: 10.1002/j.1460-2075.1990.tb07422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breer H., Boekhoff I., Tareilus E. Rapid kinetics of second messenger formation in olfactory transduction. Nature. 1990 May 3;345(6270):65–68. doi: 10.1038/345065a0. [DOI] [PubMed] [Google Scholar]
- Buck L., Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991 Apr 5;65(1):175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Chang M. S., Lowe D. G., Lewis M., Hellmiss R., Chen E., Goeddel D. V. Differential activation by atrial and brain natriuretic peptides of two different receptor guanylate cyclases. Nature. 1989 Sep 7;341(6237):68–72. doi: 10.1038/341068a0. [DOI] [PubMed] [Google Scholar]
- Chinkers M., Garbers D. L., Chang M. S., Lowe D. G., Chin H. M., Goeddel D. V., Schulz S. A membrane form of guanylate cyclase is an atrial natriuretic peptide receptor. Nature. 1989 Mar 2;338(6210):78–83. doi: 10.1038/338078a0. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- Dhallan R. S., Yau K. W., Schrader K. A., Reed R. R. Primary structure and functional expression of a cyclic nucleotide-activated channel from olfactory neurons. Nature. 1990 Sep 13;347(6289):184–187. doi: 10.1038/347184a0. [DOI] [PubMed] [Google Scholar]
- Domino S. E., Tubb D. J., Garbers D. L. Assay of guanylyl cyclase catalytic activity. Methods Enzymol. 1991;195:345–355. doi: 10.1016/0076-6879(91)95179-n. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Itakura M., Iwashina M., Mizuno T., Ito T., Hagiwara H., Hirose S. Mutational analysis of disulfide bridges in the type C atrial natriuretic peptide receptor. J Biol Chem. 1994 Mar 18;269(11):8314–8318. [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lowe D. G., Chang M. S., Hellmiss R., Chen E., Singh S., Garbers D. L., Goeddel D. V. Human atrial natriuretic peptide receptor defines a new paradigm for second messenger signal transduction. EMBO J. 1989 May;8(5):1377–1384. doi: 10.1002/j.1460-2075.1989.tb03518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Gold G. H. A cyclic nucleotide-gated conductance in olfactory receptor cilia. 1987 Jan 29-Feb 4Nature. 325(6103):442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- Ngai J., Dowling M. M., Buck L., Axel R., Chess A. The family of genes encoding odorant receptors in the channel catfish. Cell. 1993 Mar 12;72(5):657–666. doi: 10.1016/0092-8674(93)90395-7. [DOI] [PubMed] [Google Scholar]
- Pace U., Hanski E., Salomon Y., Lancet D. Odorant-sensitive adenylate cyclase may mediate olfactory reception. Nature. 1985 Jul 18;316(6025):255–258. doi: 10.1038/316255a0. [DOI] [PubMed] [Google Scholar]
- Raming K., Krieger J., Strotmann J., Boekhoff I., Kubick S., Baumstark C., Breer H. Cloning and expression of odorant receptors. Nature. 1993 Jan 28;361(6410):353–356. doi: 10.1038/361353a0. [DOI] [PubMed] [Google Scholar]
- Ressler K. J., Sullivan S. L., Buck L. B. A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell. 1993 May 7;73(3):597–609. doi: 10.1016/0092-8674(93)90145-g. [DOI] [PubMed] [Google Scholar]
- Rogers K. E., Dasgupta P., Gubler U., Grillo M., Khew-Goodall Y. S., Margolis F. L. Molecular cloning and sequencing of a cDNA for olfactory marker protein. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1704–1708. doi: 10.1073/pnas.84.6.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz S., Green C. K., Yuen P. S., Garbers D. L. Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell. 1990 Nov 30;63(5):941–948. doi: 10.1016/0092-8674(90)90497-3. [DOI] [PubMed] [Google Scholar]
- Schulz S., Singh S., Bellet R. A., Singh G., Tubb D. J., Chin H., Garbers D. L. The primary structure of a plasma membrane guanylate cyclase demonstrates diversity within this new receptor family. Cell. 1989 Sep 22;58(6):1155–1162. doi: 10.1016/0092-8674(89)90513-8. [DOI] [PubMed] [Google Scholar]
- Shyjan A. W., de Sauvage F. J., Gillett N. A., Goeddel D. V., Lowe D. G. Molecular cloning of a retina-specific membrane guanylyl cyclase. Neuron. 1992 Oct;9(4):727–737. doi: 10.1016/0896-6273(92)90035-c. [DOI] [PubMed] [Google Scholar]
- Sklar P. B., Anholt R. R., Snyder S. H. The odorant-sensitive adenylate cyclase of olfactory receptor cells. Differential stimulation by distinct classes of odorants. J Biol Chem. 1986 Nov 25;261(33):15538–15543. [PubMed] [Google Scholar]
- Stults J. T., O'Connell K. L., Garcia C., Wong S., Engel A. M., Garbers D. L., Lowe D. G. The disulfide linkages and glycosylation sites of the human natriuretic peptide receptor-C homodimer. Biochemistry. 1994 Sep 20;33(37):11372–11381. doi: 10.1021/bi00203a036. [DOI] [PubMed] [Google Scholar]
- Vassar R., Ngai J., Axel R. Spatial segregation of odorant receptor expression in the mammalian olfactory epithelium. Cell. 1993 Jul 30;74(2):309–318. doi: 10.1016/0092-8674(93)90422-m. [DOI] [PubMed] [Google Scholar]
- Yang R. B., Foster D. C., Garbers D. L., Fülle H. J. Two membrane forms of guanylyl cyclase found in the eye. Proc Natl Acad Sci U S A. 1995 Jan 17;92(2):602–606. doi: 10.1073/pnas.92.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemiecki A., Harpur A. G., Wilks A. F. JAK protein tyrosine kinases: their role in cytokine signalling. Trends Cell Biol. 1994 Jun;4(6):207–212. doi: 10.1016/0962-8924(94)90143-0. [DOI] [PubMed] [Google Scholar]
- de Sauvage F. J., Camerato T. R., Goeddel D. V. Primary structure and functional expression of the human receptor for Escherichia coli heat-stable enterotoxin. J Biol Chem. 1991 Sep 25;266(27):17912–17918. [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]




