Abstract
Background.
Capecitabine is used mainly with oxaliplatin to treat metastatic colorectal cancer (mCRC). Results from capecitabine plus irinotecan (XELIRI) with or without bevacizumab (BV) have been reported in Europe but not in Japan. Consequently, the safety and efficacy of XELIRI plus BV in Japanese patients with mCRC were assessed in a single-arm phase II study.
Methods.
Eligible patients had had prior chemotherapy containing BV for mCRC and wild-type or heterozygous UGT1A1. Therapy in each 21-day treatment cycle consisted of capecitabine (800 mg/m2 twice daily on days 1–15), irinotecan (200 mg/m2 on day 1), and BV (7.5 mg/kg on day 1). The primary endpoint was dose-limiting toxicity in phase I and progression-free survival (PFS) in phase II.
Results.
A total of 34 patients (6 in phase I, 28 in phase II) were enrolled from May 2010 to June 2011. Baseline characteristics included a median age of 60 years (range: 22–74 years) for 24 men and 10 women. No dose-limiting toxicities appeared in phase I. Median PFS was 240 days (95% confidence interval: 179–311 days). Overall response rate was 18.1%, and the disease-control rate was 90.9%. The incidence of adverse events frequently associated with irinotecan and capecitabine were neutropenia (any grade, 55.9%; grade 3 or 4, 11.8%), diarrhea (any grade, 50%; grade 3 or 4, 5.9%), and hand-foot syndrome (any grade, 61.8%; grade 3 or 4, 5.9%).
Conclusion.
Our results suggest that XELIRI plus BV is well tolerated and effective as a second-line treatment for mCRC in Japanese patients. This regimen could be especially appropriate for patients resistant to oxaliplatin-based regimens.
Abstract
摘要
背景. 卡培他滨主要与奥沙利铂联合治疗转移性结直肠癌(mCRC)。欧洲已经报告了卡培他滨+伊立替康(XELIRI)联合或不联合贝伐珠单抗(BV)的结果,但日本还没有这方面的数据。因此,我们开展了一项单臂 II 期研究,以评估 XELIRI 联合 BV 用于日本患者的安全性和疗效。
方法. mCRC 患者入组标准为既往接受过含 BV 的化疗,UGT1A1 野生型或杂合子。每 21 天为一个治疗周期,方案为卡培他滨(800 mg/m2,每日 2 次,第 1∼15 天)、伊立替康(200 mg/m2,第 1 天)、BV(7.5 mg/kg,第 1 天)。I 期研究主要终点为剂量限制性毒性反应,II 期研究主要终点为无进展生存(PFS)。
结果. 2010 年 5 月∼2011 年 6 月共入组 34 例患者(I 期 6 例,II 期 28 例)。基线特征为中位年龄 60 岁(22∼74 岁),男性 24 例,女性 10 例。I 期未发现剂量限制性毒性反应。中位 PFS 为 240 天(95% 可信区间:179∼311 天)。总缓解率为 18.1%,疾病控制率为 90.9%。伊立替康与卡培他滨相关的不良事件多为中性粒细胞减少(任何等级 55.9%;3 或 4 级 11.8%)、腹泻(任何等级 50%;3 或 4 级 5.9%)以及手足综合征(任何等级 61.8%;3 或 4 级 5.9%)。
结论. 本研究结果提示,XELIRI 联合 BV 用于日本 mCRC 患者二线治疗具有良好的耐受性和有效性。该方案特别适合对以奥沙利铂为基础的方案耐药的患者。The Oncologist 2014;19: 1131-1132
Author Summary
Discussion
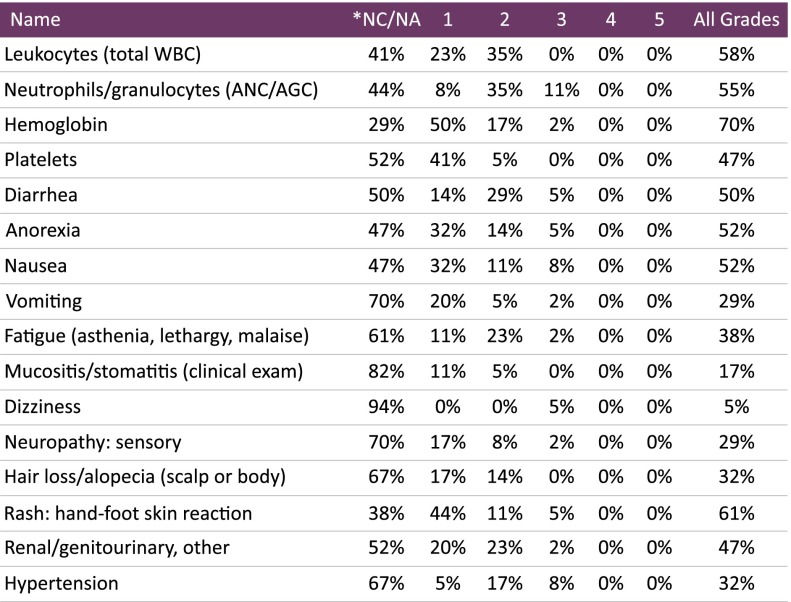
In this prospective trial for Japanese patients with metastatic colorectal cancer, capecitabine plus irinotecan (XELIRI) plus bevacizumab as a second-line regimen achieved longer progression-free survival (240 days) and a higher overall response rate (18.1%) than other reported regimens, with an acceptable tolerability profile (Fig. 1). Unlike FOLFIRI, XELIRI doses do not require a long infusion process or an infuser pump, providing a great advantage to patients. The key finding in this study was that XELIRI plus bevacizumab demonstrated promising results beyond progression in Japanese patients.
Figure 1.
Adverse events during phase I/II treatment. No serious adverse events were reported.
Abbreviations: *, no change from baseline/no adverse event; AGC, absolute granulocyte count; ANC, absolute neutrophil count; WBC, white blood cells.
Supplementary Material
Footnotes
Access the full results at: Hamamoto_Yamada-14-159.theoncologist.com
ClinicalTrials.gov Identifier: UMIN000003482
Sponsor(s): Epidemiological and Clinical Research Information Network: ECRIN (Kyoto, Japan)
Principal Investigator: Yasuhide Yamada
IRB Approved: Yes
For Further Reading:Herbert I. Hurwitz, Niall C. Tebbutt, Fairooz Kabbinavar et al. Efficacy and Safety of Bevacizumab in Metastatic Colorectal Cancer: Pooled Analysis From Seven Randomized Controlled Trials. The Oncologist 2013;18:1004–1012.
Implications for Practice:Several randomized trials of bevacizumab have been conducted to address specific questions regarding its use for patients with metastatic colorectal cancer (mCRC); however, because of their sample size limitations, subgroup analyses are frequently of limited power. By pooling individual patient data from seven randomized trials, more comprehensive analyses of the efficacy and safety of bevacizumab were made possible because of the large number of included patients. In addition, outcomes in clinically relevant subgroups were examined, and the data from these subgroups were consistent with those reported in the overall analyses. The results of this pooled analysis help further the clinician's understanding of the overall risks and benefits associated with adding bevacizumab to chemotherapy for patients with mCRC.
Author disclosures available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.