Abstract
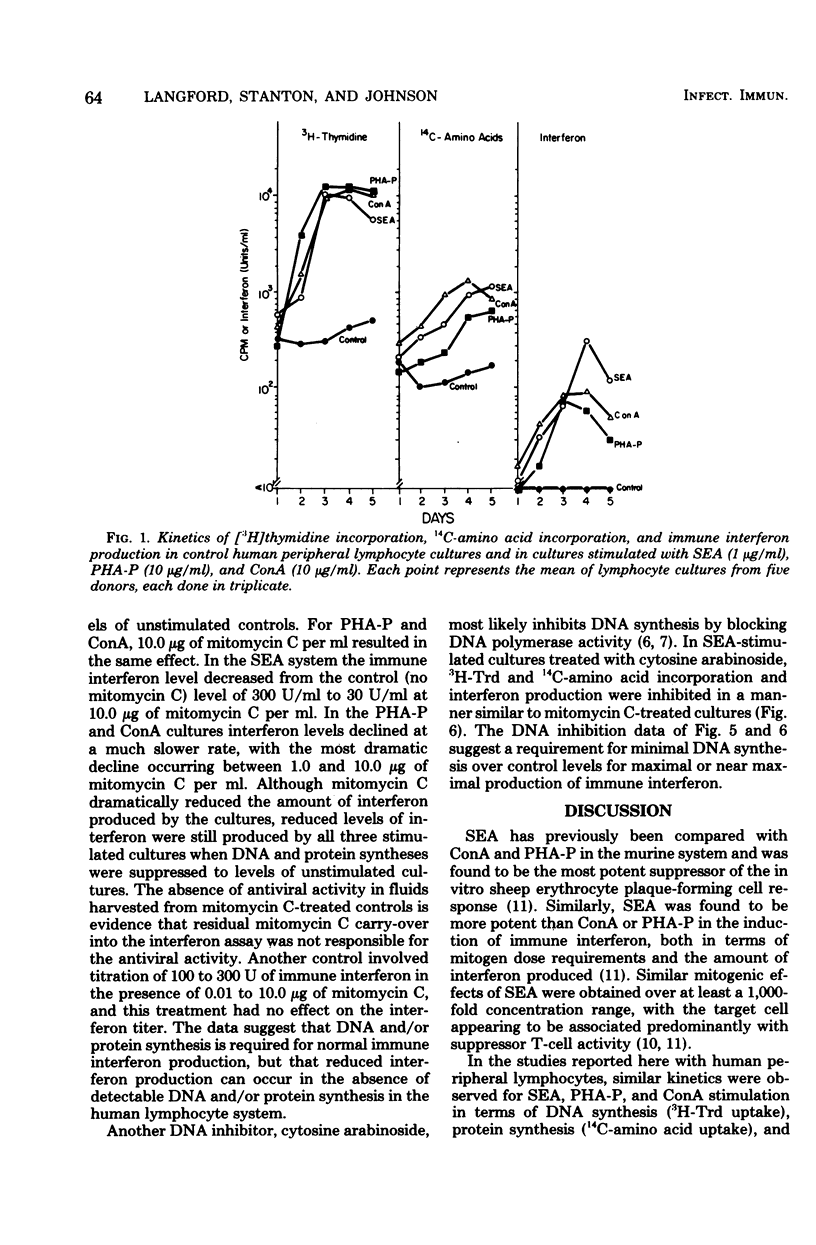
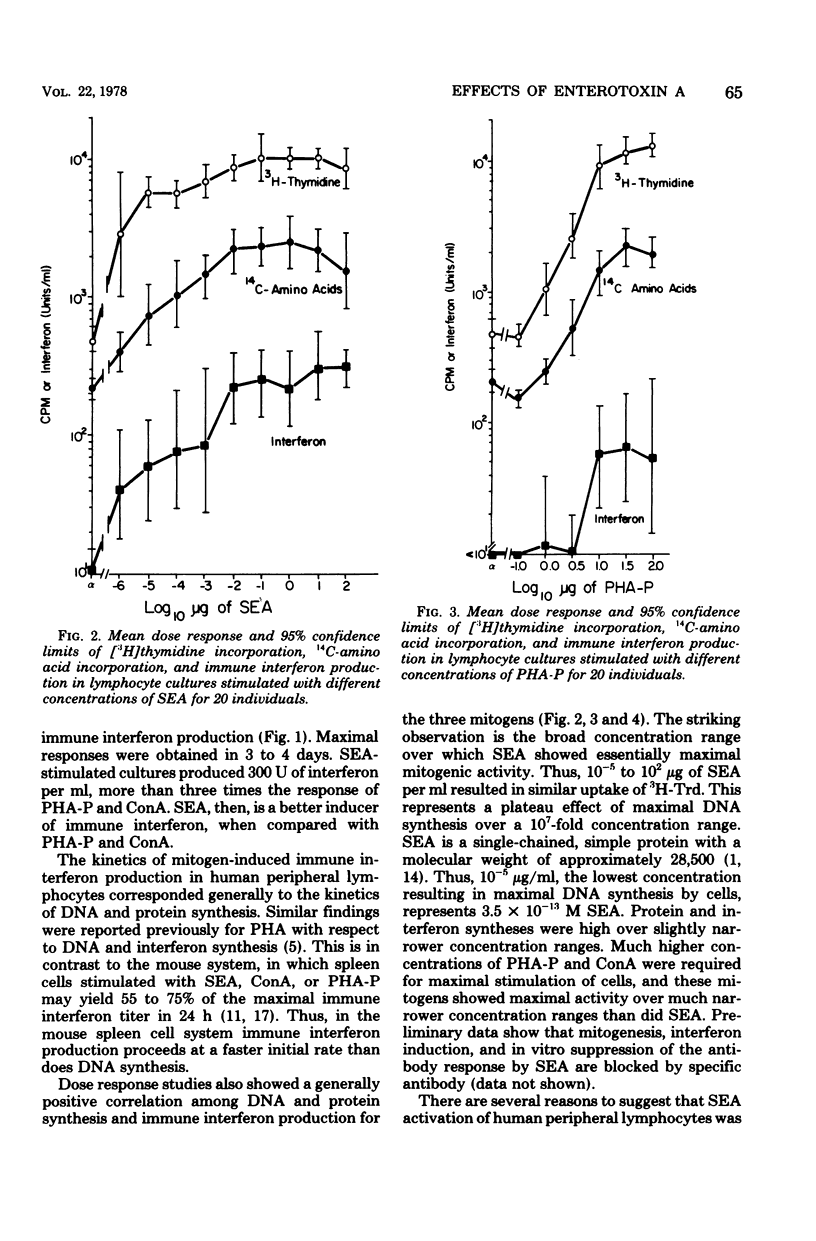
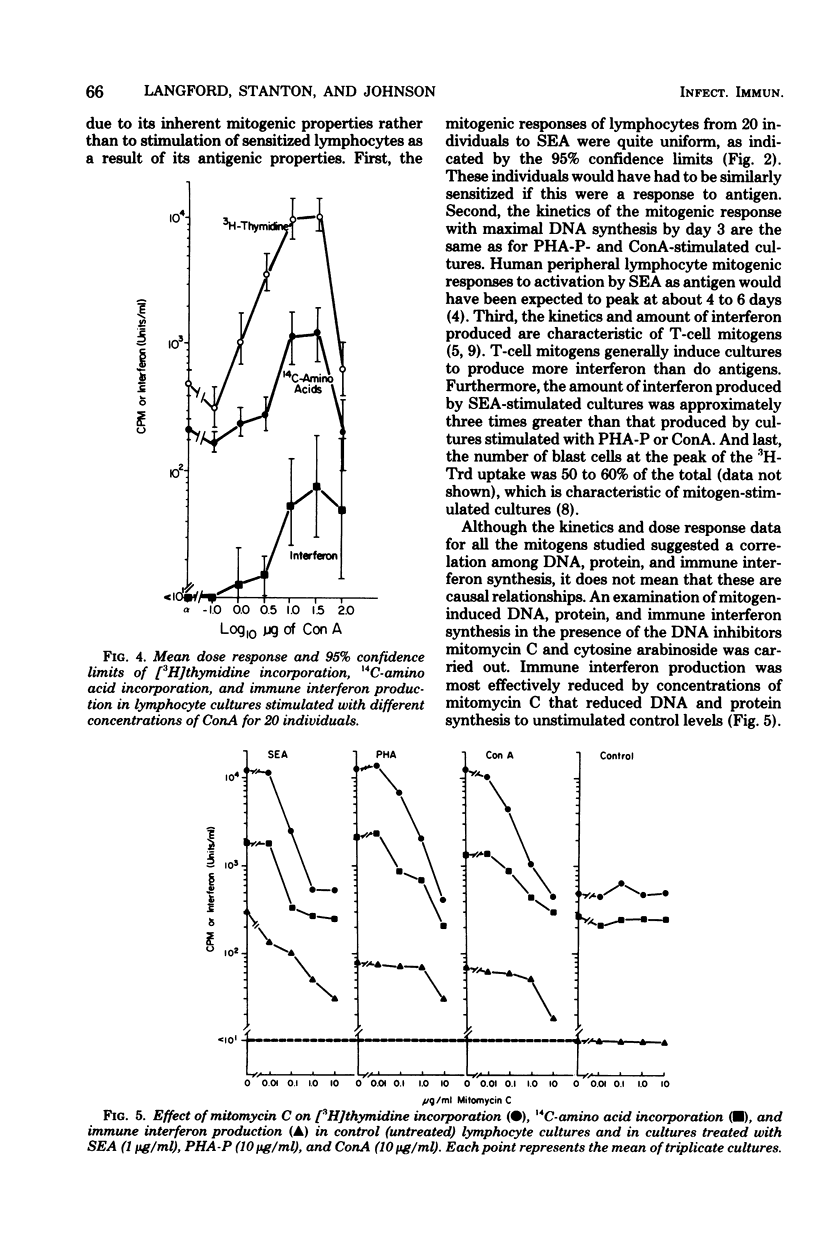
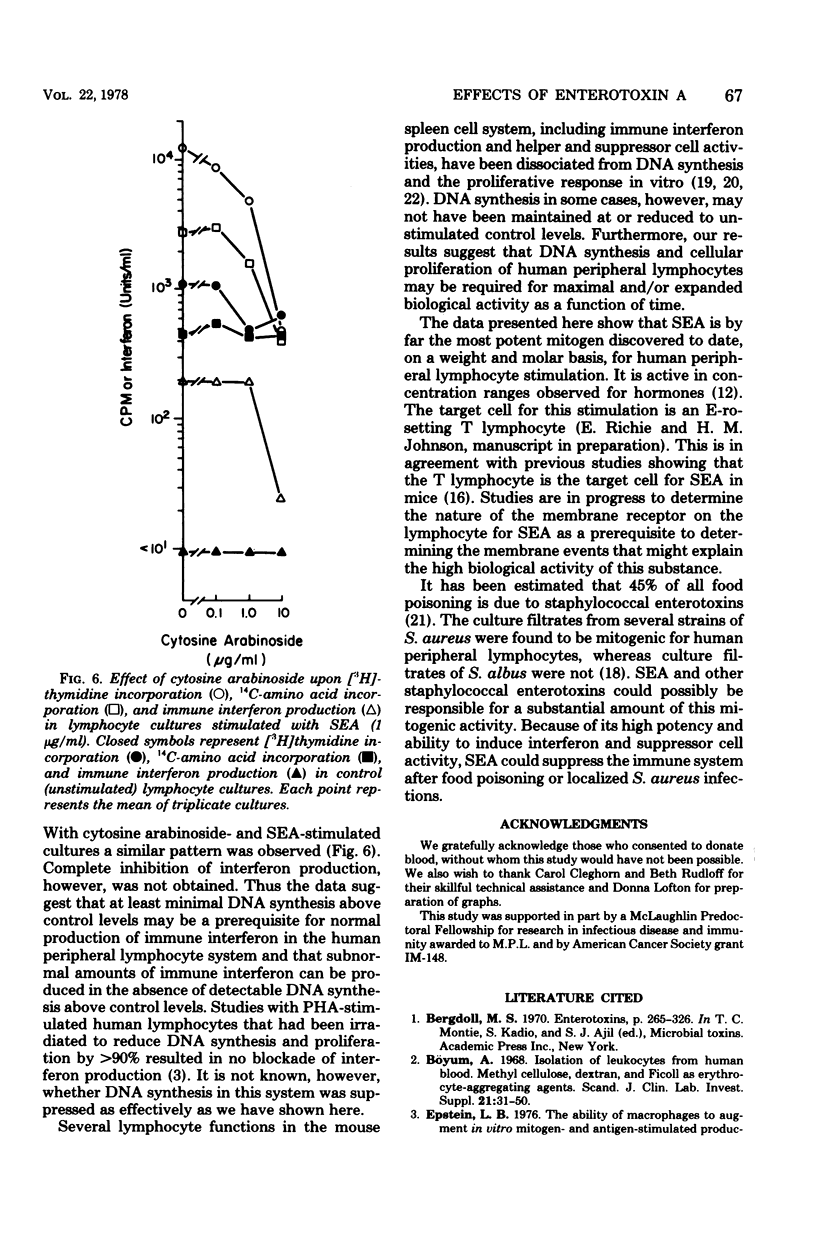
The mitogenicity, ability to induce immune interferon, and relationship between interferon synthesis and cell proliferative response were studied using human peripheral lymphocytes stimulated by staphylococcal enterotoxin A (SEA), phytohemagglutinin-P (PHA-P), and concanavalin A (ConA). Maximum cell proliferative responses ([3H]thymidine incorporation) and protein synthesis (14C-amino acid incorporation) occurred on days 3 and 4, respectively, after stimulation by each of the three mitogens. Maximal immune interferon levels were found 3 or 4 days after mitogen stimulation. SEA-treated cultures produced approximately three times more interferon than did cultures stimulated with PHA-P or ConA. Furthermore, SEA stimulated maximal cell proliferation over a much broader concentration range than did PHA-P and ConA (SEA, 10−5 to 102 μg/ml; PHA-P, 101 to 102 μg/ml; ConA, 101 to 101.5 μg/ml). Interferon was also produced at maximal or near maximal levels over a broad concentration range of SEA (10−2 to 102 μg/ml). Also, we found that inhibition of mitogen-induced DNA and protein synthesis to control levels by mitomycin C or cytosine arabinoside partially reduced interferon production. The DNA inhibitor studies indicate that immune interferon synthesis occurs maximally in association with at least some proliferative response and that submaximal levels of interferon production occur in mitogen-treated cultures in the absence of detectable proliferation. The ability of SEA to stimulate maximal DNA and immune interferon synthesis at concentrations of 3.5 × 10−13 M and 3.5 × 10−10 M, respectively, puts it in a potency range similar to that of hormones. Thus, SEA may play an important role in gut immunity and Staphylococcus aureus infections at concentrations well below those required for emetic effects.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Epstein L. B., Cline M. J., Merigan T. C. PPD-stimulated interferon: in vitro macrophage-lymphocyte interaction in the production of a mediator of cellular immunity. Cell Immunol. 1971 Dec;2(6):602–613. doi: 10.1016/0008-8749(71)90008-6. [DOI] [PubMed] [Google Scholar]
- Epstein L. B., Kreth H. W., Herzenberg L. A. Fluorescence-activated cell sorting of human T and B lymphocytes. II. Identification of the cell type responsible for interferon production and cell proliferation in response to mitogens. Cell Immunol. 1974 Jun;12(3):407–421. doi: 10.1016/0008-8749(74)90097-5. [DOI] [PubMed] [Google Scholar]
- Furlong N. B., Gresham C. Inhibition of DNA synthesis but not of poly-dAT synthesis by the arabinose analogue of cytidine in vitro. Nature. 1971 Oct 13;233(5320):212–214. [PubMed] [Google Scholar]
- Furth J. J., Cohen S. S. Inhibition of mammalian DNA polymerase by the 5'-triphosphate of 1-beta-d-arabinofuranosylcytosine and the 5'-triphosphate of 9-beta-d-arabinofuranoxyladenine. Cancer Res. 1968 Oct;28(10):2061–2067. [PubMed] [Google Scholar]
- Greaves M., Janossy G. Elicitation of selective T and B lymphocyte responses by cell surface binding ligands. Transplant Rev. 1972;11:87–130. doi: 10.1111/j.1600-065x.1972.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Green J. A., Cooperband S. R., Kibrick S. Immune specific induction of interferon production in cultures of human blood lymphocytes. Science. 1969 Jun 20;164(3886):1415–1417. doi: 10.1126/science.164.3886.1415. [DOI] [PubMed] [Google Scholar]
- Johnson H. M., Stanton G. J., Baron S. Relative ability of mitogens to stimulate production of interferon by lymphoid cells and to induce suppression of the in vitro immune response. Proc Soc Exp Biol Med. 1977 Jan;154(1):138–141. [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz E. J., Roessler W. G., Woodburn M. J., Lynch J. M., Jacoby H. M., Silverman S. J., Gorman J. C., Spero L. Purification and some chemical and physical properties of staphylococcal enterotoxin A. Biochemistry. 1972 Feb 1;11(3):360–366. doi: 10.1021/bi00753a009. [DOI] [PubMed] [Google Scholar]
- Schmalstieg F. C., Rudloff H. B., Goldblum R. M., Goldman A. S. The motility of human T lymphocytes. J Reticuloendothel Soc. 1976 Nov;20(5):331–339. [PubMed] [Google Scholar]
- Smith B. G., Johnson H. M. The effect of staphylococcal enterotoxins on the primary in vitro immune response. J Immunol. 1975 Aug;115(2):575–578. [PubMed] [Google Scholar]
- Stobo J., Green I., Jackson L., Baron S. Identification of a subpopulation of mouse lymphoid cells required for interferon production after stimulation with mitogens. J Immunol. 1974 Apr;112(4):1589–1593. [PubMed] [Google Scholar]
- Taranta A. Lymphocyte mitogens of staphylococcal origin. Ann N Y Acad Sci. 1974 Jul 31;236(0):362–375. doi: 10.1111/j.1749-6632.1974.tb41503.x. [DOI] [PubMed] [Google Scholar]
- Tse H. Y., Dutton R. W. Separation of helper and suppressor T lymphocytes. II. Ly phenotypes and lack of DNA synthesis requirement for the generation of concanavalin A helper and suppressor cells. J Exp Med. 1977 Sep 1;146(3):747–758. doi: 10.1084/jem.146.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse H., Dutton R. W. Separation of helper and suppressor T lymphocytes on a ficoll velocity sedimentation gradient. J Exp Med. 1976 May 1;143(5):1199–1210. doi: 10.1084/jem.143.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen W. C., Dean J. H., Lucas D. O. Interferon and the cellular immune response: separation of interferon-producing cells from DNA-synthetic cells. Cell Immunol. 1973 Jan;6(1):110–122. doi: 10.1016/0008-8749(73)90011-7. [DOI] [PubMed] [Google Scholar]


