Abstract
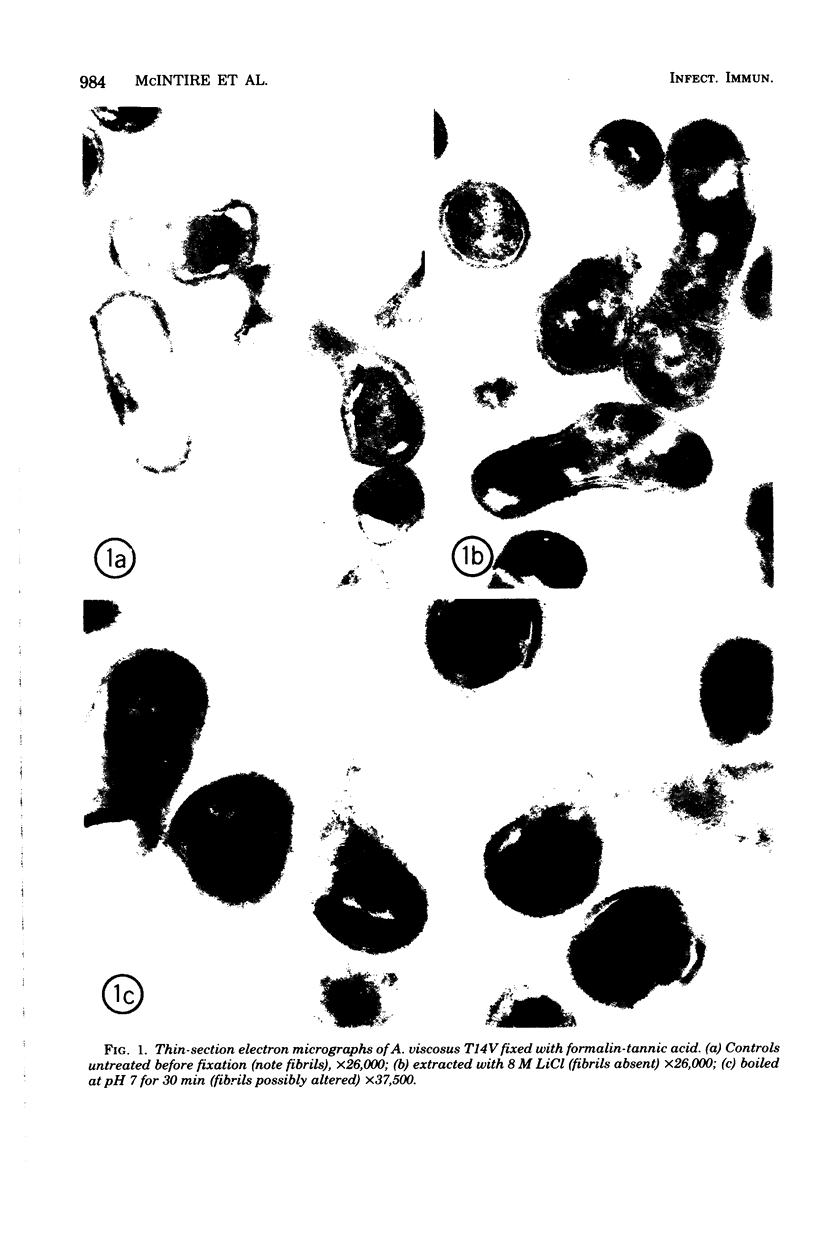
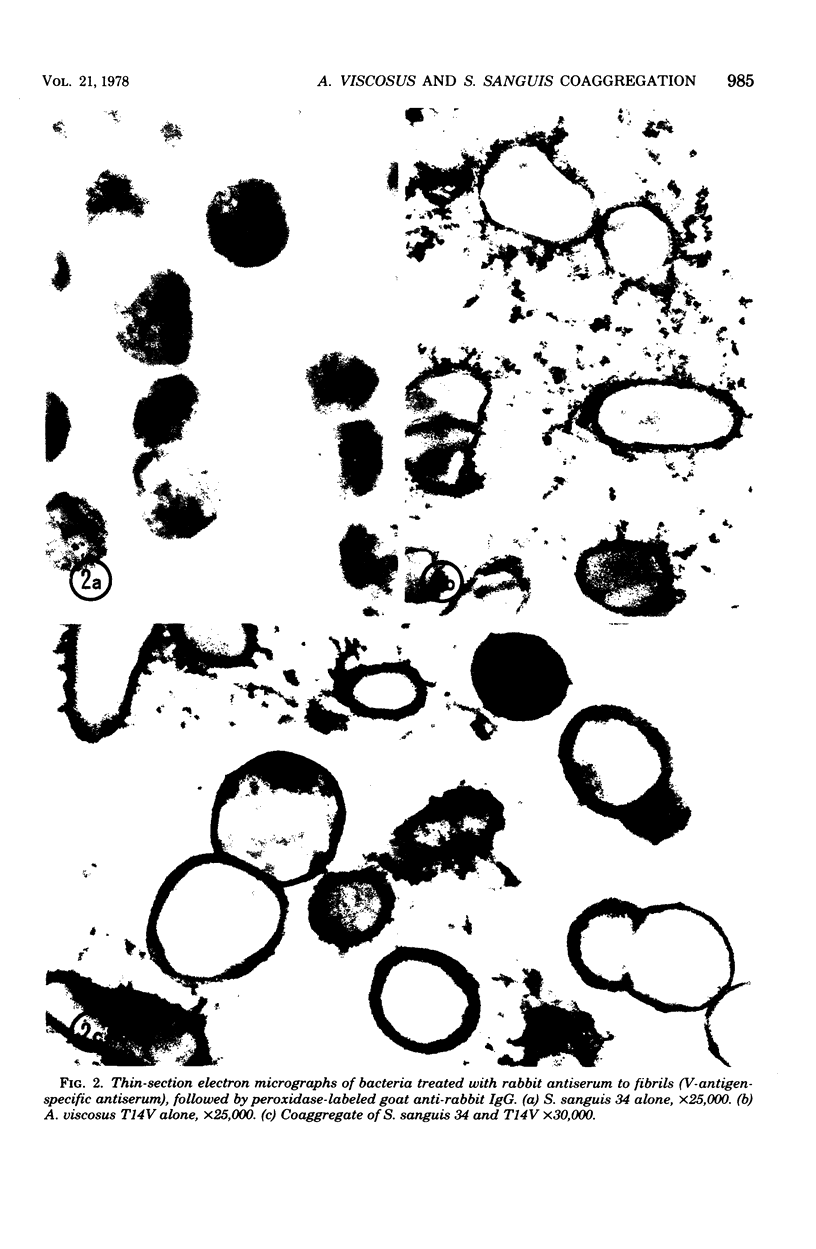
Actinomyces viscosus T14V and Streptococcus sanguis 34 coaggregate by a mechanism which is not inhibited by 1 M NaCl, is dextran independent, requires calcium, is pH dependent with an optimum at pH 8.0 to 8.5, and appears to require the interaction of a protein or glycoprotein on A. viscosus with a carbohydrate on S. sanguis. The coaggregation is inhibited more than 80% by 0.01 M lactose, 0.02 M beta-methyl-D-galactoside, or 0.05 M D-galactose; inhibition of coaggregation was less than 10% in 0.1 M alpha-methyl-D-galactoside, melibiose, maltose, cellobiose, sucrose, and a number of monosaccharides. At very high concentrations of enzyme, protease from S. griseus destroyed the reactive site on A. viscosus but not on S. sanguis. Both were totally resistant to dextranase. Periodate (0.01 M; pH 4) inactivated both bacteria. The ability of S. sanguis to coaggregate with A. viscosus was not destroyed by phenol-water extraction at 65 degrees C for 15 min. When the bacteria were cultured under specified conditions, the coaggregation was highly reproducible. Under the same conditions, T14AV, the avirulent mutant of A. viscosus T14V, did not coaggregate with S. sanguis 34. Electron microscopic studies of coaggregates, labeled immunochemically with antibody to A. viscosus, indicated that fibrils on A. viscosus may be involved in the coaggregation.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohlool B. B., Schmidt E. L. Lectins: a possible basis for specificity in the Rhizobium--legume root nodule symbiosis. Science. 1974 Jul 19;185(4147):269–271. doi: 10.1126/science.185.4147.269. [DOI] [PubMed] [Google Scholar]
- Bourgeau G., McBride B. C. Dextran-mediated interbacterial aggregation between dextran-synthesizing streptococci and Actinomyces viscosus. Infect Immun. 1976 Apr;13(4):1228–1234. doi: 10.1128/iai.13.4.1228-1234.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd H., Leach S. J., Milligan B. N-acylsuccinimides as acylating agents for proteins: the selective acylation of lysine residues. Int J Pept Protein Res. 1972;4(2):117–122. doi: 10.1111/j.1399-3011.1972.tb03407.x. [DOI] [PubMed] [Google Scholar]
- Bryant R. E., Jenkins D. E., Jr Calcium requirements for complement dependent hemolytic reactions. J Immunol. 1968 Oct;101(4):664–668. [PubMed] [Google Scholar]
- Buchanan T. M., Pearce W. A. Pili as a mediator of the attachment of gonococci to human erythrocytes. Infect Immun. 1976 May;13(5):1483–1489. doi: 10.1128/iai.13.5.1483-1489.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Grahnén H., Jonsson G. Lactobacilli and streptococci in the mouth of children. Caries Res. 1975;9(5):333–339. doi: 10.1159/000260166. [DOI] [PubMed] [Google Scholar]
- Cisar J. O., Vatter A. E., McIntire F. C. Identification of the virulence-associated antigen on the surface fibrils of Actinomyces viscosus T14. Infect Immun. 1978 Jan;19(1):312–319. doi: 10.1128/iai.19.1.312-319.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. M., Calandra G. B., Huff E., Nugent K. M. Attributes of potential utility in differentiating among "group H" streptococci or Streptococcus sanguis. J Dent Res. 1976 Jan;55:A142–A153. doi: 10.1177/002203457605500106011. [DOI] [PubMed] [Google Scholar]
- Crandall M. A., Brock T. D. Molecular basis of mating in the yeast hansenula wingei. Bacteriol Rev. 1968 Sep;32(3):139–163. doi: 10.1128/br.32.3.139-163.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Prez R. M., Bryan C. S., Hawiger J., Colley D. G. Function of the classical and alternate pathways of human complement in serum treated with ethylene glycol tetraacetic acid and MgCl2-ethylene glycol tetraacetic acid. Infect Immun. 1975 Jun;11(6):1235–1243. doi: 10.1128/iai.11.6.1235-1243.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Balcerzak-Raczkowski I. B. Interbacterial aggregation of Actinomyces naeslundii and dental plaque streptococci. J Periodontal Res. 1977 Jan;12(1):11–20. doi: 10.1111/j.1600-0765.1977.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Fuller R. Nature of the determinant responsible for the adhesion of lactobacilli to chicken crop epithelial cells. J Gen Microbiol. 1975 Apr;87(2):245–250. doi: 10.1099/00221287-87-2-245. [DOI] [PubMed] [Google Scholar]
- Galbraith W., Goldstein I. J. Phytohemagglutinins: A new class of metalloproteins. Isolation, purification, and some properties of the lectin from Phaseolus lunatus. FEBS Lett. 1970 Aug 17;9(4):197–201. doi: 10.1016/0014-5793(70)80354-4. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Interbacterial aggregation of plaque bacteria. Arch Oral Biol. 1970 Dec;15(12):1397–1400. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. On the formation of dental plaques. J Periodontol. 1973 Jun;44(6):347–360. doi: 10.1902/jop.1973.44.6.347. [DOI] [PubMed] [Google Scholar]
- Girard A. E., Jacius B. H. Ultrastructure of Actinomyces viscosus and Actinomyces naeslundii. Arch Oral Biol. 1974 Jan;19(1):71–79. doi: 10.1016/0003-9969(74)90228-3. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Hammond B. F., Steel C. F., Peindl K. S. Antigens and surface components associated with virulence of Actinomyces viscosus. J Dent Res. 1976 Jan;55:A19–A25. doi: 10.1177/002203457605500111011. [DOI] [PubMed] [Google Scholar]
- Henkart P., Humphreys S., Humphreys T. Characterization of sponge aggregation factor. A unique proteoglycan complex. Biochemistry. 1973 Jul 31;12(16):3045–3050. doi: 10.1021/bi00740a016. [DOI] [PubMed] [Google Scholar]
- Jordan H. V., Fitzgerald R. J., Stanley H. R. Plaque formation and periodontal pathology in gnotobiotic rats infected with an oral actinomycete. Am J Pathol. 1965 Dec;47(6):1157–1167. [PMC free article] [PubMed] [Google Scholar]
- Jordan H. V., Hammond B. F. Filamentous bacteria isolated from human root surface caries. Arch Oral Biol. 1972 Sep;17(9):1333–1342. doi: 10.1016/0003-9969(72)90166-5. [DOI] [PubMed] [Google Scholar]
- Jordan H. V., Keyes P. H., Bellack S. Periodontal lesions in hamsters and gnotobiotic rats infected with actinomyces of human origin. J Periodontal Res. 1972;7(1):21–28. doi: 10.1111/j.1600-0765.1972.tb00627.x. [DOI] [PubMed] [Google Scholar]
- Lis H., Sharon N. The biochemistry of plant lectins (phytohemagglutinins). Annu Rev Biochem. 1973;42(0):541–574. doi: 10.1146/annurev.bi.42.070173.002545. [DOI] [PubMed] [Google Scholar]
- McBride B. C., Bourgeau G. Dextran-induced aggregation of Actinomyces viscosus. Arch Oral Biol. 1975 Dec;20(12):837–841. doi: 10.1016/0003-9969(75)90063-1. [DOI] [PubMed] [Google Scholar]
- Nagy B., Moon H. W., Isaacson R. E. Colonization of porcine intestine by enterotoxigenic Escherichia coli: selection of piliated forms in vivo, adhesion of piliated forms to epithelial cells in vitro, and incidence of a pilus antigen among porcine enteropathogenic E. coli. Infect Immun. 1977 Apr;16(1):344–352. doi: 10.1128/iai.16.1.344-352.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottow J. C. Ecology, physiology, and genetics of fimbriae and pili. Annu Rev Microbiol. 1975;29:79–108. doi: 10.1146/annurev.mi.29.100175.000455. [DOI] [PubMed] [Google Scholar]
- Richardson C. E., Behnke W. D. Physical-chemical studies on the role of the metal ions in concanavalin A. J Mol Biol. 1976 Apr 15;102(3):441–451. doi: 10.1016/0022-2836(76)90326-0. [DOI] [PubMed] [Google Scholar]
- Rosan B. Antigens of Streptococcus sanguis. Infect Immun. 1973 Feb;7(2):205–211. doi: 10.1128/iai.7.2.205-211.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosan B. Relationship of the cell wall composition of group H streptococci and Streptococcus sanguis to their serological properties. Infect Immun. 1976 Apr;13(4):1144–1153. doi: 10.1128/iai.13.4.1144-1153.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S. D., Reitherman R. W., Barondes S. H. Distinct lectin activities from six species of cellular slime molds. Exp Cell Res. 1975 Oct 1;95(1):159–166. doi: 10.1016/0014-4827(75)90621-7. [DOI] [PubMed] [Google Scholar]
- Rölla G., Kilian M. Haemagglutination activity of plaque-forming bacteria. Caries Res. 1977;11(2):85–89. doi: 10.1159/000260253. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Hemagglutination by purified type I Escherichia coli pili. J Exp Med. 1977 Nov 1;146(5):1169–1181. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Type I Escherichia coli pili: characterization of binding to monkey kidney cells. J Exp Med. 1977 Nov 1;146(5):1182–1194. doi: 10.1084/jem.146.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M. Galloylglucoses of low molecular weight as mordant in electron microscopy. I. Procedure, and evidence for mordanting effect. J Cell Biol. 1976 Sep;70(3):608–621. doi: 10.1083/jcb.70.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M. Galloylglucoses of low molecular weight as mordant in electron microscopy. II. The moiety and functional groups possibly involved in the mordanting effect. J Cell Biol. 1976 Sep;70(3):622–633. doi: 10.1083/jcb.70.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky S. S., Hubersak C., Propas D. Induction of periodontal destruction in gnotobiotic rats by a human oral strain of Actinomyces naeslundii. Arch Oral Biol. 1970 Oct;15(10):993–995. doi: 10.1016/0003-9969(70)90095-6. [DOI] [PubMed] [Google Scholar]
- Springer G. F., Desai P. R., Adye J. C. Lectin and agglutinin receptors of red cell components. Ann N Y Acad Sci. 1974;234(0):312–331. doi: 10.1111/j.1749-6632.1974.tb53045.x. [DOI] [PubMed] [Google Scholar]
- Theilade E., Fejerskov O., Prachyabrued W., Kilian M. Microbiologic study on developing plaque in human fissures. Scand J Dent Res. 1974;82(6):420–427. doi: 10.1111/j.1600-0722.1974.tb00396.x. [DOI] [PubMed] [Google Scholar]
- Tinanoff N., Gross A., Brady J. M. Development of plaque on enamel. Parallel investigations. J Periodontal Res. 1976 Jul;11(4):197–209. doi: 10.1111/j.1600-0765.1976.tb00071.x. [DOI] [PubMed] [Google Scholar]