The authors report the results of the EACH study for the subgroup of Chinese patients with advanced hepatocellular carcinoma (HCC) who are ineligible for curative resection or local treatment. FOLFOX4 significantly improved response and disease-control rates and prolonged survival compared with doxorubicin. Systemic chemotherapy with oxaliplatin-based regimens may play an important role in the treatment of Chinese patients with advanced HCC.
Keywords: Hepatocellular carcinoma, Oxaliplatin, FOLFOX4 regimen, Systemic chemotherapy
Abstract
Background.
The EACH study assessed the efficacy of oxaliplatin, 5-fluorouracil, and leucovorin (the FOLFOX4 regimen) compared with doxorubicin alone in terms of overall survival (OS), progression-free survival (PFS), and safety in patients with advanced hepatocellular carcinoma (HCC). We present the results of this study in Chinese patients.
Methods.
In a multicenter, open-label, randomized, phase III study (NCT00471965), 371 patients (279 patients from the People’s Republic of China) were randomized 1:1 to receive either FOLFOX4 or doxorubicin until disease progression, intolerable toxicity, death, or surgical resection.
Results.
Baseline characteristics of the Chinese patients enrolled in the study were similar for the 2 treatment groups and in comparison with the whole EACH cohort. Median OS at the prespecified time point of treatment was 5.7 months with FOLFOX4 and 4.3 months with doxorubicin (hazard ratio [HR]: 0.74; 95% confidence interval [CI]: 0.55–0.98; p = .03). At the end of the follow-up period, median OS was 5.9 months with FOLFOX4 and 4.3 months with doxorubicin (HR: 0.75; 95% CI: 0.58–0.98; p = .03). Median PFS was 2.4 months and 1.7 months in the FOLFOX4 and doxorubicin groups, respectively (HR: 0.55; 95% CI: 0.45–0.78; p = .0002). The response rate (RR) and disease control rate (DCR) were significantly higher in the FOLFOX4 group than in the doxorubicin group (RR: 8.6% vs. 1.4%, p = .006; DCR: 47.1% vs. 26.6%, p = .0004). Hematological toxicity was more frequently reported in the FOLFOX4 group.
Conclusion.
For Chinese HCC patients enrolled in the EACH study, FOLFOX4 significantly improved the RR and DCR and prolonged survival compared with doxorubicin. Systemic chemotherapy with oxaliplatin-based regimens may play an important role in the treatment of Chinese patients with advanced HCC.
Abstract
摘要
背景. EACH 研究评估了 FOLFOX4 方案(奥沙利铂、5-氟尿嘧啶和亚叶酸)与多柔比星单药治疗进展期肝细胞癌(HCC)患者的有效性 [包括总生存(OS)和无进展生存(PFS)]和安全性。本文介绍了 EACH 研究的中国患者结果。
方法. EACH 研究是一项多中心、开放标签的 III 期随机临床试验(NCT00471965),371 例患者(其中 279 例来自中国大陆)按 1:1 比例随机分配,接受 FOLFOX4 方案或多柔比星单药治疗,直至疾病进展、发生不可耐受的毒性事件、死亡或接受手术切除。
结果. 中国招募的患者中两个治疗组的基线特征相似,中国患者人群的基线特征与 EACH 研究总队列也相似。在计划的治疗时间内,FOLFOX4 方案与多柔比星的中位 OS 分别为 5.7 个月和 4.3 个月 [风险比(HR):0.74;95% 可信区间(CI):0.55 ∼ 0.98,P = 0.03]。随访结束时,FOLFOX4 方案与多柔比星的中位 OS 分别为 5.9 个月和 4.3 个月(HR:0.75,95%CI :0.58 ∼ 0.98;P = 0.03)。FOLFOX4 组和多柔比星组的中位 PFS 分别为 2.4 个月和 1.7 个月(HR:0.55;95%CI :0.45 ∼ 0.78;P = 0.0002)。FOLFOX4 组的缓解率(RR)和疾病控制率(DCR)显著高于多柔比星组(RR:8.6% vs. 1.4%,P = 0.006 ;DCR:47.1% vs. 26.6%,P = 0.0004)。FOLFOX4 方案组中血液学毒性更常见。
结论. 与多柔比星相比,FOLFOX4 方案显著提高 EACH 研究入组的中国 HCC 患者的 RR 和 DCR,并延长了生存期。以奥沙利铂为基础的全身化疗方案在中国进展期 HCC 患者的治疗方面可能具有重要作用。The Oncologist 2014;19: 1169-1178
Implications for Practice:
We report the results of the EACH study for the subgroup of Chinese patients with advanced hepatocellular carcinoma (HCC) who are ineligible for curative resection or local treatment. We showed that an oxaliplatin-based chemotherapy regimen (FOLFOX4; oxaliplatin, 5-fluorouracil, and leucovorin) significantly improved the response rate and the disease control rate and prolonged survival compared with doxorubicin in Chinese patients. Oxaliplatin was approved by the China Food and Drug Administration for systemic chemotherapy of HCC. The FOLFOX4 regimen is an affordable treatment option for most advanced HCC patients in the People’s Republic of China, and it may play an important role in the treatment of these patients.
Introduction
Worldwide, hepatocellular carcinoma (HCC) is the fifth most common cancer in men and the seventh in women and is responsible for 9.2% (696,000) of cancer deaths overall [1]. In 2008, more than 50% of all new HCC cases reported worldwide were diagnosed in the People’s Republic of China [1]. This high incidence can be attributed primarily to a high prevalence of hepatitis B virus (HBV) infection [2]. A serosurvey performed in China in 2006, 14 years after the introduction of vaccination against hepatitis B in infants, reported a hepatitis B surface antigen prevalence of 7.2% in participants aged 1–59 years [3]. Additional environmental risk factors have also been described in Chinese populations that affect either HCC prevalence directly or progression from hepatitis B infection to HCC. These factors include exposure to aflatoxin [4, 5], contamination of drinking water with blue-green algae, nitrite or organochlorine pesticides [6], coinfection with hepatitis C virus (HCV) [7], and excessive alcohol consumption. A number of genetic polymorphisms have also been associated with the progression to HCC among persons infected with hepatitis B virus, including the susceptibility locus (rs17401966) in the kinesin family member 1B gene (KIF1B) at chromosome 1p36.22 [8], rs9272105 (HLA-DQA1/DRB1), and rs455804 (GRIK1) [9].
To date, tumor resection, liver transplantation, and local ablation are regarded as potentially curative therapeutic options for early stages of HCC and are associated with 5-year survival rates of 40%–70% [10–12]. Among noncurative therapies, transarterial chemoembolization has been shown to positively affect survival [13]. Recent advances have focused on identification of at-risk populations, early detection, and treatment of early stage disease. However, the majority of Asian HCC patients have locally advanced or metastatic disease at diagnosis and thus are not eligible for resection or local treatments [14].
Previously, HCC was considered resistant to common anticancer chemotherapy [15]. Prior to the introduction of sorafenib in 2008, no effective systemic therapy for advanced HCC was available. Although, doxorubicin was initially shown to prolong survival when compared with best supportive care [16], two analyses that included patients enrolled in clinical studies showed a low overall response rate (RR) when doxorubicin was used as single agent or in combination with other agents [17]. The demonstration of the efficiency of sorafenib, a multikinase inhibitor, was an important milestone in the treatment of patients with advanced HCC [10]. Sorafenib has been shown to improve overall survival (OS) in both Asian and white patients [18, 19]. In an Asia-Pacific randomized phase III study enrolling 271 patients with HCC and no previous systemic therapy, treatment with sorafenib significantly improved median OS compared with placebo: 6.5 months (95% confidence interval [CI]: 5.56–7.56) versus 4.2 months (95% CI: 3.75–5.46), with a hazard ratio (HR) of 0.68 (95% CI: 0.50–0.93; p = .014) [17].
Oxaliplatin (Eloxatin; Sanofi, Paris France, http://en.sanofi.com), a platinum-based cytotoxic agent, has been shown to be active against several cisplatin-resistant cell lines, colorectal carcinoma, and other solid tumors that are not responsive to cisplatin [20]. In addition, the combination of oxaliplatin with 5-fluorouracil (5-FU) has been demonstrated to have synergistic antiproliferative activity in several in vivo tumor models [21].The activity of oxaliplatin-containing regimens in advanced HCC has been documented in a series of phase I and II trials [22–26]. For example, a phase I trial using oxaliplatin plus 5-FU and leucovorin (the FOLFOX4 regimen) administered to Chinese patients with advanced HCC showed an improvement in clinical symptoms and Karnofsky performance status in 8 of 10 enrolled patients, with 1 patient showing partial response and 4 patients showing stable disease and a time to tumor progression of 1.3–5.6 months [26]. A subsequent phase II trial in Chinese patients with advanced HCC using the FOLFOX4 regimen showed a RR of 18.2% with an acceptable safety profile [24].
The EACH study was conducted to assess efficacy and safety in terms of OS of the FOLFOX4 regimen compared with a single-agent doxorubicin regimen in advanced HCC patients ineligible for curative resection or local treatment. We present the results of the subgroup analysis of the EACH study in Chinese patients.
Materials and Methods
Study Design
The EACH study, an international, multicenter, open-label, randomized, phase III study (NCT00471965), was conducted between March 2007 and May 2010 at 38 study centers in mainland China, Taiwan, the Republic of Korea, and Thailand [27]. Of these, 23 participating centers were located in mainland China, and 2 centers were in Taiwan; 76% of enrolled patients were Chinese. The study design was previously described in the paper presenting the EACH study results [27]. Eligible patients were randomized 1:1 to receive either the FOLFOX4 regimen or doxorubicin alone as systemic chemotherapy [27]. Randomization was centralized and stratified by country, Barcelona Clinic Liver Cancer (BCLC) stage, and disease status (locally advanced or metastatic). The treatment phase started within 7 days of randomization and continued until disease progression, intolerable toxicity, death, patient withdrawal of consent, or the patient became eligible for surgical resection, whichever occurred first [27]. The patients from the FOLFOX4 treatment group received 1 cycle of treatment every 2 weeks; those from the doxorubicin group received 1 cycle every 3 weeks. After the treatment phase, patients were followed every 2 months until either death or study termination.
The cut-off date for the prespecified final analysis of the treatment phase was planned to be triggered either when 249 events occurred or after enrollment of the 440th patient, whichever occurred first. The cut-off date for the prespecified final analysis of the treatment phase was May 31, 2009; the cut-off date for the analysis of the data collected during the follow-up (post hoc analysis) was December 31, 2009. The analysis of the results of the Chinese patients enrolled in the EACH study was a planned subgroup analysis and was predefined in the statistical analysis plan.
Tumor evaluation, by imaging techniques (computed tomography [CT] and/or magnetic resonance imaging [MRI] scans) and assessment of serum α-fetoprotein levels, was performed at the screening visit, at randomization, every 6 weeks during the treatment phase, and at each study visit during follow-up. Toxicity and safety were monitored throughout the study.
Written informed consent was obtained from all patients prior to conducting any study-related procedures. The study was conducted in accordance with good clinical practices guidelines, the Declaration of Helsinki, and local laws, regulations, and applicable guidelines of the countries in which the study was conducted. All study-related documents were approved by institutional review boards or independent ethics committees.
Sanofi was the funding source and was involved in all stages of the study conduct and analysis. The study was designed by the principal academic investigators in conjunction with Sanofi’s medical department. Data collection and statistical analysis were performed by Sanofi’s medical department. Data were managed in parallel by the sponsor and the principal investigators. Sanofi and the study steering committee were responsible for the decision to publish the results of the study. All authors had full access to all analyses performed; they took full responsibility for these analyses and for the interpretation of the results.
Objectives
The primary objective of the study was to assess and compare OS in patients treated with either FOLFOX4 or doxorubicin [27]. The secondary objectives were to assess and compare efficacy in terms of progression-free survival (PFS), RR, disease control rate (DCR), secondary resection rate, and safety, for both treatment groups [27]. We aim to assess these objectives in the Chinese patients enrolled into the study.
Study Population
Enrolled patients were aged 18–75 years; had histologically, cytologically, or clinically diagnosed unresectable HCC; were ineligible for or unwilling to receive local invasive treatment (e.g., chemoembolism, ablation); had at least one measurable lesion (≥2 cm on common CT; ≥1 cm on spiral CT or MRI); had not received previous cancer treatment (except for surgery) or had disease progression after a previous interventional or local therapy, with Karnofsky performance status ≥70, life expectancy ≥3 months, and BCLC stage B/C; and showed adequate organ and marrow function. Clinically diagnosed patients had to meet three criteria: α-fetoprotein ≥400 μg/L, CT or MRI evidence of hypervascular liver tumor, and cirrhosis or evidence of infection with hepatitis B or C (e.g., HBV- or HCV-antigen positive). The full list of inclusion criteria was provided in the paper describing the EACH study results [27].
Patients with previous interventional therapy involving chemotherapeutic agents and patients on anticancer herbal treatment were enrolled if the treatment was completed ≥4 weeks prior to randomization; patients with previous adjuvant chemotherapy were eligible if the treatment was completed ≥12 months prior to randomization.
Exclusion criteria included allergy to platinum compounds or other study drugs; any previous oxaliplatin or doxorubicin treatment (except adjuvant treatment more than 12 months before the randomization); previous liver transplantation; concomitant administration of any other anticancer therapy, including interferon-α and herbal medicines and excluding radiotherapy to a nontarget lesion; central nervous system metastasis; history of other malignant disease; pregnancy; lactation; history of other serious illness; or medical conditions.
Study Treatment and Administration
Treatment regimens were administered as follows. The FOLFOX4 regimen included oxaliplatin 85 mg/m2 in 250 mL glucose 5% administered as 2-hour infusion on day 1; leucovorin 200 mg/m2 (dextrorotatory/levorotatory [dl], or dl isoforms) or 100 mg/m2 (levorotatory, or l isoform) in 5% glucose solution administered by infusion on days 1 and 2; a bolus of 400 mg/m2 5-FU; and a 22-hour continuous infusion of 600 mg/m2 5-FU on days 1 and 2, repeated every 2 weeks. Doxorubicin 50 mg/m2 was administered as an intravenous infusion every 3 weeks. The cumulative dose limit for doxorubicin was 450 mg/m2.
Oxaliplatin (Eloxatin; Sanofi) is a lyophilized powder (50 mg per vial) manufactured by Thissen Laboratories (Braine-l'Alleud, Belgium, http://www.cenexi.com/English/2/Cenexi_Thissen_Lab/8). It was packed and labeled by the study sponsor. Leucovorin, 5-U, and doxorubicin were purchased in each participating country and provided to the study centers.
All doses of the study medications were calculated in milligrams of each drug per square meter of body surface area (BSA) as measured at baseline (mg/m2) and rounded to the nearest 5 mg. Dose adjustments during treatment were based on adverse events (AEs). The BSA was recalculated if body weight changed by >5% (i.e., grade 1 according to National Cancer Institute Common Terminology Criteria [NCI-CTC 3.0]). Weight loss was considered an AE. The maximum BSA used to calculate oxaliplatin, 5-FU, leucovorin, and doxorubicin doses was 2 m2. Dose reductions during treatment were based on AEs. Dose re-escalation was not allowed in patients with previous dose reduction related to toxicity. In cases of repeated grade 4 toxicity despite dose reduction or if a dose interruption of more than 2 weeks in the FOLFOX4 group or more than 3 weeks in the doxorubicin group was required because of toxicity, the study treatment was discontinued and the patient continued the study only for follow-up.
Assessment of Efficacy
Efficacy analyses were performed on the intention-to-treat (ITT) cohort, which included all randomized patients, regardless of the number of treatment cycles received. The primary endpoint was OS, measured from the date of randomization until the date of death from any cause. If death was not confirmed, survival time was censored at the last date the patient was known to have been alive or at the cut-off date, whichever came first.
The secondary endpoints were PFS, RR, DCR, and secondary resection rate. PFS was measured from the date of randomization to documentation of disease progression or death from any cause. Patients lost to follow-up or who received other anticancer therapy before progression were censored. Complete and partial responses were assessed by investigators according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 [28] and based on tumor imaging (CT scans and/or MRI) performed every 6 weeks during the treatment phase and at each study visit during follow-up. The number of patients receiving secondary resection after chemotherapy was also recorded. DCR was calculated as the ratio between the number of patients with a complete response, partial response, or stable disease and the number of patients with measurable tumor.
Safety and Toxicity Assessment
Safety analyses were performed on the safety cohort, according to the treatment received. The safety cohort included all patients who received at least one cycle of the study treatment.
Safety data recorded were AEs, serious AEs (SAEs), hematological toxicity, general physical examination, special examinations (chest x-ray, electrocardiogram, echocardiography) and laboratory data. The toxicity profile was assessed according to NCI-CTC version 3.0 and was recorded at every visit after baseline. Blood samples for hematology and biochemistry evaluations (hemoglobin, whole blood cell count, sodium, potassium, calcium, albumin, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, total bilirubin, serum creatinine, glucose, creatinine clearance) were collected at each study visit. The AEs were graded according to NCI-CTC version 3.0 criteria, and the grade of each event was the worst grade that occurred during a two-cycle regimen.
Statistical Analysis
Considering 80% power and a type I error of 5%, it was estimated that the inclusion of a minimum of 200 patients in each group would allow detection of a difference between an OS of 43% in the FOLFOX4 group and 30% in the doxorubicin group at 1 year, assuming a constant HR of 0.701, an accrual period of 12 months, and a total maximum follow-up period of 18 months. The calculation took into account two interim analyses of OS using a group sequential approach with efficacy boundaries based on O’Brien and Fleming’s α spending function [29]. The prespecified final analysis of OS was planned to be triggered after 249 deaths or after enrollment of the 440th patient. Assuming a drop-out rate of 10%, the total sample was planned to include a maximum of 440 patients. OS and PFS were compared between the treatment groups using a stratified log-rank test at a significance level in the final analysis of 5.0% (p ≤ .05). BCLC staging and disease status as specified at the time of randomization were used for stratification. The survival curves were estimated using Kaplan-Meier methods. Medians and corresponding 95% CIs were also provided by treatment groups. RR, DCR, and secondary resection rate were compared between the two treatment arms using the Cochran-Mantel-Haenszel test stratified by BCLC stage and disease status. The chi-square test was used to compare the incidence of AEs between study groups.
Results
Patients
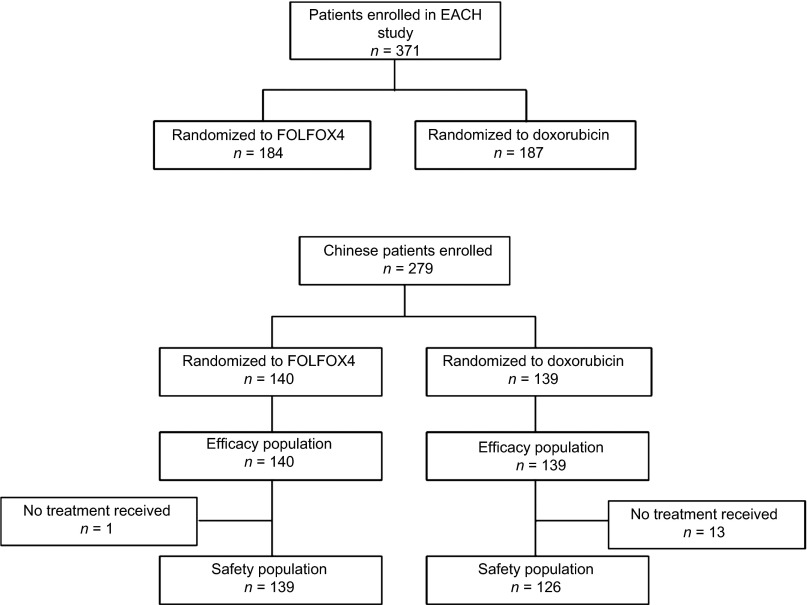
Of the 371 patients enrolled and randomized in the study overall, 279 patients were enrolled and randomized from study centers in China, including 259 from mainland China and 20 from Taiwan (Fig. 1). All randomized Chinese patients (n = 279) were included in the ITT population. At the cut-off date for final analysis, 231 Chinese patients had died (FOLFOX4: 117; doxorubicin: 114); 13 patients were still on study treatment (FOLFOX4: 7; doxorubicin: 6). Fourteen patients had not received any study medication and thus were excluded from the safety population (FOLFOX4: 1; doxorubicin: 13), and 252 patients had discontinued study medication (FOLFOX4: 132; doxorubicin: 120). The most common reason for discontinuation was disease progression, which occurred in 47.1% (66 of 140) of patients in the FOLFOX4 group and 53.2% (74 of 139) of patients in the doxorubicin group. Major protocol deviations were violation of inclusion or exclusion criteria (FOLFOX4: 11; doxorubicin: 13); administration of concomitant anticancer medication or other investigational treatments, as prohibited in the study protocol (FOLFOX4: 1; doxorubicin: 2); randomization error (incorrect stratification; FOLFOX4: 17; doxorubicin: 25); and dose modification error with patients receiving <80% or >120% of the planned dose (FOLFOX4: 2; doxorubicin: 0).
Figure 1.
Disposition of Chinese patients.
Abbreviation: FOLFOX4, oxaliplatin, 5-fluorouracil, and leucovorin.
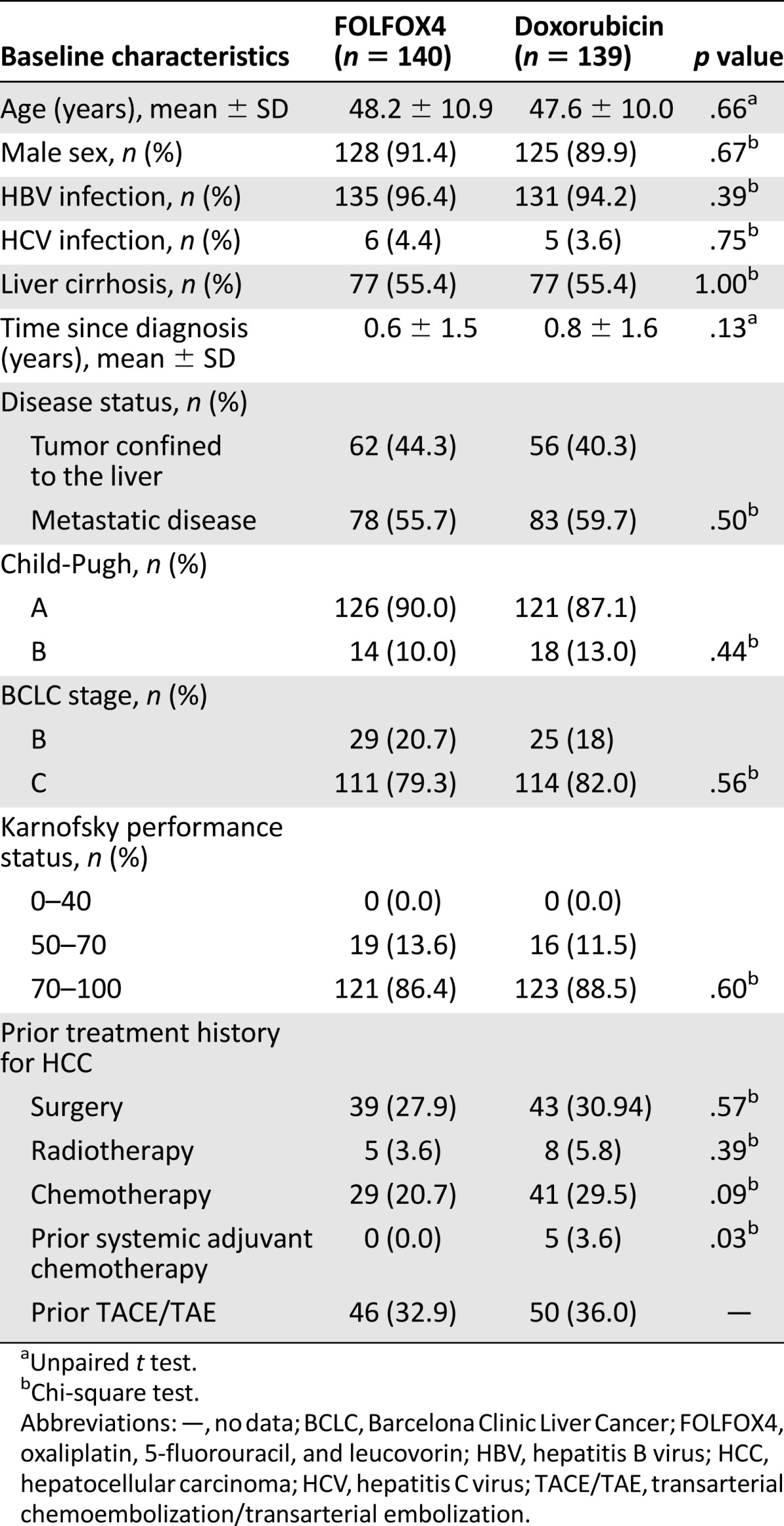
Except for previous administration of systemic adjuvant chemotherapy, there were no significant differences in baseline characteristics between the FOLFOX 4 and doxorubicin study groups in the Chinese population (Table 1). The median number of treatment cycles administered during the study was 3 cycles (range: 1–18) in the FOLFOX4 group and 2 cycles (range: 1–11) in the doxorubicin group.
Table 1.
Baseline characteristics of Chinese patients enrolled
Efficacy Analysis in the Chinese ITT Cohort
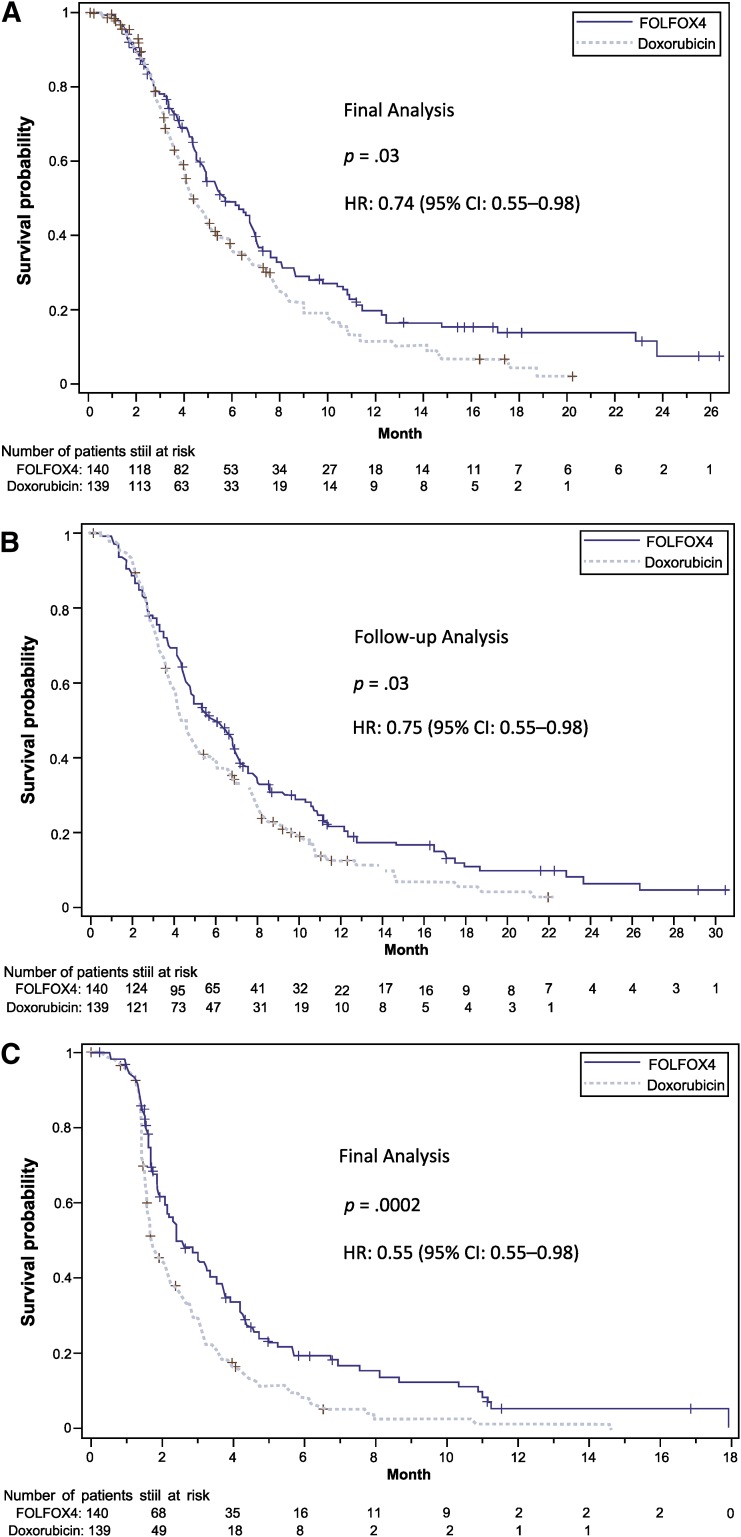
Median OS at the end of treatment was significantly longer in the FOLFOX4 group than in the doxorubicin group (5.7 months [95% CI: 4.8–6.9] vs. 4.3 months [95% CI: 4.0–5.3]), with a 26.4% risk reduction in mortality (HR: 0.74 [95% CI: 0.55–0.98]; p = .03) (Table 2; Fig. 2A). This difference remained statistically significant at the end of the follow-up period, at 5.9 months in the FOLFOX4 group versus 4.3 months in the doxorubicin group (HR: 0.75 [95% CI: 0.58–0.98]; p = .03). Median PFS was 2.4 months (95% CI: 2.1–3.3) and 1.7 months (95% CI: 1.6–2.2) months in the FOLFOX4 and doxorubicin groups, respectively, with a 45% risk reduction in disease progression (HR: 0.55 [95% CI: 0.45–0.78]; p = .0002) (Table 2; Fig. 2B).
Table 2.
Overall and progression-free survival in the Chinese intention-to-treat population
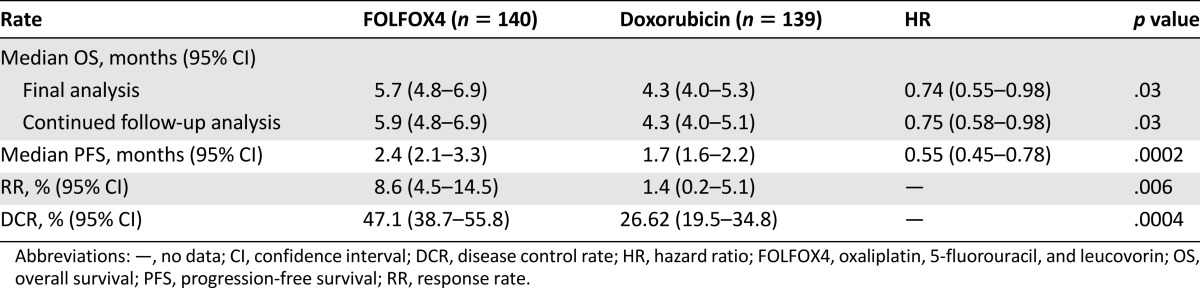
Figure 2.
Kaplan-Meier survival curves for the FOLFOX4 group versus the doxorubicin group (intention-to-treat cohort). (A): Overall survival at final analysis. (B): Overall survival at follow-up analysis. (C): Progression-free survival. +, Stratified log-rank test.
Abbreviations: CI, confidence interval; FOLFOX4, oxaliplatin, 5-fluorouracil, and leucovorin; HR, hazard ratio.
In the FOLFOX4 group, 12 patients (8.6%) had partial response and 54 (38.6%) had stable disease; in the doxorubicin group, 2 patients (1.4%) had partial response and 35 (25.2%) stable disease. There were no complete responses in either group. The RR and the DCR were significantly higher in the FOLFOX4 group than in the doxorubicin group (RR: 8.6% vs. 1.4%, p = .006; DCR: 47.1% vs. 26.6%, p = .0004) (Table 2; Fig. 2C).
Safety Evaluation in the Chinese ITT Cohort
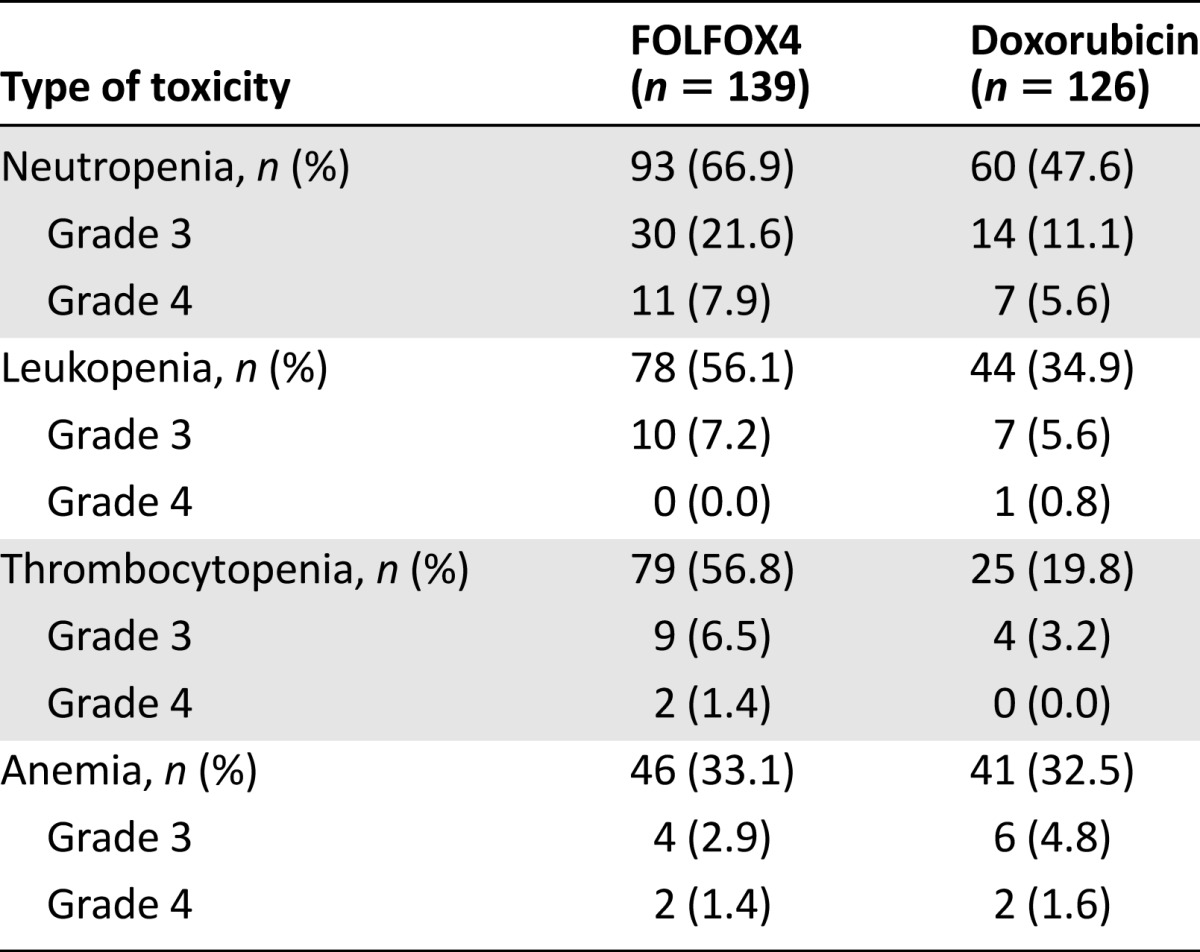
During the treatment period, 93.5% of patients in the FOLFOX4 group and 89.7% in the doxorubicin group experienced at least 1 AE (p = .21) (Table 3). Moreover, 91.4% of patients in the FOLFOX4 group and 86.5% of patients in the doxorubicin group reported AEs considered as possibly related to the study medication (Table 3). A total of 24 patients (15 in the FOLFOX4 group and 9 in the doxorubicin group) reported at least 1 SAE. Of these, 4 patients in the FOLFOX4 group and 2 patients in the doxorubicin group reported SAEs considered as possibly related to the study medication (Table 3). The most frequently reported SAEs were hemorrhage (upper gastrointestinal, gastrointestinal not otherwise specified; FOLFOX4: 4.32%; doxorubicin: 3.17%) followed by pulmonary-other (respiratory failure; FOLFOX4: 2.16%; doxorubicin: 0.00%). The most frequently reported nonhematological AEs were nausea (reported by 41.7% of patients in the FOLOFOX4 group) and alopecia (reported by 38.9% of patients in the doxorubicin group) (Table 4). The most frequently reported hematological toxicity was neutropenia (FOLFOX4: 66.91%; doxorubicin 47.62%) (Table 5).
Table 3.
Incidence of adverse events during study period in both treatment groups (safety cohort)
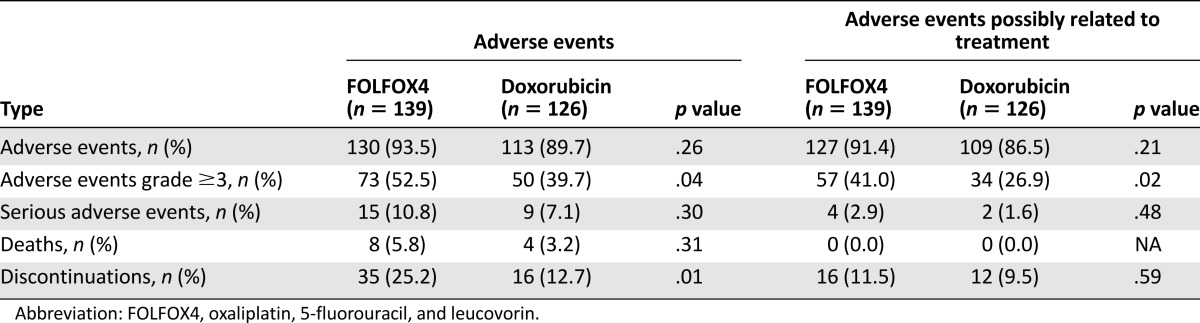
Table 4.
Nonhematological adverse events and adverse events possibly related to treatment (safety cohort)
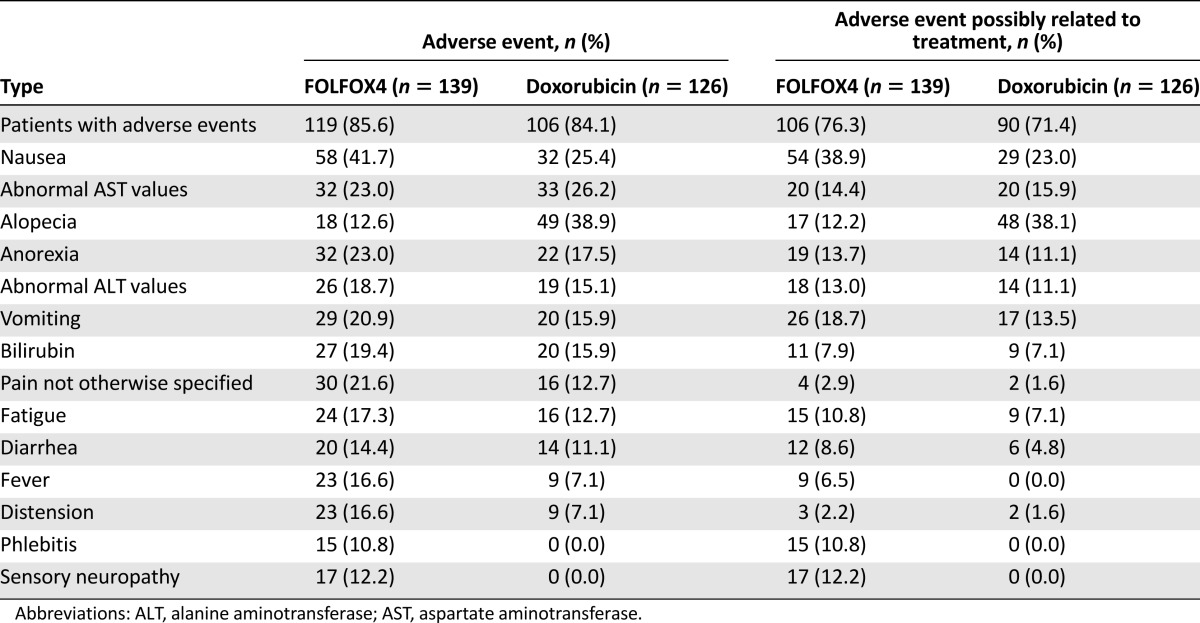
Table 5.
Hematological drug related adverse events (safety cohort)
The rate of discontinuation of study drugs due to AEs was higher in the FOLFOX4 group than in the doxorubicin group: 35 patients (25.2%) and 16 patients (12.7%), respectively. Of these, 16 (11.6%) and 12 (9.6%) patients in the FOLFOX4 and doxorubicin groups, respectively, had their medication withdrawn due to events that were considered by the investigator to be causally related to the study treatment.
Comparison of Chinese and Non-Chinese Populations
The baseline characteristics of the Chinese and non-Chinese populations are presented in supplemental online Table 1. Except for age and incidence of HBV or HCV infection, all baseline characteristics were similar in the Chinese and non-Chinese populations. The mean age of the Chinese population was significantly lower than the mean age of the non-Chinese population (47.9 ± 10.5 vs. 54.1 ± 10.5 years, p < .0001). The prevalence of HBV or HCV infection at baseline was significantly higher in Chinese patients than in non-Chinese patients (96.77% vs. 89.13%, p = .0039).
Median OS was higher in the FOLFOX4 group than in the doxorubicin group at the two cut-off dates in both Chinese and non-Chinese populations (supplemental online Table 2). In the FOLFOX4 group, median OS was not statistically significant between the Chinese and non-Chinese populations. However, there was a significant difference between Chinese and non-Chinese patients in the doxorubicin group, favoring non-Chinese patients (p = .0134 and p = .0183 at the first and second cut-off dates, respectively). No statistically significant differences were observed between the Chinese and non-Chinese patients in terms of PFS for both groups.
For the non-Chinese population, no statistically significant difference was observed between the FOLFOX4 and the doxorubicin groups in terms of OS and PFS (p = .98 and p = 0.55, respectively). The RR in this population was similar in both treatment groups: 6.8% in the FOLFOX4 group and 6.3% in the doxorubicin group (p = 1.00).
Discussion
For the Chinese HCC patients included in the EACH study, the patients treated with FOLFOX4 had significantly longer median OS at both cut-off dates than those treated with doxorubicin alone. It was also observed that there was greater median PFS (2.4 vs. 1.7 months), RR (8.6% vs. 1.4%), and DCR (47.1% vs. 26.6%) in the FOLFOX4 group.
The previously published Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) and Oriental trials [18, 19] showed that molecularly targeted therapy is a feasible approach to treat advanced HCC. Both trials evaluated the potential effect of sorafenib on OS in HCC patients. In the SHARP trial [19], 602 patients with unresectable advanced HCC and without prior systemic therapy were randomly assigned to receive either oral sorafenib or placebo until radiological and symptomatic progression. Sorafenib significantly prolonged median OS (10.7 vs. 7.9 months; HR: 0.69 [95% CI: 0.55–0.87]; p < .001), and the median time to radiological progression (5.5 vs. 2.8 months; p < .001). No difference between the 2 study groups was observed in terms of the time to symptomatic progression (4.1 vs. 4.9 months) [19]. The Oriental trial [18] confirmed the efficacy of sorafenib in patients from the Asia-Pacific region. Median OS was 6.5 months in the sorafenib group and 4.2 months in the placebo group (HR: 0.68 [95% CI: 0.50–0.93]; p = .014). Sorafenib significantly improved the median PFS: 2.8 months (range: 2.63−3.58) in the sorafenib group versus 1.4 months (range: 1.35−1.55) in the placebo group (HR: 0.57 [95% CI: 0.42–0.79]; p = .0005) [18]. In terms of efficacy, the results of the subgroup analysis of the EACH study presented in this paper were of similar magnitude in terms of PFS to those reported in the Oriental trial but showed a smaller effect than in the SHARP trial, whereas the EACH study [27] showed better results in terms of RR and DCR than those reported in the Oriental trial.
In our trial, the Chinese patients treated with FOLFOX4 tended to show greater benefit in terms of survival compared with the entire EACH study population [27] and the non-Chinese population. Indeed, in the whole ITT cohort of the EACH study, the difference between study groups in terms of OS was not statistically significant at the cut-off date for the final analysis of the treatment period (6.4 months in the FOLFOX4 group vs. 4.9 months in the doxorubicin group, p = .06) [27]. However, the median OS for the Chinese patients (5.7 months in the FOLFOX4 and 4.3 months in the doxorubicin group) was shorter than that for the EACH study population overall and for the non-Chinese population for both groups. This lower median OS in the Chinese patients can probably be attributed to the higher proportion of patients with a history of HBV infection at baseline in these patients (the vast majority of Chinese patients had a history of HBV infection) than in the EACH study overall (95.34% vs. 91.37%) or in the non-Chinese population (89.1% had HBC or HCV infection at baseline). Although the prognostic significance of viral etiology in the treatment of advanced HCC is unclear, it remains a potential key factor influencing the clinical manifestation, treatment, and progression of HCC [30, 31].
The FOLFOX4 regimen was generally very well tolerated by the Chinese HCC patients, with no statistically significant differences in the number of patients reporting AEs or SAEs between the study groups, in line with findings for the EACH study cohort overall [27]. The most frequent AEs observed were consistent with those reported for the whole EACH cohort [27] and in previous clinical trials of FOLFOX4 in patients with colorectal cancer [32]. No unexpected AEs were observed in the study presented in this paper.
We observed a higher number of cases of neutropenia, leukopenia, and thrombocytopenia in the FOLFOX group than in the doxorubicin group, in line with previous studies showing frequent hematological toxicity during FOLFOX4 treatment [33], with higher rates in older patients [32].
There was no statistically significant difference in the number of deaths due to SAEs between the FOLFOX4 and doxorubicin groups in the Chinese population. None of these deaths were assessed by investigators to be treatment related. Similar observations were reported for the entire EACH trial cohort [27].
In this study, the control group was given doxorubicin at a dose of 50mg/m2, which was lower than the dose administered in previous studies on chemotherapy [34, 35]. The rationale for the selection of this dose was previously discussed in the report of the EACH study [27]. The usual dose of doxorubicin used for HCC treatment ranges from 40 to 75 mg/m2 [16, 17]. We chose 50 mg/m2 mainly for safety reasons. Asian patients with advanced HCC often have HBV infection and liver cirrhosis with impaired liver function, and thus the tolerance to chemotherapy is poor. In addition, it has been reported that the administration of doxorubicin at doses of 60–75 mg/m2 was associated with a drug-related mortality rate of 25% in Asian patients [16].
This subset analysis has several limitations that may have influenced the study results. First, more than 90% of patients in both treatment groups had HBV infection, but no data on HBV treatment and the reactivation rate was collected during the study. Another limitation is the lack of data collection on the subsequent therapies recommended for the patients and the response to these therapies. In addition, compared with the FOLFOX4 group, the proportion of patients who received prior systemic adjuvant chemotherapy was higher in the doxorubicin group (0.0% vs. 3.6%), and this imbalance may have influenced the results.
Currently sorafenib is the only molecularly targeted therapy available, with strong evidence supporting its indication for the treatment of HCC. However, its efficiency as a single-agent therapy in patients from the Asia-Pacific region is limited, with a low RR of 2%–3% and a median survival period of 2.3–2.8 months. Several mechanisms responsible for the HCC resistance to the sorafenib therapy have been hypothesized. Among these is the genetic heterogeneity of HCC cells, probably reflecting that the etiology of HCC was linked to the primary resistance to sorafenib and may explain its limited efficacy in Asian patients [36]. A recent study has shown that HBV-positive HCC cells exhibit lower expression of microRNA-193b targeting urokinase plasminogen activator and higher myeloid cell leukemia-1 protein levels, and increasing the expression of microRNA-193b increased the HCC cells response to sorafenib treatment [37]. In addition, treatment with sorafenib is associated with high incidence of toxicity events (e.g., diarrhea, hand-foot skin reaction, bleeding) and high associated costs. It was estimated that in China, because of its costs (approximately $8,000 per month), only <1.0% of the more than 400,000 newly diagnosed HCC cases each year can be treated with sorafenib. Until recently, all other new molecularly targeted therapies developed after sorafenib have failed to show an improvement in the survival of patients with advanced HCC in phase III trials [38, 39]. A number of monoclonal antibodies that bind the vascular endothelial growth factor and inhibit angiogenesis are currently under evaluation in phase II and III clinical trials [40–43].
Other systemic therapy drugs such as doxorubicin, cisplatin, and 5-FU have been used for the treatment of HCC but with no evidence of improved survival [44, 45], probably due to HCC resistance to these therapies [15], their increased toxicity counteracting the benefit [45, 46], and the surrogate endpoints used to predict survival [47, 48].
The EACH study was designed to confirm previous findings from several phase II clinical studies suggesting that HCC is sensitive to the FOLFOX4 treatment regimen, with manageable toxicity, in Chinese patients [24, 25]. Currently, an oxaliplatin-based regimen is widely used in China and other countries [23, 49–52]. Oxaliplatin was approved by the China Food and Drug Administration for the systemic chemotherapy of HCC on March 12, 2013, and the FOLFOX4 regimen was included in the “Diagnosis and Treatment Guideline of Primary Liver Cancer” published by the China Ministry of Health on October 24, 2011. Compared with sorafenib, oxaliplatin is less expensive; therefore, the FOLFOX regimen is an affordable treatment option for most advanced HCC patients in China. Based on the costs and the results from clinical studies showing positive results in Chinese HCC patients, systemic chemotherapy with an oxaliplatin-based regimen represents an accessible therapy that deserves further study, especially during its use in clinical practice.
Conclusion
In contrast with the whole population of the EACH study, the results from the Chinese subgroup analysis show that the FOLFOX4 regimen significantly prolonged survival in Chinese patients. These results may be due to ethnic differences in etiology. Consequently, advanced HCC may be sensitive to new cytotoxic agents with higher efficacy and lower toxicity in Chinese populations. Systemic chemotherapy with oxaliplatin-containing regimens may play an important role in the treatment of advanced HCC in the future. Further studies are needed to investigate the role of systemic chemotherapy in the treatment of HCC, the potential effect of the association between chemotherapy and molecularly targeted therapy, the optimal combination of drugs, and the potential beneficial effects of different drug combinations in the treatment of HCC.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
We thank all study participants and the general practitioners, study nurses, and personnel who contributed to the study. We also thank Adriana Rusu (XPE Pharma & Science) for providing writing assistance during the development of the manuscript. This study was sponsored by Sanofi. Sanofi took responsibility for all costs associated with this study (ClinicalTrials.gov identifier NCT00471965) and for the development and publishing of this report.
Footnotes
Editor's Note: See the related commentary by Stephen L. Chan on pages 1115–1117 of this issue.
Author Contributions
Conception/Design: Shukui Qin, Ying Cheng, Jun Liang, Lin Shen, Yuxian Bai, Jianfeng Li, Jia Fan, Lijian Liang, Yaqi Zhang, Gang Wu, Kun-Ming Rau, Tsai-Shen Yang, Zhixiang Jian, Houjie Liang, Yan Sun
Provision of study material or patients: Shukui Qin, Ying Cheng, Jun Liang, Lin Shen, Yuxian Bai, Jianfeng Li, Jia Fan, Lijian Liang, Yaqi Zhang, Gang Wu, Kun-Ming Rau, Tsai-Shen Yang, Zhixiang Jian, Houjie Liang, Yan Sun
Collection and/or assembly of data: Shukui Qin
Data analysis and interpretation: Shukui Qin, Jianfeng Li
Manuscript writing: Shukui Qin, Ying Cheng, Jun Liang, Lin Shen, Yuxian Bai, Jianfeng Li, Jia Fan, Lijian Liang, Yaqi Zhang, Gang Wu, Kun-Ming Rau, Tsai-Shen Yang, Zhixiang Jian, Houjie Liang, Yan Sun
Final approval of manuscript: Shukui Qin, Ying Cheng, Jun Liang, Lin Shen, Yuxian Bai, Jianfeng Li, Jia Fan, Lijian Liang, Yaqi Zhang, Gang Wu, Kun-Ming Rau, Tsai-Shen Yang, Zhixiang Jian, Houjie Liang, Yan Sun
Disclosures
Lin Shen: Roche, Amgen (C/A, RF); Jianfeng Li: Sanofi (E). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Ferlay J, Shin HR, Bray F et al. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC CancerBase No. 11. International Agency for Research on Cancer; 2013. Available at http://globocan.iarc.fr. Accessed December 10, 2013.
- 2.Zhou JY, Zhang L, Li L, et al. High hepatitis B virus load is associated with hepatocellular carcinomas development in Chinese chronic hepatitis B patients: A case control study. Virol J. 2012;9:16. doi: 10.1186/1743-422X-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang X, Bi S, Yang W, et al. Epidemiological serosurvey of hepatitis B in China—declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27:6550–6557. doi: 10.1016/j.vaccine.2009.08.048. [DOI] [PubMed] [Google Scholar]
- 4.London WT, Evans AA, McGlynn K, et al. Viral, host and environmental risk factors for hepatocellular carcinoma: A prospective study in Haimen City, China. Intervirology. 1995;38:155–161. doi: 10.1159/000150426. [DOI] [PubMed] [Google Scholar]
- 5.Sun Z, Lu P, Gail MH, et al. Increased risk of hepatocellular carcinoma in male hepatitis B surface antigen carriers with chronic hepatitis who have detectable urinary aflatoxin metabolite M1. Hepatology. 1999;30:379–383. doi: 10.1002/hep.510300204. [DOI] [PubMed] [Google Scholar]
- 6.Gao J, Xie L, Yang WS, et al. Risk factors of hepatocellular carcinoma—current status and perspectives. Asian Pac J Cancer Prev. 2012;13:743–752. doi: 10.7314/apjcp.2012.13.3.743. [DOI] [PubMed] [Google Scholar]
- 7.Shi J, Zhu L, Liu S, et al. A meta-analysis of case-control studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma in China. Br J Cancer. 2005;92:607–612. doi: 10.1038/sj.bjc.6602333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Zhai Y, Hu Z, et al. Genome-wide association study identifies 1p36.22 as a new susceptibility locus for hepatocellular carcinoma in chronic hepatitis B virus carriers. Nat Genet. 2010;42:755–758. doi: 10.1038/ng.638. [DOI] [PubMed] [Google Scholar]
- 9.Li S, Qian J, Yang Y, et al. GWAS identifies novel susceptibility loci on 6p21.32 and 21q21.3 for hepatocellular carcinoma in chronic hepatitis B virus carriers. PLoS Genet. 2012;8:e1002791. doi: 10.1371/journal.pgen.1002791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruix J, Sherman M. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: Resection versus transplantation. Hepatology. 1999;30:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 13.Pinter M, Hucke F, Graziadei I, et al. Advanced-stage hepatocellular carcinoma: Transarterial chemoembolization versus sorafenib. Radiology. 2012;263:590–599. doi: 10.1148/radiol.12111550. [DOI] [PubMed] [Google Scholar]
- 14.Fan J, Yang G-S, Fu ZR, et al. Liver transplantation outcomes in 1,078 hepatocellular carcinoma patients: A multi-center experience in Shanghai, China. J Cancer Res Clin Oncol. 2009;135:1403–1412. doi: 10.1007/s00432-009-0584-6. [DOI] [PubMed] [Google Scholar]
- 15.Yau T, Chan P, Epstein R, et al. Management of advanced hepatocellular carcinoma in the era of targeted therapy. Liver Int. 2009;29:10–17. doi: 10.1111/j.1478-3231.2008.01916.x. [DOI] [PubMed] [Google Scholar]
- 16.Lai CL, Wu PC, Chan GC, et al. Doxorubicin versus no antitumor therapy in inoperable hepatocellular carcinoma. A prospective randomized trial. Cancer. 1988;62:479–483. doi: 10.1002/1097-0142(19880801)62:3<479::aid-cncr2820620306>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 17.Burroughs A, Hochhauser D, Meyer T. Systemic treatment and liver transplantation for hepatocellular carcinoma: Two ends of the therapeutic spectrum. Lancet Oncol. 2004;5:409–418. doi: 10.1016/S1470-2045(04)01508-6. [DOI] [PubMed] [Google Scholar]
- 18.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 19.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 20.Summary of product characteristics: Eloxatin. Available at http://www.sanofi-aventis.co.uk/products/Eloxatin_SPC.pdf. Accessed May 27, 2011.
- 21.Raymond E, Chaney SG, Taamma A, et al. Oxaliplatin: A review of preclinical and clinical studies. Ann Oncol. 1998;9:1053–1071. doi: 10.1023/a:1008213732429. [DOI] [PubMed] [Google Scholar]
- 22.Boige V, Raoul JL, Pignon JP, et al. Multicentre phase II trial of capecitabine plus oxaliplatin (XELOX) in patients with advanced hepatocellular carcinoma: FFCD 03-03 trial. Br J Cancer. 2007;97:862–867. doi: 10.1038/sj.bjc.6603956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louafi S, Boige V, Ducreux M, et al. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC): Results of a phase II study. Cancer. 2007;109:1384–1390. doi: 10.1002/cncr.22532. [DOI] [PubMed] [Google Scholar]
- 24.Qin SK, Chao MR, Qian J, et al. Treatment of advanced primary liver cancer patients with FOLFOX4 regimen containing oxaliplatin. Chin Clin Oncol. 2005;10:58–60. [Google Scholar]
- 25.Qin SK, Wang YJ, Wu Q, et al. Preliminary results of a phase II trial of FOLFOX4 regimen in Chinese patients with unresectable primary liver cancer. J Clin Oncol. 2006;24:629s, 14065a. [Google Scholar]
- 26.Yen Y, Lim DW, Chung V, et al. Phase II study of oxaliplatin in patients with unresectable, metastatic, or recurrent hepatocellular cancer: A California Cancer Consortium Trial. Am J Clin Oncol. 2008;31:317–322. doi: 10.1097/COC.0b013e318162f57d. [DOI] [PubMed] [Google Scholar]
- 27.Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31:3501–3508. doi: 10.1200/JCO.2012.44.5643. [DOI] [PubMed] [Google Scholar]
- 28.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 29.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 30.Ohkubo K, Kato Y, Ichikawa T, et al. Viral load is a significant prognostic factor for hepatitis B virus-associated hepatocellular carcinoma. Cancer. 2002;94:2663–2668. doi: 10.1002/cncr.10557. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Y, Si X, Wu L, et al. Influence of viral hepatitis status on prognosis in patients undergoing hepatic resection for hepatocellular carcinoma: A meta-analysis of observational studies. World J Surg Oncol. 2011;9:108. doi: 10.1186/1477-7819-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldberg RM, Tabah-Fisch I, Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24:4085–4091. doi: 10.1200/JCO.2006.06.9039. [DOI] [PubMed] [Google Scholar]
- 33.Kochi M, Ichikawa W, Meguro E, et al. Phase II study of FOLFOX4 with “wait and go” strategy as first-line treatment for metastatic colorectal cancer. Cancer Chemother Pharmacol. 2011;68:1215–1222. doi: 10.1007/s00280-011-1605-0. [DOI] [PubMed] [Google Scholar]
- 34.Abou-Alfa GK, Johnson P, Knox JJ, et al. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: A randomized trial. JAMA. 2010;304:2154–2160. doi: 10.1001/jama.2010.1672. [DOI] [PubMed] [Google Scholar]
- 35.Yeo W, Mok TS, Zee B, et al. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532–1538. doi: 10.1093/jnci/dji315. [DOI] [PubMed] [Google Scholar]
- 36.Zhai B, Sun XY. Mechanisms of resistance to sorafenib and the corresponding strategies in hepatocellular carcinoma. World J Hepatol. 2013;5:345–352. doi: 10.4254/wjh.v5.i7.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mao K, Zhang J, He C, et al. Restoration of miR-193b sensitizes hepatitis B virus-associated hepatocellular carcinoma to sorafenib. Cancer Lett. 2014 doi: 10.1016/j.canlet.2014.07.004. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Cheng A, Kang Y, Lin D, et al. Phase III trial of sunitinib (Su) versus sorafenib (So) in advanced hepatocellular carcinoma (HCC) J Clin Oncol. 2011;29(suppl):4000a. [Google Scholar]
- 39.Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: Results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31:3517–3524. doi: 10.1200/JCO.2012.48.4410. [DOI] [PubMed] [Google Scholar]
- 40.Park JW, Finn RS, Kim JS, et al. Phase II, open-label study of brivanib as first-line therapy in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2011;17:1973–1983. doi: 10.1158/1078-0432.CCR-10-2011. [DOI] [PubMed] [Google Scholar]
- 41.Llovet JM, Decaens T, Raoul JL, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: Results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31:3509–3516. doi: 10.1200/JCO.2012.47.3009. [DOI] [PubMed] [Google Scholar]
- 42.Toh H, Chen P, Carr B, et al. A phase II study of ABT-869 in hepatocellular carcinoma (HCC): Interim analysis. J Clin Oncol. 2009;27(suppl):4581a. [Google Scholar]
- 43.Alberts SR, Fitch TR, Kim GP, et al. Cediranib (AZD2171) in patients with advanced hepatocellular carcinoma: A phase II North Central Cancer Treatment Group Clinical Trial. Am J Clin Oncol. 2012;35:329–333. doi: 10.1097/COC.0b013e3182118cdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 45.Lopez PM, Villanueva A, Llovet JM. Systematic review: Evidence-based management of hepatocellular carcinoma—an updated analysis of randomized controlled trials. Aliment Pharmacol Ther. 2006;23:1535–1547. doi: 10.1111/j.1365-2036.2006.02932.x. [DOI] [PubMed] [Google Scholar]
- 46.Simonetti RG, Liberati A, Angiolini C, et al. Treatment of hepatocellular carcinoma: A systematic review of randomized controlled trials. Ann Oncol. 1997;8:117–136. doi: 10.1023/a:1008285123736. [DOI] [PubMed] [Google Scholar]
- 47.Zhu AX. New agents on the horizon in hepatocellular carcinoma. Ther Adv Med Oncol. 2013;5:41–50. doi: 10.1177/1758834012458480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu Q, Qin SK. Features and treatment options of Chinese hepatocellular carcinoma. Chin Clin Oncol. 2013;2:38–43. doi: 10.3978/j.issn.2304-3865.2013.09.07. [DOI] [PubMed] [Google Scholar]
- 49.Yang LQ, Qin SK, Zhao NL, et al. Clinical study of FOLFOX4 regimen as systemic chemotherapy for advanced primary liver carcinoma. Chin Clin Oncol. 2013;18:108–113. [Google Scholar]
- 50.Yang CX, Qin SK. Clinical study progression of oxaliplatin for advanced primary hepatic carcinoma. Chin Clin Oncol. 2010;15:845–855. [Google Scholar]
- 51.Assenat E, Boige V, Thézenas S, et al. Sorafenib (S) alone versus S combined with gemcitabine and oxaliplatin (GEMOX) in first-line treatment of advanced hepatocellular carcinoma (HCC): Final analysis of the randomized phase II GONEXT trial (UNICANCER/FFCD PRODIGE 10 trial) J Clin Oncol. 2013;31(suppl):4028a. [Google Scholar]
- 52.Zaanan A, Williet N, Hebbar M, et al. Gemcitabine plus oxaliplatin in advanced hepatocellular carcinoma: A large multicenter AGEO study. J Hepatol. 2013;58:81–88. doi: 10.1016/j.jhep.2012.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.