Abstract
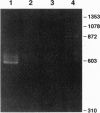
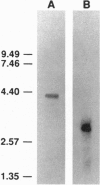
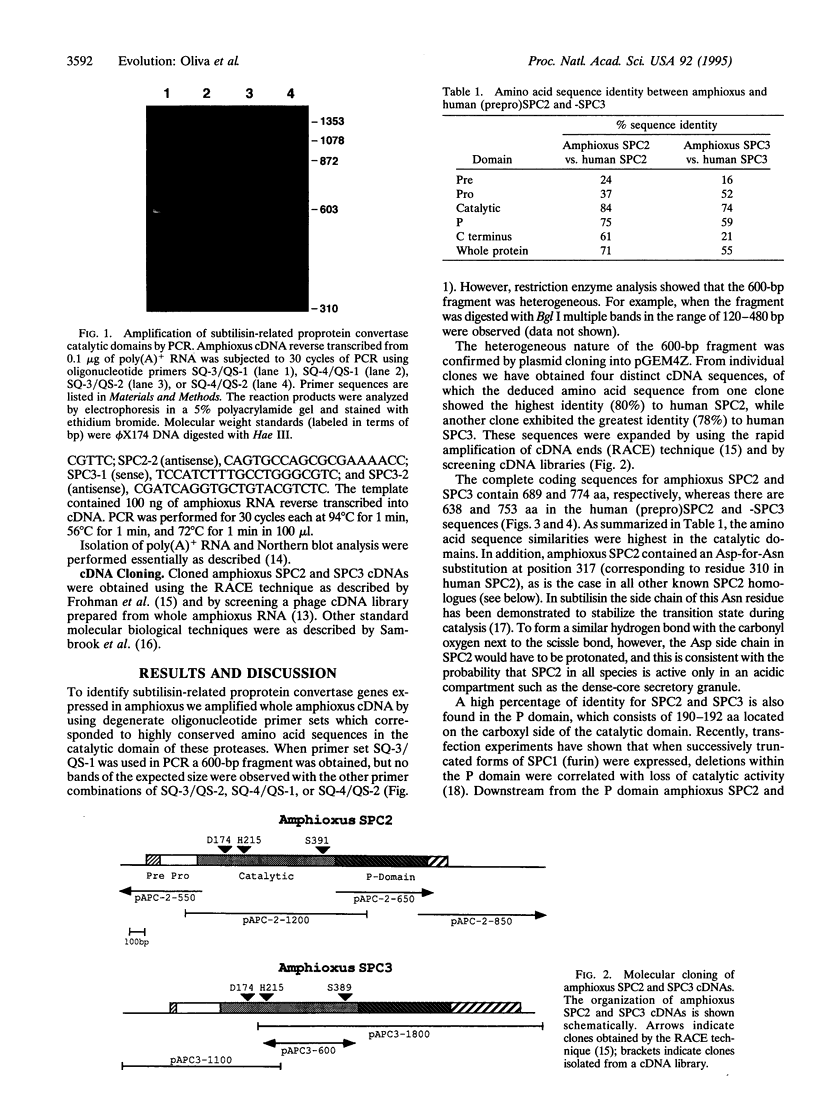
SPC2 and SPC3 are two members of a family of subtilisin-related proteases which play essential roles in the processing of prohormones into their mature forms in the pancreatic B cell and many other neuroendocrine cells. To investigate the phylogenetic origins and evolutionary functions of SPC2 and SPC3 we have identified and cloned cDNAs encoding these enzymes from amphioxus (Branchiostoma californiensis), a primitive chordate. The amino acid sequence of preproSPC2 contains 689 aa and is 71% identical to human SPC2. In contrast, amphioxus prproSPC3 consists of 774 aa and exhibits 55% identity to human SPC3. These results suggest that the primary structure of SPC2 has been more highly conserved during evolution than that of SPC3. To further investigate the function(s) of SPC2 and SPC3 in amphioxus, we have determined the regional expression of these genes by using a reverse transcriptase-linked polymerase chain reaction (RT-PCR) assay. Whole amphioxus was dissected longitudinally into four equal-length segments and RNA was extracted. Using RT-PCR to simultaneously amplify SPC2 and SPC3 DNA fragments, we found that the cranial region (section 1) expressed equal amounts of SPC2 and SPC3 mRNAs, whereas in the caudal region (section 4) the SPC2-to-SPC3 ratio was 5:1. In the mid-body sections 2 and 3 the SPC2-to-SPC3 ratio was 1:5. By RT-PCR we also determined that amphioxus ILP, a homologue of mammalian insulin/insulin-like growth factor, was expressed predominately in section 3. These results suggest that the relative levels of SPC2 and SPC3 mRNAs are specifically regulated in various amphioxus tissues. Furthermore, the ubiquitous expression of these mRNAs in the organism indicates that they are involved in the processing of other precursor proteins in addition to proILP.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braks J. A., Guldemond K. C., van Riel M. C., Coenen A. J., Martens G. J. Structure and expression of Xenopus prohormone convertase PC2. FEBS Lett. 1992 Jun 22;305(1):45–50. doi: 10.1016/0014-5793(92)80652-w. [DOI] [PubMed] [Google Scholar]
- Chan S. J., Cao Q. P., Steiner D. F. Evolution of the insulin superfamily: cloning of a hybrid insulin/insulin-like growth factor cDNA from amphioxus. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9319–9323. doi: 10.1073/pnas.87.23.9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. J., Oliva A. A., Jr, LaMendola J., Grens A., Bode H., Steiner D. F. Conservation of the prohormone convertase gene family in metazoa: analysis of cDNAs encoding a PC3-like protein from hydra. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6678–6682. doi: 10.1073/pnas.89.15.6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J. Y., Korner J., Kreiner T., Scheller R. H., Axel R. The function and differential sorting of a family of aplysia prohormone processing enzymes. Neuron. 1994 Apr;12(4):831–844. doi: 10.1016/0896-6273(94)90336-0. [DOI] [PubMed] [Google Scholar]
- Field K. G., Olsen G. J., Lane D. J., Giovannoni S. J., Ghiselin M. T., Raff E. C., Pace N. R., Raff R. A. Molecular phylogeny of the animal kingdom. Science. 1988 Feb 12;239(4841 Pt 1):748–753. doi: 10.1126/science.3277277. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. S., Sterne R. E., Thorner J. Enzymes required for yeast prohormone processing. Annu Rev Physiol. 1988;50:345–362. doi: 10.1146/annurev.ph.50.030188.002021. [DOI] [PubMed] [Google Scholar]
- Hatsuzawa K., Nagahama M., Takahashi S., Takada K., Murakami K., Nakayama K. Purification and characterization of furin, a Kex2-like processing endoprotease, produced in Chinese hamster ovary cells. J Biol Chem. 1992 Aug 15;267(23):16094–16099. [PubMed] [Google Scholar]
- Kiefer M. C., Tucker J. E., Joh R., Landsberg K. E., Saltman D., Barr P. J. Identification of a second human subtilisin-like protease gene in the fes/fps region of chromosome 15. DNA Cell Biol. 1991 Dec;10(10):757–769. doi: 10.1089/dna.1991.10.757. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Kim W. S., Torii S., Hosaka M., Nakagawa T., Ikemizu J., Baba T., Murakami K. Identification of the fourth member of the mammalian endoprotease family homologous to the yeast Kex2 protease. Its testis-specific expression. J Biol Chem. 1992 Mar 25;267(9):5897–5900. [PubMed] [Google Scholar]
- Ouimet T., Mammarbachi A., Cloutier T., Seidah N. G., Castellucci V. F. cDNA structure and in situ localization of the Aplysia californica pro-hormone convertase PC2. FEBS Lett. 1993 Sep 20;330(3):343–346. doi: 10.1016/0014-5793(93)80901-6. [DOI] [PubMed] [Google Scholar]
- Roebroek A. J., Schalken J. A., Leunissen J. A., Onnekink C., Bloemers H. P., Van de Ven W. J. Evolutionary conserved close linkage of the c-fes/fps proto-oncogene and genetic sequences encoding a receptor-like protein. EMBO J. 1986 Sep;5(9):2197–2202. doi: 10.1002/j.1460-2075.1986.tb04484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah N. G., Gaspar L., Mion P., Marcinkiewicz M., Mbikay M., Chrétien M. cDNA sequence of two distinct pituitary proteins homologous to Kex2 and furin gene products: tissue-specific mRNAs encoding candidates for pro-hormone processing proteinases. DNA Cell Biol. 1990 Jul-Aug;9(6):415–424. doi: 10.1089/dna.1990.9.415. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Marcinkiewicz M., Benjannet S., Gaspar L., Beaubien G., Mattei M. G., Lazure C., Mbikay M., Chrétien M. Cloning and primary sequence of a mouse candidate prohormone convertase PC1 homologous to PC2, Furin, and Kex2: distinct chromosomal localization and messenger RNA distribution in brain and pituitary compared to PC2. Mol Endocrinol. 1991 Jan;5(1):111–122. doi: 10.1210/mend-5-1-111. [DOI] [PubMed] [Google Scholar]
- Smeekens S. P., Avruch A. S., LaMendola J., Chan S. J., Steiner D. F. Identification of a cDNA encoding a second putative prohormone convertase related to PC2 in AtT20 cells and islets of Langerhans. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):340–344. doi: 10.1073/pnas.88.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S. P., Montag A. G., Thomas G., Albiges-Rizo C., Carroll R., Benig M., Phillips L. A., Martin S., Ohagi S., Gardner P. Proinsulin processing by the subtilisin-related proprotein convertases furin, PC2, and PC3. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8822–8826. doi: 10.1073/pnas.89.18.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S. P. Processing of protein precursors by a novel family of subtilisin-related mammalian endoproteases. Biotechnology (N Y) 1993 Feb;11(2):182–186. doi: 10.1038/nbt0293-182. [DOI] [PubMed] [Google Scholar]
- Smeekens S. P., Steiner D. F. Identification of a human insulinoma cDNA encoding a novel mammalian protein structurally related to the yeast dibasic processing protease Kex2. J Biol Chem. 1990 Feb 25;265(6):2997–3000. [PubMed] [Google Scholar]
- Smit A. B., Spijker S., Geraerts W. P. Molluscan putative prohormone convertases: structural diversity in the central nervous system of Lymnaea stagnalis. FEBS Lett. 1992 Nov 9;312(2-3):213–218. doi: 10.1016/0014-5793(92)80938-d. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Smeekens S. P., Ohagi S., Chan S. J. The new enzymology of precursor processing endoproteases. J Biol Chem. 1992 Nov 25;267(33):23435–23438. [PubMed] [Google Scholar]
- Van de Ven W. J., Roebroek A. J., Van Duijnhoven H. L. Structure and function of eukaryotic proprotein processing enzymes of the subtilisin family of serine proteases. Crit Rev Oncog. 1993;4(2):115–136. [PubMed] [Google Scholar]