Abstract
Background
While colorectal cancer is a disease characterized by sequential accumulation of mutations in epithelial cells, mechanisms leading to genomic vulnerability contributing to tumor initiation remain undefined. GUCY2C has emerged as an intestine-specific tumor suppressor controlling epithelial homeostasis through circuits canonically disrupted in cancer. Surprisingly, the GUCY2C tumor suppressor is universally over-expressed by human colorectal cancer cells. This apparent paradox likely reflects silencing of GUCY2C through loss of its paracrine hormone guanylin. Here, we quantified expression of guanylin mRNA and protein in tumors and normal epithelia from patients with colorectal cancer.
Methods
Guanylin mRNA was quantified in tumors and normal adjacent epithelia from 281 patients by the reverse transcriptase-polymerase chain reaction. Separately, guanylin protein was quantified by immunohistochemistry in 54 colorectal tumors and 30 specimens of normal intestinal epithelium.
Results
Guanylin mRNA in colorectum varied over a 100-fold across the population. Guanylin mRNA was reduced 100- to-1,000-fold in >85% of tumors compared to matched normal adjacent mucosa (p<0.001). Loss of guanylin mRNA was greatest in tumors from patients <50 years old (p<0.005) and with the highest expression in normal adjacent mucosa (Spearman’s correlation coefficient=0.61, p<0.001). In a separate validation cohort, guanylin protein was detected in all 30 normal colorectal mucosa specimens, but in none of 54 colorectal tumors.
Conclusions
Colorectal cancer may initiate as a disease of paracrine hormone insufficiency through loss of guanylin expression, silencing the GUCY2C tumor suppressor and disrupting homeostatic mechanisms regulating colorectal epithelia cells.
Impact
Intestinal tumorigenesis may be prevented by oral GUCY2C hormone replacement therapy.
Keywords: Guanylin, GUCY2C, colorectal cancer, tumor suppressor, chemoprevention
Introduction
While sporadic colorectal cancer is the fourth leading cause of cancer and the second leading cause of cancer-related death worldwide (1), mechanisms underlying tumor initiation remain incompletely defined. The oncogenomic view suggests that colorectal cancer is a disease of sequential accumulation of mutations in a defined set of genes in the single layer of epithelial cells lining the colon and rectum (2). In turn, these mutations contribute to the spatiotemporal continuum of transformation, from loss of normal growth control mediating the formation of benign adenoma, to the ability of tumor cells to invade and metastasize (3). Although these steps in tumor progression have been characterized, initiating mechanisms at the center of colorectal cancer pathogenesis that establish the susceptibility of intestinal epithelial cells to undergo sequential mutagenesis remain unknown.
Guanylyl cyclase C (GUCY2C) is a member of the membrane-bound guanylyl cyclase family of transmembrane receptor-enzymes that is selectively expressed in brush border membranes of intestinal epithelial cells from the gastroduodenal junction to the end of the rectum (4). The cognate ligands for this receptor include the endogenous paracrine hormones uroguanylin and guanylin, expressed in small and large intestine, respectively, and the exogenous heat-stable enterotoxin (STs) produced by diarrheagenic enterotoxigenic bacteria (5). Interaction of these ligands with the extracellular receptor binding domain is required to activate the cytoplasmic catalytic domain which synthesizes cyclic GMP (cGMP) from GTP (4). In turn, accumulating cGMP activates a cascade of intracellular signaling events resulting in the net secretion of fluid and electrolytes which can manifest as diarrhea (6).
Beyond secretion, GUCY2C has emerged as a modulator of the dynamic crypt-villus and crypt-surface axes in small and large intestine, respectively (5). The GUCY2C hormone axis regulates the cell cycle and proliferation of transit amplifying cells in crypts, the size of the proliferating crypt compartment, DNA damage sensing and repair, the balance between glycolytic and mitochondrial oxidative metabolism, differentiation along the secretory lineage of epithelial cells, and epithelial-mesenchymal interactions underlying desmoplasia (7–11). These homeostatic processes regulated by GUCY2C organizing the crypt-surface axis also are the canonical processes universally corrupted in tumorigenesis (12). Indeed, genetic silencing of GUCY2C potentiates tumorigenesis in genetic and carcinogen-induced mouse models of colorectal cancer (13, 14). Conversely, enforced ligand activation of GUCY2C inhibits intestinal tumorigenesis in mice (15). Taken together these observations establish GUCY2C is an intestinal tumor suppressor with the potential to contribute to the initiation and progression of colorectal cancer (5, 15, 16).
In the context of GUCY2C as a tumor suppressor, preliminary studies suggest that guanylin expression is commonly lost in colorectal cancer (15, 17–21). In those studies, guanylin mRNA expression was substantially reduced in tumors compared to matched normal adjacent tissues (17, 19–21). Moreover, expression of this hormone also is uniformly lost in intestinal neoplasia in mouse models (15, 20). Guanylin loss in intestinal tumorigenesis in humans, and the conservation of this mechanism across species, suggests that colorectal cancer might initiate as a disease of paracrine hormone insufficiency, silencing the GUCY2C tumor suppressor, disrupting essential homeostatic mechanisms that alter the susceptibility of intestinal epithelial cells to transform (5, 15, 16, 22). The present study for the first time explores the expression of guanylin in primary tumors and matched normal adjacent tissues in a large cohort of patients with colorectal cancer, at mRNA and protein levels. This study establishes the near universality of guanylin loss, silencing the GUCY2C tumor suppressor, in colorectal cancer.
Materials and Methods
Patients and Tissues
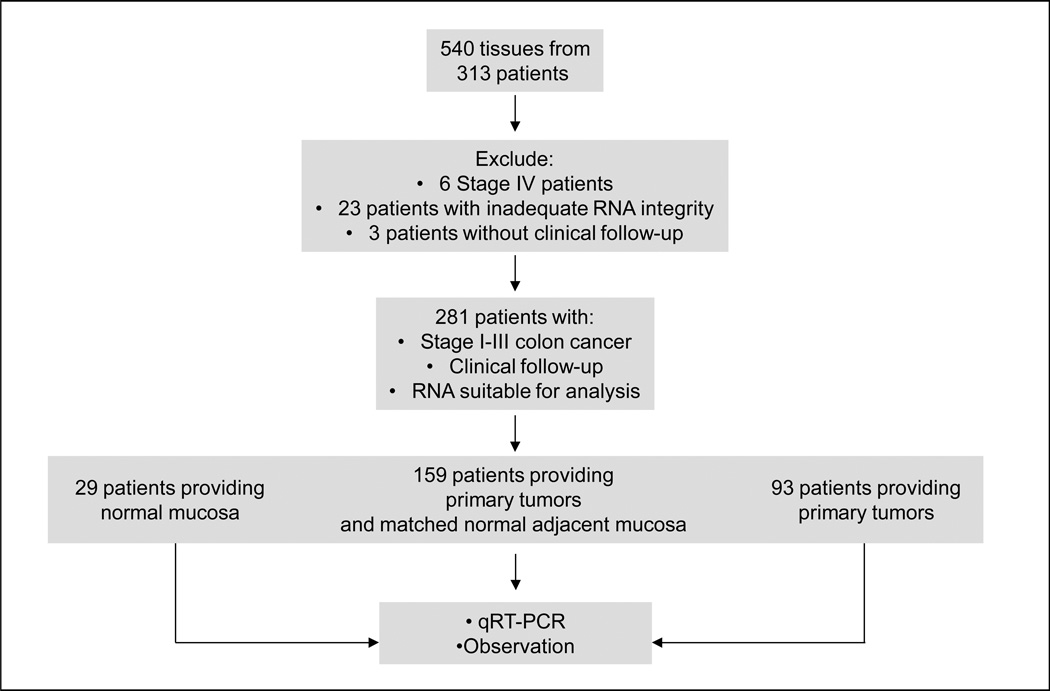
For studies of mRNA expression, tissues were collected from patients participating in a prospective observational trial of the utility of GUCY2C quantitative RT-PCR in regional lymph nodes for staging patients with colorectal cancer (23). Between January 2003 and June 2007, we enrolled 313 patients with colorectal cancer at one of 7 academic medical centers and 2 community hospitals in the U.S. and Canada (Fig. 1). Tumor specimens and/or normal mucosa were dissected from colon and rectal resections and frozen at −80°C within one hour of surgery to minimize warm ischemia. Of 540 tumor and/or normal mucosa specimens collected, 440 tissue samples from 281 patients (29 with normal adjacent mucosa only; 159 with matched tumors and normal adjacent mucosa; 93 with tumor only; Fig. 1) provided viable RNA quantified by the reverse transcriptase polymerase chain reaction (qRT-PCR) of β-actin (ACTB), where threshold cycles (CT) were <40 (23). Exclusions in this cohort included 6 patients with stage IV disease, 3 who had no clinical follow-up, and 23 who provided specimens yielding degraded RNA (Fig. 1). Disease status, obtained in routine follow-up by treating physicians, was provided for all patients through December 2007. For studies of guanylin protein expression, tumors and normal adjacent tissues were obtained from de-identified archived formalin-fixed paraffin-embedded specimens formulated into tissue microarrays for immunohistochemical analysis (24). Protein expression was evaluated in adenocarcinomas (n=54) and, where available, their matched normal epithelia (n=30). Among these adenocarcinomas, 8% displayed muscular invasion, 23% displayed pericolic fat invasion, and 15% had metastasized to regional lymph nodes. Adenocarcinomas were well-differentiated (24%), moderately differentiated (63%) or poorly differentiated (13%).
Figure 1. Patient selection for guanylin qRT-PCR analysis.
RNA Isolation
RNA was extracted from tissues by a modification of the acid guanidinium thiocyanate-phenol-chloroform extraction method (25, 26). Briefly, individual tissues were pulverized in 1.0 mL Tri-Reagent (Molecular Research Center, Cincinnati, OH) with 12–14 sterile 2.5 mm zirconium beads in a bead mill (Biospec, Bartlesville, OK) for 1–2 min. Phase separation was performed with 0.1 mL bichloropropane, and the aqueous phase re-extracted with 0.5 mL chloroform. RNA was precipitated with 50% isopropanol and washed with 70% ethanol. Air-dried RNA was dissolved in water, concentration determined by spectrophotometry, and stored at −80°C.
RT-PCR
The EZ RT-PCR kit (Applied Biosystems, Foster City, CA) was employed to amplify target mRNA from total RNA in a 50 µL reaction. Optical strip-tubes were used for all reactions, which were conducted in an ABI 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). In addition to the kit components [50 mM Bicine (pH 8.2), 115 mM KOAc, 10 µM EDTA, 60 nM ROX, 8% glycerol, 3 mM Mg(OAc)2, 300 µM each dATP, dCTP, and dGTP, 600 µM dUTP, 0.5 U uracil N-glycosylase, and 5 U rTth DNA polymerase], the reaction master mix contained target-specific forward and reverse primers and Taqman probe (Applied Biosystems, Foster City, CA), and 1 µg RNA template. The thermocycler program employed for RT included: 50° × 2 min, 60° × 30 min, 95° × 5 min; and for PCR: 45 cycles of 94° × 20 sec, 62° × 1 min. Reactions were performed at least in duplicate and results averaged.
Immunohistochemistry
Sections of tissue microarrays (24) representing formalin-fixed, paraffin-embedded normal epithelia and adenocarcinomas were deparaffinized and heated in pH 9.0 antigen retrieval buffer (Dako, Carpinteria, CA) and processed in a Dako Autostainer using rabbit polyclonal anti-guanylin antibody (Sigma Prestige Antibodies catalogue #HPA018215) at 1:60 dilution at RT for 30 min. Antigen visualization was performed using HRP-conjugated anti-rabbit secondary antibody and diaminobenzidine as the chromogen.
Statistical Methods
Target transcripts for guanylin were quantified relative to the intestinal epithelial cell marker villin (VIL) by RT-qPCR employing relative logistic regression models fit to fluorescence data from each well for replicate amplification reactions (27). Villin was chosen for normalization because it is a selective product of epithelial, compared to other cells, in intestine and is persistently and reliably expressed during tumorigenesis (28–30). Therefore, normalizing to villin permits estimates of epithelial cell-specific transcripts (e.g., guanylin) without tissue microdissection. For viable samples (ACTB qRT-PCR CT<40 cycles), target transcript expression was defined as the log relative expression (R) by:
where m̂R and m̂r are defined by the estimate of m obtained from the logistic model (equation 1) for the reference gene (VIL) and the target gene (guanylin), respectively.
| (Equation 1) |
Each sample was analyzed at least in duplicate for each target gene for each tissue sample.
Standard paradigms for qRT-PCR analyses assign maximum CT values (e.g. >45 cycles) to samples with undetectable target gene expression. However, this approach biases estimates of quantity and underestimates variance, inflating alpha levels in hypothesis testing. Here, analyses incorporated a multiple imputation algorithm, which selects random values from the lower tail of the distribution rather than assigning a fixed value of zero to samples with target gene expression below the lower limit of quantification (LOQ). This process, validated in studies quantifying HIV mRNA in plasma, limits bias in estimates of quantity and variance (31). In these analyses, 10 multiple imputations were implemented by SAS code (available on request) based on the likelihood of the truncated normal distribution (truncated at the LOQ), and those combined using Proc Mianalyze in SAS v 9.2.
Assessment of association of guanylin expression with clinical and demographic covariates was completed utilizing linear mixed models based on the 10 imputed datasets as described above. Values of loss (NAT-Tumor) were computed based on linear contrasts, with confidence intervals and p values as estimated through Proc Mianalyze. Survival estimates were plotted using the Kaplan-Meier method, and differences estimated across strata based on the logrank test. The association of NAT with NAT/Tumor was assessed via Spearman correlation.
Results
Patient Characteristics
Of the 281 patients providing viable specimens, 159 provided primary colorectal tumors and matched normal adjacent mucosa while 29 patients provided only normal mucosa and 93 provided only tumor (Fig. 1, Table 1). Clinicopathologic features, including depth of tumor penetration (T1/2, T3, T4), grade, and tumor anatomical location (right, left, sigmoid colon) were similar to national experience (32–34). There were no statistically significant differences in baseline characteristics of patients included and those excluded from analysis. Patients exhibited the well-established direct relationships between time to recurrence (p<0.039) and stage (Supplementary Fig. 1) (32–34). Twenty-two percent of patients with pN0 and 76% with stage III, colon cancer received adjuvant 5-fluorouracil-based chemotherapy.
Table 1.
Patient characteristics.
| All Patients | ||
|---|---|---|
| N | % | |
| Totals | 281 | |
| Age, years | ||
| <50 | 18 | 6.4 |
| 50–75 | 177 | 63.2 |
| >75 | 85 | 30.4 |
| Sex | ||
| Male | 149 | 53.0 |
| Female | 132 | 47.0 |
| T Stage | ||
| T1/T2 | 89 | 31.7 |
| T3 | 158 | 56.2 |
| T4 | 34 | 12.1 |
| Nodal Status | ||
| N0 | 212 | 75.4 |
| N1 | 42 | 15.0 |
| N2 | 27 | 9.6 |
| Grade | ||
| Well | 23 | 8.2 |
| Moderate | 210 | 74.7 |
| Poor/Unknown | 48 | 17.1 |
| Chemotherapy | ||
| Yes | 101 | 35.9 |
| No | 180 | 64.1 |
| Tumor Site | ||
| Left Colon | 24 | 8.6 |
| Right Colon | 126 | 45.0 |
| Sigmoid Colon | 97 | 34.6 |
| Rectum | 33 | 11.8 |
| Number of lymph nodes harvested | ||
| ≤12 | 66 | 23.4 |
| >12 | 215 | 76.5 |
Guanylin mRNA Expression in Normal Mucosa
Across the population, basal guanylin expression varied over a 100-fold range, reflecting interindividual variability in gene expression, rather than differential expression along the rostral-caudal axis (Figs. 2, 3). Moreover, there is an inverse relationship between age and hormone expression reflected by a substantial decrease in basal expression of guanylin in patients greater than 50 years old (yo; p<0.01; Table 2). Basal expression of guanylin in normal epithelium was not significantly associated with tumor characteristics, including T, N or AJCC stage (Table 2).
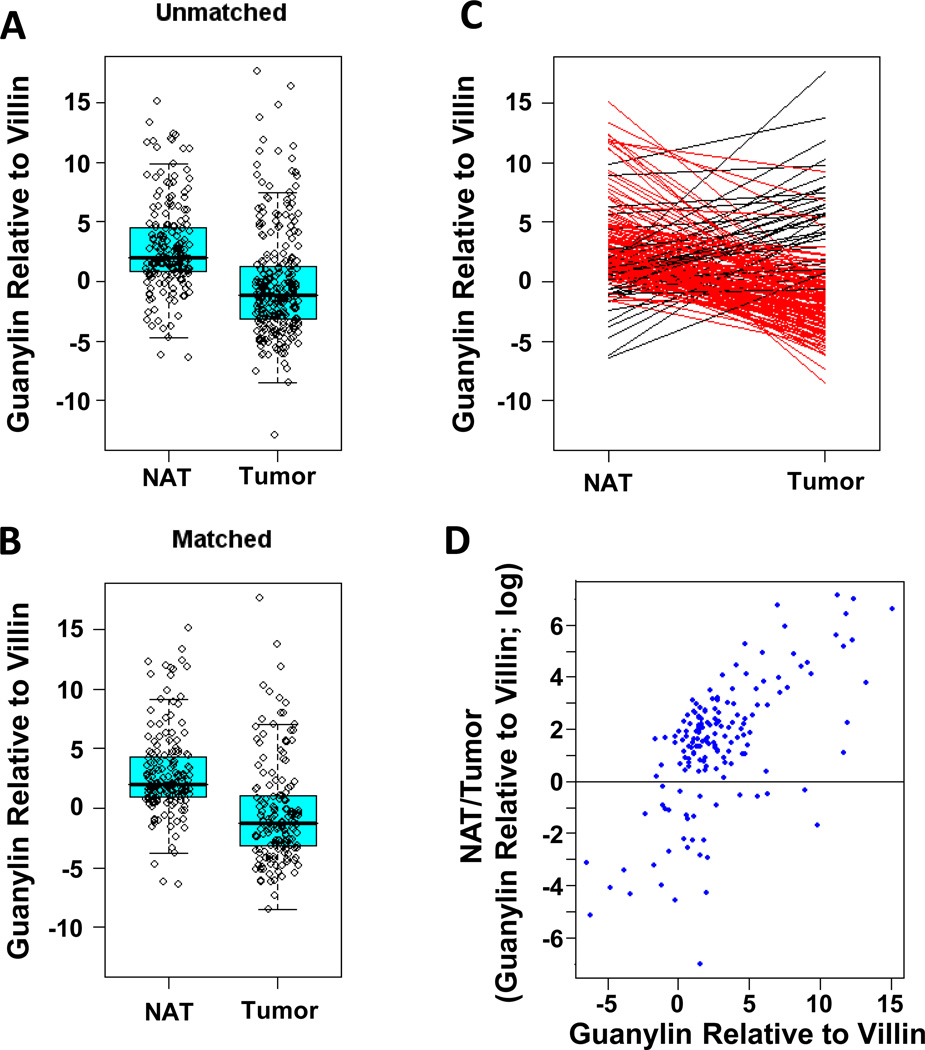
Figure 2. Guanylin mRNA expression in normal adjacent tissues (NAT) and tumors from the colorectum.
(A) Distribution of guanylin mRNA expression in all 188 NAT and 252 tumors. (B) Distribution of guanylin mRNA expression in 159 matched NAT and tumor specimens. (C) Change in guanylin mRNA expression in 159 tumors, compared to their matched NATs. Red lines connect NATs with adjacent tumor specimens with decreased guanylin mRNA expression. Black lines connect NATs with adjacent tumor specimens with increased guanylin mRNA expression. (D) Relationship between basal guanylin expression in 159 NAT (X axis) and loss of guanylin expression in associated tumors (NAT/tumor; Y axis). Tissues were obtained and processed, mRNA extracted, and guanylin mRNA expression quantified by RT-PCR analysis as described.
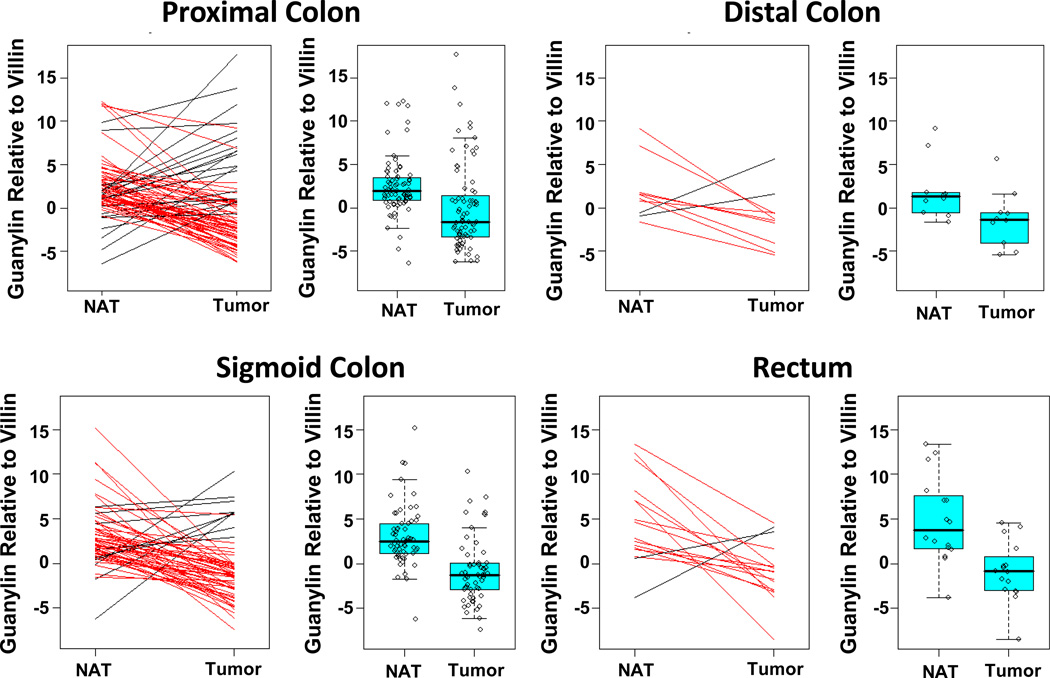
Figure 3. Guanylin mRNA expression in matched normal adjacent tissues (NAT) and tumors resected from different anatomical segments.
Tissues were obtained and processed, mRNA extracted, and guanylin mRNA quantified by RT-PCR analysis as described. For each anatomical segment, the change in guanylin mRNA expression in tumors is compared to their matched NATs, where red lines connect NATs with adjacent tumor specimens with decreased guanylin mRNA expression while black lines connect NATs with adjacent tumor specimens with increased guanylin mRNA expression. Similarly, the distribution of guanylin mRNA expression in matched NAT and tumor specimens is plotted for each anatomical segment.
Table 2.
Guanylin expression in normal adjacent tissue and tumors.a
| Patient Characteristic |
Relative Expression NATb |
Relative Expression Tumorb |
Loss (NAT-Tumor)b,c |
Ln (Fold Change)d |
pe |
|---|---|---|---|---|---|
| Age | |||||
| <50 | 5.79⊥ (3.86, 7.71) | −1.80 (−4.02, 0.43) | 7.58† (4.62, 10.54) | referent | --- |
| 50–75 | 2.61 ⊥ (1.94, 3.29) | −0.29 (−1.00, 0.42) | 2.91† (1.93, 3.89) | 4.67 (1.55, 7.80) | 0.003 |
| >75 | 2.53 ⊥ (1.56, 3.49) | −0.27 (−1.26, 0.72) | 2.80† (1.42, 4.18) | 4.79 (1.54, 8.03) | 0.004 |
| Sex | |||||
| Male | 2.95 ⊥ (2.20, 3.70) | −0.06 (−0.83, 0.72) | 3.00† (1.92, 4.09) | referent | --- |
| Female | 2.71 ⊥ (1.92, 3.51) | −0.73 (−1.52, 0.07) | 3.44† (2.32, 4.56) | 0.43 (−1.13, 2.00) | 0.587 |
| T Stage | |||||
| T1/T2 | 3.31 ⊥ (2.32, 4.31) | −0.38 (−1.37, 0.61) | 3.69† (2.30, 5.09) | referent | --- |
| T3 | 2.57 ⊥ (1.83, 3.30) | −0.55 (−1.28, 0.18) | 3.12† (2.08, 4.15) | −0.58 (−2.31, 1.15) | 0.512 |
| T4 | 2.86 ⊥ (1.43, 4.29) | 0.36 (−1.17, 1.89) | 2.49† (0.42, 4.58) | −1.20 (−3.69, 1.29) | 0.346 |
| Grade | |||||
| Well | 1.24 | 0.09 (−1.92, 2.10) | 1.15 (−1.69, 3.98) | referent | --- |
| Moderate | 2.77 ⊥ (2.14, 3.39) | −0.37 (−0.97, 0.25) | 3.14† (2.26, 4.01) | 1.99 (−0.98, 4.96) | 0.189 |
| Poor/Unknown | 4.00 ⊥ (2.54, 5.45) | −0.47 (−1.95, 1.01) | 4.46† (2.39, 6.53) | 3.32 (−0.20, 6.84) | 0.065 |
| Tumor Site | |||||
| Left | 2.41 ⊥ (0.44, 4.37) | −2.20 (−4.21, −0.20) | 4.61† (1.82, 3.69) | referent | --- |
| Right | 2.52 ⊥ (1.72, 3.33) | −0.02 (−0.84, 0.80) | 2.54† (1.39, 3.69) | −2.07 (−5.09, 0.95) | 0.179 |
| Rectal | 4.15 ⊥ (2.58, 5.71) | −0.54 (−2.28, 1.20) | 4.69 † (2.36, 7.02) | 0.08 (−3.58, 3.71) | 0.965 |
| Sigmoid | 2.89 ⊥ (1.96, 3.83) | −0.40 (−1.30, 0.50) | 3.29† (2.00, 4.59) | −1.31 (−4.39, 1.77) | 0.402 |
| Node Status | |||||
| Node Negative | 2.90 ⊥ (2.28, 3.52) | −0.56 (−1.18, 0.08) | 3.45† (2.56, 4.33) | referent | --- |
| Node Positive | 2.64 ⊥ (1.50, 3.77) | 0.17 (−0.96, 1.30) | 2.46† (0.86, 2.82) | −0.99 (−2.82, 0.85) | 0.292 |
GUCA1A expression, normalized to VIL, reflects mean (95% confidence interval).
Values reflect the natural log (Ln) of the GUCA1A/VIL ratio.
Values reflect the difference of the natural log (Ln) of the GUCA1A/VIL ratio in normal adjacent tissue (NAT) and tumor.
Values reflect the natural log (Ln) of the fold change in GUCA1A/VIL ratio in normal adjacent tissue (NAT) and tumor.
p reflects the comparison of Ln(Fold Change) of the given category to the referent category.
Indicates that expression is significantly greater than 0.
Indicates that NAT-Tumor (Loss) is significantly greater than 0.
Guanylin mRNA Expression in Tumors
Profiling mRNA transcripts in small cohorts of patients suggested that guanylin expression is reduced during colon tumorigenesis (17–21). Indeed, guanylin mRNA expression was lower in tumors compared to normal adjacent tissue (p<0.001; Fig. 2A–C; Table 2). In that context, 88% of tumors exhibited significantly attenuated expression of guanylin mRNA, compared to matched normal adjacent tissues, regardless of their anatomical site of origin (Figs. 2B–C, 3; Tables 2). The quantity of hormone loss was greatest in tumors arising from NAT with the highest guanylin expression (Spearman’s correlation coefficient=0.61, p<0.001; Fig. 2D). There was an inverse relationship between loss of guanylin expression in tumors and age, and patients <50 yo exhibited the greatest loss, ~1,959-fold, while patients >50 yo exhibited the least attenuation in expression, ~107-fold (p<0.005; Table 2). As with basal hormone levels in normal tissue, attenuated expression of guanylin was not associated with tumor histopathologic characteristics, including T, N or AJCC stage (Table 2).
Guanylin Protein Expression in Tumors
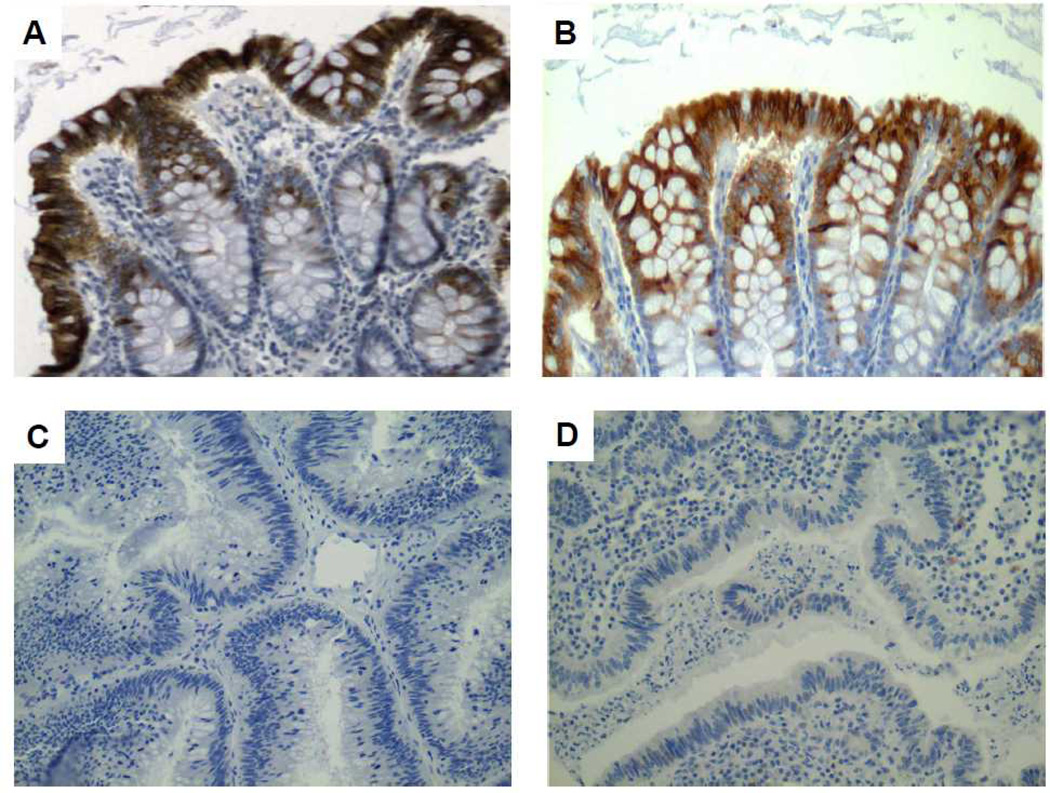
The availability of reliable polyclonal antisera for immunohistochemistry of human tissues permitted interrogation of colorectal tumors and matched normal adjacent tissues for guanylin expression by tissue microarray analysis. Guanylin protein expression was strongly positive in all 30 specimens of normal epithelium (Fig. 4). Conversely, guanylin protein was absent in all 54 adenocarcinomas examined (Fig. 4). These immunohistochemical analyses confirm qRT-qPCR studies which demonstrate near-universal attenuation of guanylin expression in colorectal tumorigenesis.
Figure 4. Guanylin protein expression is lost in adenocarcinomas.
Immunohistochemistry of representative cases illustrates the presence of guanylin in all normal epithelia (A, B), but its universal loss in adenocarcinomas (C, D). Brown chromogen is diaminobenzidine.
Discussion
Colorectal cancer is a disease of sequential accumulation of mutations in the single layer of epithelial cells lining the rostral-caudal axis of the large intestine (2, 3). Mutations in APC and β-catenin, which are present in nearly 100% of tumors, lead to loss of normal growth control and the formation of small polyps (2). Further mutations in genes, including KRAS and BRAF, lead to exuberant hyperproliferation and the formation of large premalignant adenoma (2, 3). Terminal mutations in p53, or the TGF-β signaling components SMAD4 or the TGF-β receptor, drive transformation to invasive adenocarcinoma with metastatic potential (2, 3). While this sequence of mutations is well-defined, mechanisms underlying the susceptibility of intestinal epithelial cells to accumulate mutations driving tumorigenesis remain undefined.
The intestinal epithelium comprises a highly dynamic structure organized as crypt-villus units in small intestine and crypt-surface units in the colorectum (35). In these continuously regenerating structures, proliferating transit amplifying cells arising from stem cells at the base and migrate up the wall of the crypt. With their continuous cycles of chromosomal replication and cell division, these cells depend on unceasing DNA damage sensing and repair. At a point along their migration, these cells are reprogrammed, halting proliferation and undergoing differentiation into the canonical cell lineages of the intestine, including enterocytes, goblet cells, enteroendocrine cells, and in small intestine Paneth cells at the base of the crypt. This shift from proliferation to differentiation reflects coordination of key homeostatic mechanisms including reprogramming of metabolism from glycolysis to oxidative phosphorylation and reciprocal epithelial-mesenchymal signaling. Following differentiation, cells continue their migration to the tip of the villus in small intestine or the crypt-surface junction in the large intestine, where they undergo apoptosis and shed into the fecal stream. This tight choreography of homeostatic processes is central to maintaining the structural integrity of the intestine in the face of complete epithelial renewal every 3–5 days.
GUCY2C has recently emerged as a key regulator of these essential homeostatic processes organizing the intestinal epithelium. Activation of GUCY2C and accumulation of its downstream second messenger, cGMP, decreases key drivers and increases inhibitors of the cell cycle to maintain the size of the proliferating crypt compartment (8, 10, 11, 13, 14). Similarly, GUCY2C signaling drives DNA damage sensing and repair, maintaining DNA integrity to prevent accumulation of mutations in key oncogenes and tumor suppressors (8, 14). Further, GUCY2C drives epithelial cell differentiation, specifically supporting the development of the secretory lineage including Paneth and goblet cells (8). Additionally, GUCY2C signaling orchestrates metabolic reprogramming, from glycolysis characterizing proliferation to mitochondrial oxidative phosphorylation essential for differentiated cell function (14).
Beyond its role in maintaining epithelial homeostasis, GUCY2C has emerged as a tumor suppressor whose silencing induces dysfunction in canonical pathways underlying transformation. Silencing GUCY2C disrupts the DNA damage sensing and repair machinery, promoting mutations in key tumor suppressors, like APC, and oncogenes like β-catenin (8, 14). Also, attenuating GUCY2C signaling corrupts normal mechanisms regulating proliferation, enhancing expression of the drivers, while reducing expression of inhibitors, of the cell cycle, expanding the proliferating crypt compartment (8, 10, 11, 13, 14). Conversely, there is a contraction of the differentiated epithelial cell compartment, with specific losses in cells of the secretory lineage including goblet and Paneth cells (8). Further, silencing GUCY2C and cGMP production blocks metabolic plasticity, imposing glycolytic programming across the entire crypt-surface axis that precisely mimics the Warburg metabolic phenotype pathognomonic of neoplasia (14). Finally, attenuating GUCY2C signaling reprograms bidirectional interactions between epithelial and mesenchymal compartments, creating maladaptive circuits that drive the formation of desmoplasia, a defining feature of tumorigenesis (36). Indeed, silencing the GUCY2C tumor suppressor and disrupting normal homeostatic mechanisms induces many of the hallmark pathways considered essential for cancer (12). In that context, attenuating GUCY2C signaling substantially amplifies the incidence and burden of intestinal tumorigenesis in mouse models of genetic or carcinogen-induced colorectal cancer (8, 14, 15).
Surprisingly, while GUCY2C functions as a tumor suppressor, its expression is universally preserved in primary and metastatic colorectal tumors. Indeed, most colorectal tumors over-express GUCY2C 2–10-fold compared to normal adjacent tissue (37). This apparent paradox can best be appreciated in the context of changes in the expression of GUCY2C hormone during tumorigenesis. Preliminary studies in small samples of patients demonstrated that guanylin mRNA was one of the most commonly lost gene products in colorectal tumorigenesis (17, 19–21). Further, this hormone was lost early in the process of tumorigensis, at the premalignant adenoma stage of transformation (19). Moreover, elimination of guanylin mRNA expression during transformation was conserved across species, and colorectal tumors in mice also lose guanylin expression (15, 20).
These preliminary studies offer a potential explanation for the paradox of universal over-expression of the GUCY2C tumor suppressor in colorectal cancer. They suggest that silencing of this tumor suppressor during transformation is functional reflecting loss of guanylin, rather than oncogenomic reflecting tumor suppressor mutations (5, 38). Studies here confirm this hypothesis in a large cohort of patients with colorectal cancer. They demonstrate that the expression of mRNA transcripts encoding guanylin is reduced, or lost, in >85% of tumors and this occurs in all anatomical locations across the colorectum and in all disease stages. Moreover, these studies for the first time confirm guanylin mRNA loss at the protein level by immunohistochemistry, demonstrating that the expression of guanylin protein is completely eliminated in 100% of tumors examined, compared to normal adjacent tissues, in a separate validation cohort. Together, these observations support the suggestion that the functional silencing of the tumor suppressor GUCY2C through loss of paracrine hormone expression is a universal mechanistic step in sporadic colorectal carcinogenesis.
While loss of guanylin expression appears to be universally associated with intestinal neoplasia in animals and humans, molecular mechanisms underlying hormone loss remain unclear. To date, genetic or epigenetic mechanisms of hormone silencing have not emerged. Clearly, there are transcriptional or post-transcriptional mechanisms involved, reflected in the reduction in guanylin mRNA broadly across most tumors. Interestingly, in contrast to mRNA, which is reduced in most tumors, guanylin protein expression is completely lost in 100% of tumors, compared to normal adjacent tissues. This could reflect the greater sensitivity of RT-qPCR with its ability for near-single transcript resolution, compared to immunohistochemistry (37). Alternatively, this hormone might be silenced by independent mechanisms at the levels of transcription (mRNA) and translation (protein), creating reinforcing mechanisms that ultimately silence the GUCY2C tumor suppressor. These considerations highlight the importance of defining the molecular mechanisms silencing hormone expression, in order to determine their reversibility to prevent tumorigenesis.
The present study supports the working hypothesis that silencing the GUCY2C tumor suppressor by eliminating paracrine hormone expression contributes to the pathophysiology of colorectal cancer. Indeed, loss of guanylin and silencing GUCY2C early, and the resulting disruption of the cell cycle and DNA damage sensing and repair, may contribute to accumulation of mutations underlying the oncogenomic basis of tumorigenesis. In that context, it is tempting to speculate that the substantial reduction (>95%; see Table 2) in guanylin expression in normal epithelia in people >50 yo revealed here might contribute mechanistically to the established epidemiological vulnerability of this population to colorectal cancer (1, 39). These mechanistic considerations suggest that colorectal cancer might initiate as a disease of paracrine hormone insufficiency (5, 38). Like other diseases of endocrine insufficiency reflecting hormone loss, but preservation of receptor expression, silencing GUYC2C might be prevented by therapeutic replacement of GUCY2C ligands. These translational considerations are underscored by the recent regulatory approval of the oral GUCY2C ligand linaclotide to treat patients with irritable bowel syndrome-constipation type (40), and the initiation of a clinical program to explore its utility in preventing colorectal transformation in humans (ClinicalTrials.gov Identifier:NCT01950403) (41).
Supplementary Material
Acknowledgements
We would like to acknowledge the essential contribution of Stephanie Schulz, Ph.D. (deceased) to the design, conduct, and interpretation of this study.
Funding/Support: These studies were supported by grants from the National Institutes of Health (R01 CA75123, R01 CA95026, RC1 CA146033, to SA Waldman, co-investigator T Hyslop; and R01 CA170533 to SA Waldman), (P30 CA56036); the Pennsylvania Department of Health (SAP #4100059197, SAP #4100051723) to SA Waldman, co-investigator AE Snook; and Targeted Diagnostic and Therapeutics Inc. to SA Waldman. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. C Wilson, JE Lin, JP Gong, and P Li were supported by NIH institutional award T32 GM08562 for Postdoctoral Training in Clinical Pharmacology. JE Lin is the recipient of the Young Investigator Award from the American Society for Clinical Pharmacology and Therapeutics (ASCPT). C Wilson, P Li, and JP Gong were enrolled in the NIH-supported institutional K30 Training Program in Human Investigation (K30 HL004522). AE Snook is the recipient of the Measey Foundation Fellowship. SA Waldman is the Samuel MV Hamilton Professor of Thomas Jefferson University.
Footnotes
Disclosure of Potential Conflicts of Interest: S.A.W. is the Chair of the Data Safety Monitoring Board for the Chart-1 Trial™ sponsored by Cardio3 Biosciences, and the Chair (uncompensated) of the Scientific Advisory Board of Targeted Diagnostics & Therapeutics, Inc. which provided research funding that, in part, supported this work and has a license to commercialize inventions related to this work.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 3.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, et al. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000;52:375–414. [PubMed] [Google Scholar]
- 5.Pitari GM, Li P, Lin JE, Zuzga D, Gibbons AV, Snook AE, et al. The paracrine hormone hypothesis of colorectal cancer. Clin Pharmacol Ther. 2007;82:441–447. doi: 10.1038/sj.clpt.6100325. [DOI] [PubMed] [Google Scholar]
- 6.Fiskerstrand T, Arshad N, Haukanes BI, Tronstad RR, Pham KD, Johansson S, et al. Familial diarrhea syndrome caused by an activating GUCY2C mutation. N Engl J Med. 366:1586–1595. doi: 10.1056/NEJMoa1110132. [DOI] [PubMed] [Google Scholar]
- 7.Li P, Lin JE, Chervoneva I, Schulz S, Waldman SA, Pitari GM. Homeostatic Control of the Crypt-Villus Axis by the Bacterial Enterotoxin Receptor Guanylyl Cyclase C Restricts the Proliferating Compartment in Intestine. Am J Pathol. 2007;171:1847–1858. doi: 10.2353/ajpath.2007.070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li P, Schulz S, Bombonati A, Palazzo JP, Hyslop TM, Xu Y, et al. Guanylyl cyclase C suppresses intestinal tumorigenesis by restricting proliferation and maintaining genomic integrity. Gastroenterology. 2007;133:599–607. doi: 10.1053/j.gastro.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 9.Lin JE, Li P, Snook AE, Kricka L, Park J, Schulz S, et al. GUCY2C establishes lineage dependence in intestinal tumorigenesis through AKT. 2008 In review. [Google Scholar]
- 10.Pitari GM, Di Guglielmo MD, Park J, Schulz S, Waldman SA. Guanylyl cyclase C agonists regulate progression through the cell cycle of human colon carcinoma cells. Proc Natl Acad Sci U S A. 2001;98:7846–7851. doi: 10.1073/pnas.141124698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitari GM, Zingman LV, Hodgson DM, Alekseev AE, Kazerounian S, Bienengraeber M, et al. Bacterial enterotoxins are associated with resistance to colon cancer. Proc Natl Acad Sci U S A. 2003;100:2695–2699. doi: 10.1073/pnas.0434905100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Li P, Lin JE, Chervoneva I, Schulz S, Waldman SA, Pitari GM. Homeostatic control of the crypt-villus axis by the bacterial enterotoxin receptor guanylyl cyclase C restricts the proliferating compartment in intestine. Am J Pathol. 2007;171:1847–1858. doi: 10.2353/ajpath.2007.070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin JE, Li P, Snook AE, Schulz S, Dasgupta A, Hyslop TM, et al. The hormone receptor GUCY2C suppresses intestinal tumor formation by inhibiting AKT signaling. Gastroenterology. 2010;138:241–254. doi: 10.1053/j.gastro.2009.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shailubhai K, Yu HH, Karunanandaa K, Wang JY, Eber SL, Wang Y, et al. Uroguanylin treatment suppresses polyp formation in the Apc(Min/+) mouse and induces apoptosis in human colon adenocarcinoma cells via cyclic GMP. Cancer Res. 2000;60:5151–5157. [PubMed] [Google Scholar]
- 16.Li P, Lin JE, Marszlowicz GP, Valentino MA, Chang C, Schulz S, et al. GCC signaling in colorectal cancer: Is colorectal cancer a paracrine deficiency syndrome? Drug News Perspect. 2009;22:313–318. doi: 10.1358/dnp.2009.22.6.1395254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birkenkamp-Demtroder K, Lotte Christensen L, Harder Olesen S, Frederiksen CM, Laiho P, Aaltonen LA, et al. Gene expression in colorectal cancer. Cancer Res. 2002;62:4352–4363. [PubMed] [Google Scholar]
- 18.Cohen MB, Hawkins JA, Witte DP. Guanylin mRNA expression in human intestine and colorectal adenocarcinoma. Laboratory Investigation. 1998;78:101–108. [PubMed] [Google Scholar]
- 19.Notterman DA, Alon U, Sierk AJ, Levine AJ. Transcriptional gene expression profiles of colorectal adenoma, adenocarcinoma, and normal tissue examined by oligonucleotide arrays. Cancer Res. 2001;61:3124–3130. [PubMed] [Google Scholar]
- 20.Steinbrecher KA, Tuohy TM, Heppner Goss K, Scott MC, Witte DP, Groden J, et al. Expression of guanylin is downregulated in mouse and human intestinal adenomas. Biochemical & Biophysical Research Communications. 2000;273:225–230. doi: 10.1006/bbrc.2000.2917. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, et al. Gene expression profiles in normal and cancer cells. Science. 1997;276:1268–1272. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]
- 22.Li P, Lin JE, Snook AE, Gibbons A, Zuzga D, Schulz S, et al. Colorectal cancer as a paracrine deficiency syndrome amenable to oral hormone replacement therapy. Clinical Translational Science. 2008;1:163–167. doi: 10.1111/j.1752-8062.2008.00040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waldman SA, Hyslop T, Schulz S, Barkun A, Nielsen K, Haaf J, et al. Association of GUCY2C expression in lymph nodes with time to recurrence and disease-free survival in pN0 colorectal cancer. Jama. 2009;301:745–752. doi: 10.1001/jama.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeBaron MJ, Crismon HR, Utama FE, Neilson LM, Sultan AS, Johnson KJ, et al. Ultrahigh density microarrays of solid samples. Nat Methods. 2005;2:511–513. doi: 10.1038/nmeth772. [DOI] [PubMed] [Google Scholar]
- 25.Cagir B, Gelmann A, Park J, Fava T, Tankelevitch A, Bittner EW, et al. Guanylyl cyclase C messenger RNA is a biomarker for recurrent stage II colorectal cancer. Ann Intern Med. 1999;131:805–812. doi: 10.7326/0003-4819-131-11-199912070-00002. [DOI] [PubMed] [Google Scholar]
- 26.Carrithers SL, Barber MT, Biswas S, Parkinson SJ, Park PK, Goldstein SD, et al. Guanylyl cyclase C is a selective marker for metastatic colorectal tumors in human extraintestinal tissues. Proc Natl Acad Sci U S A. 1996;93:14827–14832. doi: 10.1073/pnas.93.25.14827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chervoneva I, Li Y, Iglewicz B, Waldman S, Hyslop T. Relative quantification based on logistic models for individual polymerase chain reactions. Stat Med. 2007;26:5596–5611. doi: 10.1002/sim.3127. [DOI] [PubMed] [Google Scholar]
- 28.Bacchi CE, Gown AM. Distribution and pattern of expression of villin, a gastrointestinal-associated cytoskeletal protein, in human carcinomas: a study employing paraffin-embedded tissue. Laboratory Investigation. 1991;64:418–424. [PubMed] [Google Scholar]
- 29.Nishizuka S, Chen ST, Gwadry FG, Alexander J, Major SM, Scherf U, et al. Diagnostic markers that distinguish colon and ovarian adenocarcinomas: identification by genomic, proteomic, and tissue array profiling. Cancer Res. 2003;63:5243–5250. [PubMed] [Google Scholar]
- 30.West AB, Isaac CA, Carboni JM, Morrow JS, Mooseker MS, Barwick KW. Localization of villin, a cytoskeletal protein specific to microvilli, in human ileum and colon and in colonic neoplasms. Gastroenterology. 1988;94:343–352. doi: 10.1016/0016-5085(88)90421-0. [DOI] [PubMed] [Google Scholar]
- 31.Lynn HS. Maximum likelihood inference for left-censored HIV RNA data. Stat Med. 2001;20:33–45. doi: 10.1002/1097-0258(20010115)20:1<33::aid-sim640>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 32.Compton CC, Greene FL. The staging of colorectal cancer: 2004 and beyond. CA Cancer J Clin. 2004;54:295–308. doi: 10.3322/canjclin.54.6.295. [DOI] [PubMed] [Google Scholar]
- 33.Greene FL. AJCC Cancer Staging Manual. 6th ed. New York: Springer; 2002. [Google Scholar]
- 34.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics,.2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 35.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–284. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Gibbons AV, Lin JE, Kim GW, Marszalowicz GP, Li P, Stoecker BA, et al. Intestinal GUCY2C Prevents TGF-beta Secretion Coordinating Desmoplasia and Hyperproliferation in Colorectal Cancer. Cancer Res. 2013;73:6654–6666. doi: 10.1158/0008-5472.CAN-13-0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulz S, Hyslop T, Haaf J, Bonaccorso C, Nielsen K, Witek ME, et al. A validated quantitative assay to detect occult micrometastases by reverse transcriptase-polymerase chain reaction of guanylyl cyclase C in patients with colorectal cancer. Clin Cancer Res. 2006;12:4545–4552. doi: 10.1158/1078-0432.CCR-06-0865. [DOI] [PubMed] [Google Scholar]
- 38.Lin JE, Li P, Pitari GM, Schulz S, Waldman SA. Guanylyl cyclase C in colorectal cancer: susceptibility gene and potential therapeutic target. Future Oncol. 2009;5:509–522. doi: 10.2217/fon.09.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, et al. Colorectal cancer. Lancet. 2010;375:1030–1047. doi: 10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- 40.Lembo AJ, Schneier HA, Shiff SJ, Kurtz CB, MacDougall JE, Jia XD, et al. Two randomized trials of linaclotide for chronic constipation. N Engl J Med. 2011;365:527–536. doi: 10.1056/NEJMoa1010863. [DOI] [PubMed] [Google Scholar]
- 41. http://clinicaltrials.gov/ct2/show/NCT01912079?term=linaclotide&rank=1.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.