Abstract
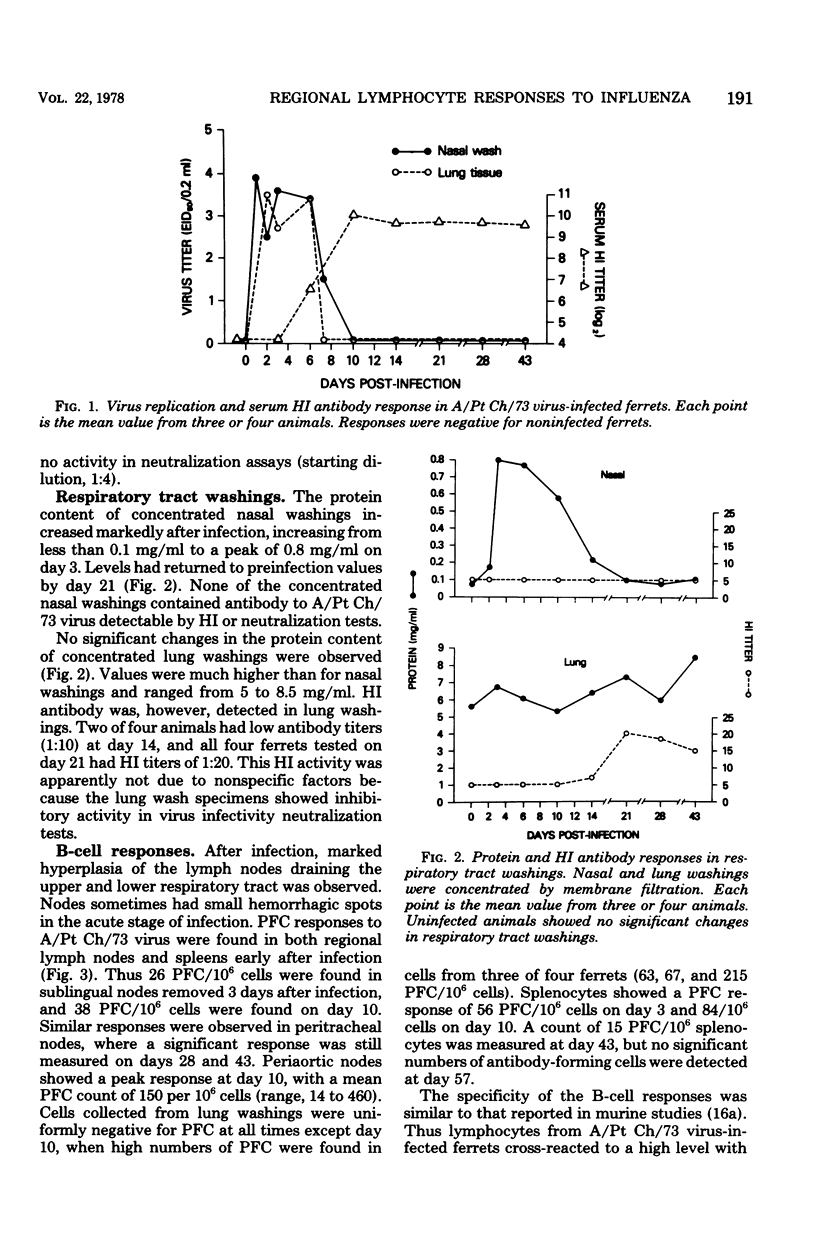
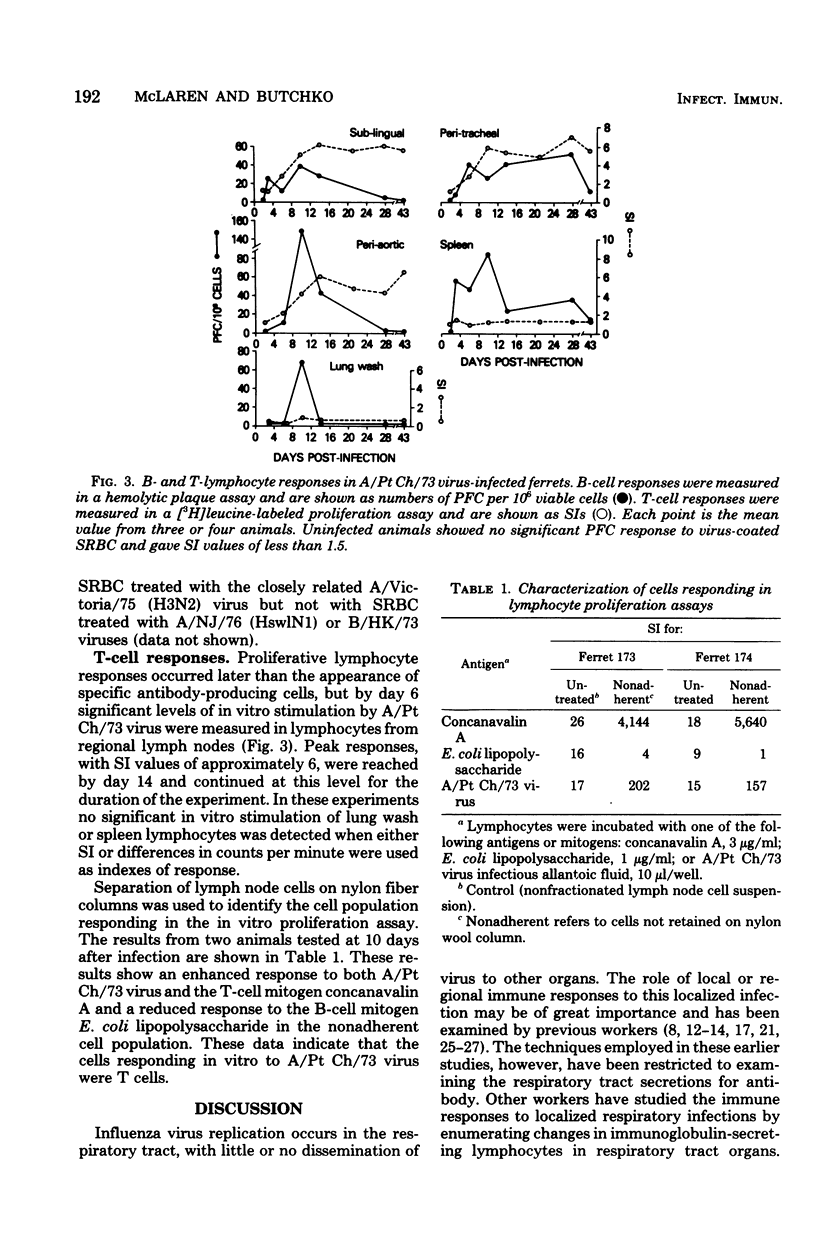
Ferrets were infected with A/Port Chalmers/72 influenza virus and the T- and B-cell responses in the spleen, in lymph nodes draining the upper and lower respiratory tract, and in lung washings were examined in vitro. Lymphocyte responses were measured by using a hemolytic plaque assay for B cells and a proliferation assay for T cells. Virus and antibody levels were measured in respiratory tract washings, and antibody titers were measured in sera from infected animals. Individual B cells secreting specific antibody to A/Port Chalmers/72 virus were detected in regional lymph node and spleen preparations as early as 3 days and as late as 43 days after infection. T-cell assays showed an in vitro response of lymph node cells to A/Port Chalmers/73 virus from day 6 to day 43. Virus was isolated from the respiratory tract up to 7 days after infection. Serum hemagglutination-inhibiting antibody was first detectable on day 6, with maximum titers reached by day 10. These results demonstrated that antibody production and a cellular immune responses were detectable at regional sites at a time when virus was still present and before serum antibody was measured.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blandford G., Cureton R. J., Heath R. B. Studies of the immune respnse in Sendai virus infection of mice. J Med Microbiol. 1971 Aug;4(3):351–356. doi: 10.1099/00222615-4-3-351. [DOI] [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- Cambridge G., Mackenzie J. S., Keast D. Cell-mediated immune response to influenza virus infections in mice. Infect Immun. 1976 Jan;13(1):36–43. doi: 10.1128/iai.13.1.36-43.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole P. J., Molyneux M. E. Lymphocyte reactivity to influenza virus in man. Immunology. 1975 Oct;29(4):749–754. [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Effros R. B., Bennink J. Heterogeneity of the cytotoxic response of thymus-derived lymphocytes after immunization with influenza viruses. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1209–1213. doi: 10.1073/pnas.74.3.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie J. C., Stuart-Harris C. H. The production of neutralizing activity in serum and nasal secretion following immunization with influenza B virus. J Hyg (Lond) 1970 Jun;68(2):233–244. doi: 10.1017/s0022172400028709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros R. B., Doherty P. C., Gerhard W., Bennink J. Generation of both cross-reactive and virus-specific T-cell populations after immunization with serologically distinct influenza A viruses. J Exp Med. 1977 Mar 1;145(3):557–568. doi: 10.1084/jem.145.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis F. A., Martin W. J., Verbonitz M. W., Butchko G. M. Specificity studies on cytotoxic thymus-derived lymphocytes reactive with influenza virus-infected cells: evidence for dual recognition of H-2 and viral hemagglutinin antigens. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3006–3010. doi: 10.1073/pnas.74.7.3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis F. A., Martin W. J., Verbonitz M. W. Hemagglutinin-specific cytotoxic T-cell response during influenza infection. J Exp Med. 1977 Sep 1;146(3):893–898. doi: 10.1084/jem.146.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedson D. S., Fulk R. V., Huber M. A., Reisberg M. A., Kasel J. A. Anti-neuraminidase antibody response in serum and nasal secretions following intranasal or subcutaneous inactivated A2-Hong Kong-68 influenza virus vaccine. J Immunol. 1971 Sep;107(3):730–737. [PubMed] [Google Scholar]
- Freestone D. S., Hamilton-Smith S., Schild G. C., Buckland R., Chinn S., Tyrrell D. A. Antibody responses and resistance to challenge in volunteers vaccinated with live attenuated, detergent split and oil adjuvant A2-Hong Kong-68 (H 3 N 2 ) influenza vaccines. A report to the Medical Research Council Committee on Influenza and other Respiratory Virus Vaccines. J Hyg (Lond) 1972 Sep;70(3):531–543. doi: 10.1017/s0022172400063117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwaltney J. M., Jr, Edmondson W. P., Jr, Rothenberg R., White P. W. A comparison of subcutaneous, nasal, and combined influenza vaccination. I. Antigenicity. Am J Epidemiol. 1971 Jun;93(6):472–479. doi: 10.1093/oxfordjournals.aje.a121281. [DOI] [PubMed] [Google Scholar]
- Jerne N. K., Henry C., Nordin A. A., Fuji H., Koros A. M., Lefkovits I. Plaque forming cells: methodology and theory. Transplant Rev. 1974;18:130–191. doi: 10.1111/j.1600-065x.1974.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- McLaren C., Grubbs G. E., Ennis F. A. Detection of cells producing surface antigen-specific antibody to influenza viruses. J Clin Microbiol. 1978 Oct;8(4):438–444. doi: 10.1128/jcm.8.4.438-444.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren C., Potter C. W., Jennings R. Immunity to influenza in ferrets. X. Intranasal immunization of ferrets with inactivated influenza A virus vaccines. Infect Immun. 1974 Jun;9(6):985–990. doi: 10.1128/iai.9.6.985-990.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter C. W., McLaren C., Jennings R. Assessment of resistance to influenza virus infection in animal models. Dev Biol Stand. 1975;28:307–318. [PubMed] [Google Scholar]
- Potter C. W., Oxford J. S., Shore S. L., McLaren C., Stuart-Harris C. Immunity to influenza in ferrets. I. Response to live and killed virus. Br J Exp Pathol. 1972 Apr;53(2):153–167. [PMC free article] [PubMed] [Google Scholar]
- Ruben F. L., Jackson G. G., Gotoff S. P. Humoral and cellular response in humans after immunization with influenza vaccine. Infect Immun. 1973 Apr;7(4):594–596. doi: 10.1128/iai.7.4.594-596.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S. M., McCahon D., Beare A. S. A single radial haemolysis technique for the measurement of influenza antibody. J Gen Virol. 1975 Apr;27(1):1–10. doi: 10.1099/0022-1317-27-1-1. [DOI] [PubMed] [Google Scholar]
- Scott G. H., Walker J. S. Immunoglobulin-bearing cells in lungs of mice infected with influenza virus. Infect Immun. 1976 May;13(5):1525–1527. doi: 10.1128/iai.13.5.1525-1527.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore S. L., Potter C. W., McLaren C. Immunity to influenza in ferrets. IV. Antibody in nasal secretions. J Infect Dis. 1972 Oct;126(4):394–400. doi: 10.1093/infdis/126.4.394. [DOI] [PubMed] [Google Scholar]
- Waldman R. H., Spencer C. S., Johnson J. E., 3rd Respiratory and systemic cellular and humoral immune responses to influenza virus vaccine administered parenterally or by nose drops. Cell Immunol. 1972 Feb;3(2):294–300. doi: 10.1016/0008-8749(72)90168-2. [DOI] [PubMed] [Google Scholar]
- Wilson A. B., Planterose D. N., Nagington J., Park J. R., Barry R. D., Coombs R. R. Influenza A antigens on human lymphocytes in vitro and probably in vivo. Nature. 1976 Feb 19;259(5544):582–584. doi: 10.1038/259582a0. [DOI] [PubMed] [Google Scholar]


