Abstract
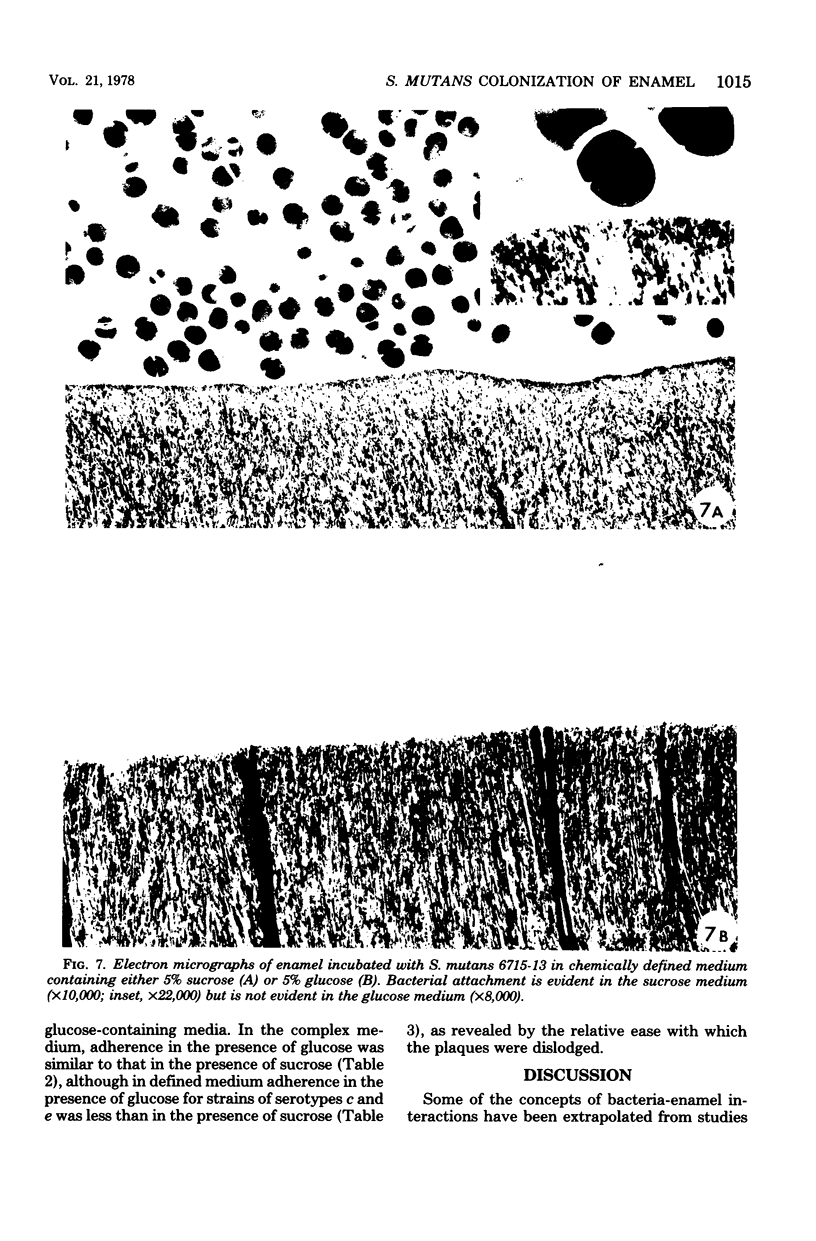
An in vitro model consisting of enamel from extracted human molars, suspended from wires in inoculated culture tubes, was used to study the adhesion of bacteria to enamel. Under conditions in which there was no macroscopically visible plaque formation, electron micrographs showed no bacterial deposits on the enamel surface. In samples where Streptococcus mutans attached to enamel, an extracellular, pellicle-like material was associated with the bacteria adjacent to the enamel. This material appeared to bind to the enamel surface and to mediate bacterial attachment. Membrane-filtered (Millipore Corp.) saliva deposited a thin surface layer on the enamel, but there were no observable alterations of S. mutans attachment to enamel pretreated with saliva. It was noted that Bratthall serotype c and e strains of S. mutans, when grown in glucose-containing medium, attached, although less tenaciously, to enamel and nichrome wires. Chemical and gas chromatographic analyses of cell-associated materials formed by serotype c and e strains cultured in glucose-containing medium revealed low amounts of glucose-positive material and no polymer linkages characteristic of glucan; yet the same strains cultured in sucrose-containing medium had relatively high amounts of glucose-positive material, with polymer linkages-characteristic of glucan. Serotype a, b, and d strains could attach only in sucrose-containing media.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkhed D., Rosell K. G. Structural studies on polysaccharides synthetised from sucrose by soluble enzymes in human dental plaque material. Odontol Revy. 1975;26(4):281–290. [PubMed] [Google Scholar]
- Bowen W. H. The induction of rampant dental caries in monkeys (Macaca irus). Caries Res. 1969;3(3):227–237. doi: 10.1159/000259597. [DOI] [PubMed] [Google Scholar]
- De Stoppelaar J. D., Van Houte J., Backer Dirks O. The relationship between extracellular polysaccharide-producing streptococci and smooth surface caries in 13-year-old children. Caries Res. 1969;3(2):190–199. doi: 10.1159/000259582. [DOI] [PubMed] [Google Scholar]
- Ebisu S., Misaki A., Kato K., Kotani S. The structure of water-insoluble glucans of cariogenic Streptococcus mutans, formed in the absence and presence of dextranase. Carbohydr Res. 1974 Dec;38:374–381. doi: 10.1016/s0008-6215(00)82375-7. [DOI] [PubMed] [Google Scholar]
- FITZGERALD R. J., KEYES P. H. Demonstration of the etiologic role of streptococci in experimental caries in the hamster. J Am Dent Assoc. 1960 Jul;61:9–19. doi: 10.14219/jada.archive.1960.0138. [DOI] [PubMed] [Google Scholar]
- Freedman M. L., Tanzer J. M. Dissociation of plaque formation from glucan-induced agglutination in mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):189–196. doi: 10.1128/iai.10.1.189-196.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M., Birked D., Granath K. Analyses of glucans from cariogenic and mutant Streptococcus mutans. Infect Immun. 1978 Jul;21(1):17–27. doi: 10.1128/iai.21.1.17-27.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Berman K. S., Knoettner P., Kapsimalis B. Dental caries and alveolar bone loss in gnotobiotic rats infected with capsule forming streptococci of human origin. Arch Oral Biol. 1966 Jun;11(6):549–560. doi: 10.1016/0003-9969(66)90220-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Synthesis of insoluble dextran and its significance in the formation of gelatinous deposits by plaque-forming streptococci. Arch Oral Biol. 1968 Oct;13(10):1249–1262. doi: 10.1016/0003-9969(68)90081-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. On the formation of dental plaques. J Periodontol. 1973 Jun;44(6):347–360. doi: 10.1902/jop.1973.44.6.347. [DOI] [PubMed] [Google Scholar]
- Guggenheim B. Enzymatic hydrolysis and structure of water-insoluble glucan produced by glucosyltransferases from a strain of streptococcus mutans. Helv Odontol Acta. 1970 Nov;14(Suppl):89+–89+. [PubMed] [Google Scholar]
- Guggenheim B., Schroeder H. E. Biochemical and morphological aspects of extracellular polysaccharides produced by cariogenic streptococci. Helv Odontol Acta. 1967 Oct;11(2):131–152. [PubMed] [Google Scholar]
- Hay D. I. Some observations on human saliva proteins and their role in the formation of the acquired enamel pellicle. J Dent Res. 1969 Sep-Oct;48(5):806–810. doi: 10.1177/00220345690480053301. [DOI] [PubMed] [Google Scholar]
- Hillman J. D., Van Houte J., Gibbons R. J. Sorption of bacteria to human enamel powder. Arch Oral Biol. 1970 Sep;15(9):899–903. doi: 10.1016/0003-9969(70)90163-9. [DOI] [PubMed] [Google Scholar]
- JORDAN H. V., FITZGERALD R. J., BOWLER A. E. Inhibition of experimental caries by sodium metabisulfite and its effect on the growth and metabolism of selected bacteria. J Dent Res. 1960 Jan-Feb;39:116–123. doi: 10.1177/00220345600390010501. [DOI] [PubMed] [Google Scholar]
- Johnson M. C., Bozzola J. J., Shechmeister I. L., Shklair I. L. Biochemical study of the relationship of extracellular glucan to adherence and cariogenicity in Streptococcus mutans and an extracellular polysaccharide mutant. J Bacteriol. 1977 Jan;129(1):351–357. doi: 10.1128/jb.129.1.351-357.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Rölla G. Initial colonization of teeth in monkeys as related to diet. Infect Immun. 1976 Oct;14(4):1022–1027. doi: 10.1128/iai.14.4.1022-1027.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasse B. Human streptococci and experimental caries in hamsters. Arch Oral Biol. 1966 Apr;11(4):429–436. doi: 10.1016/0003-9969(66)90107-5. [DOI] [PubMed] [Google Scholar]
- Littleton N. W., Kakehashi S., Fitzgerald R. J. Recovery of specific "caries-inducing" streptococci from carious lesions in the teeth of children. Arch Oral Biol. 1970 May;15(5):461–463. doi: 10.1016/0003-9969(70)90073-7. [DOI] [PubMed] [Google Scholar]
- Mayhall C. W. Concerning the composition and source of the acquired enamel pellicle of human teeth. Arch Oral Biol. 1970 Dec;15(12):1327–1341. doi: 10.1016/0003-9969(70)90021-x. [DOI] [PubMed] [Google Scholar]
- McCabe M. M. Comments on adherence of Streptococcus mutans. J Dent Res. 1976 Apr;55(Spec No):C226–C228. doi: 10.1177/002203457605500319011. [DOI] [PubMed] [Google Scholar]
- McCabe R. M., Keyes P. H., Howell A., Jr An in vitro method for assessing the plaque forming ability of oral bacteria. Arch Oral Biol. 1967 Dec;12(12):1653–1656. doi: 10.1016/0003-9969(67)90200-2. [DOI] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. I. Roles of insoluble dextran-levan synthetase enzymes and cell wall polysaccharide antigen in plaque formation. Infect Immun. 1973 Oct;8(4):555–562. doi: 10.1128/iai.8.4.555-562.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian J., Freedman M. L., Tanzer J. M., Lovelace S. M. Ultrastructure of Mutants of Streptococcus mutans with Reference to Agglutination, Adhesion, and Extracellular Polysaccharide. Infect Immun. 1974 Nov;10(5):1170–1179. doi: 10.1128/iai.10.5.1170-1179.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisizawa T., Imai S., Akada H., Hinoide M., Araya S. Extracellular glucans produced by oral streptococci. Arch Oral Biol. 1976;21(3):207–213. doi: 10.1016/0003-9969(76)90131-x. [DOI] [PubMed] [Google Scholar]
- Nisizawa T., Imai S., Araya S. Methylation analysis of extracellular glucans produced by Streptococcus mutans strain JC 2. Arch Oral Biol. 1977;22(4):281–285. doi: 10.1016/0003-9969(77)90114-5. [DOI] [PubMed] [Google Scholar]
- OGUR M., ROSEN G. The nucleic acids of plant tissues; the extraction and estimation of desoxypentose nucleic acid and pentose nucleic acid. Arch Biochem. 1950 Feb;25(2):262–276. [PubMed] [Google Scholar]
- Orstavik D., Kraus F. W., Henshaw L. C. In vitro attachment of streptococci to the tooth surface. Infect Immun. 1974 May;9(5):794–800. doi: 10.1128/iai.9.5.794-800.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce E. I. Adsorption of steptococcal extracellular polysaccharides by hydroxyapatite. Arch Oral Biol. 1976;21(9):545–549. doi: 10.1016/0003-9969(76)90020-0. [DOI] [PubMed] [Google Scholar]
- Sidebotham R. L. Dextrans. Adv Carbohydr Chem Biochem. 1974;30:371–444. doi: 10.1016/s0065-2318(08)60268-1. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Sönju T., Rölla G. Chemical analysis of the acquired pellicle formed in two hours on cleaned human teeth in vivo. Rate of formation and amino acid analysis. Caries Res. 1973;7(1):30–38. doi: 10.1159/000259822. [DOI] [PubMed] [Google Scholar]
- Tanzer J. M., Freedman M. L., Fitzgerald R. J., Larson R. H. Diminished virulence of glucan synthesis-defective mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):197–203. doi: 10.1128/iai.10.1.197-203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Slee A. M., Kamay B., Scheer E. R. In vitro evaluation of three iodine-containing compounds as antiplaque agents. Antimicrob Agents Chemother. 1977 Jul;12(1):107–113. doi: 10.1128/aac.12.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinanoff N., Glick P. L., Weber D. F. Ultrastructure of organic films on the enamel surface. Caries Res. 1976;10(1):19–32. doi: 10.1159/000260186. [DOI] [PubMed] [Google Scholar]
- Tinanoff N., Gross A., Brady J. M. Development of plaque on enamel. Parallel investigations. J Periodontal Res. 1976 Jul;11(4):197–209. doi: 10.1111/j.1600-0765.1976.tb00071.x. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Banghart S. B. Adherence as a determinant of the presence of Streptococcus salivarius and Streptococcus sanguis on the human tooth surface. Arch Oral Biol. 1970 Nov;15(11):1025–1034. doi: 10.1016/0003-9969(70)90115-9. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Upeslacis V. N., Jordan H. V., Skobe Z., Green D. B. Role of sucrose in colonization of Streptococcus mutans in conventional Sprague-Dawley rats. J Dent Res. 1976 Mar-Apr;55(2):202–215. doi: 10.1177/00220345760550020801. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Upeslacis V. N. Studies of the mechanism of sucrose-associated colonization of Streptococcus mutans on teeth of conventional rats. J Dent Res. 1976 Mar-Apr;55(2):216–222. doi: 10.1177/00220345760550020901. [DOI] [PubMed] [Google Scholar]
- Warshawsky H., Moore G. A technique for the fixation and decalcification of rat incisors for electron microscopy. J Histochem Cytochem. 1967 Sep;15(9):542–549. doi: 10.1177/15.9.542. [DOI] [PubMed] [Google Scholar]
- Zobell C. E. The Effect of Solid Surfaces upon Bacterial Activity. J Bacteriol. 1943 Jul;46(1):39–56. doi: 10.1128/jb.46.1.39-56.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Houte J., Burgess R. C., Onose H. Oral implantation of human strains of Streptococcus mutans in rats fed sucrose or glucose diets. Arch Oral Biol. 1976;21(9):561–564. doi: 10.1016/0003-9969(76)90023-6. [DOI] [PubMed] [Google Scholar]
- van Houte J., Duchin S. Streptococcus mutans in the mouths of children with congenital sucrase deficiency. Arch Oral Biol. 1975 Nov;20(11):771–773. doi: 10.1016/0003-9969(75)90050-3. [DOI] [PubMed] [Google Scholar]