Abstract
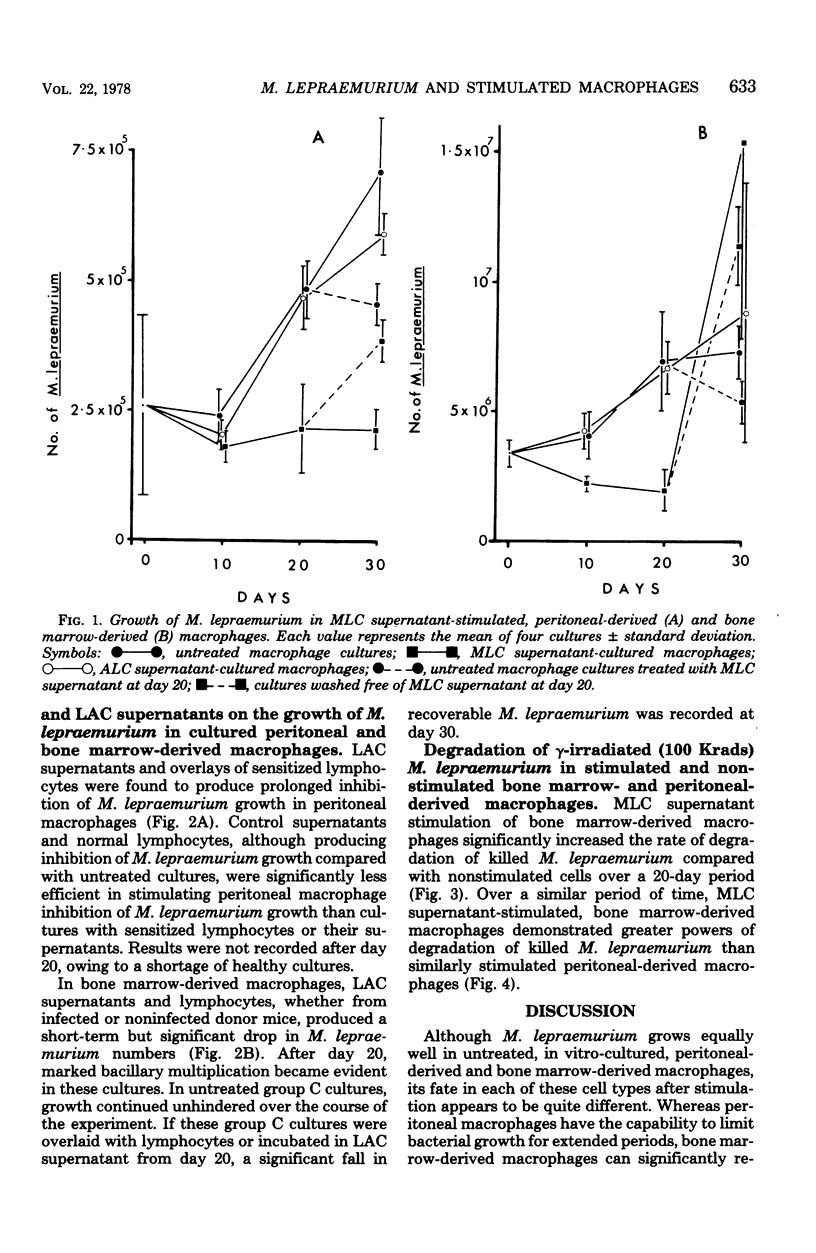
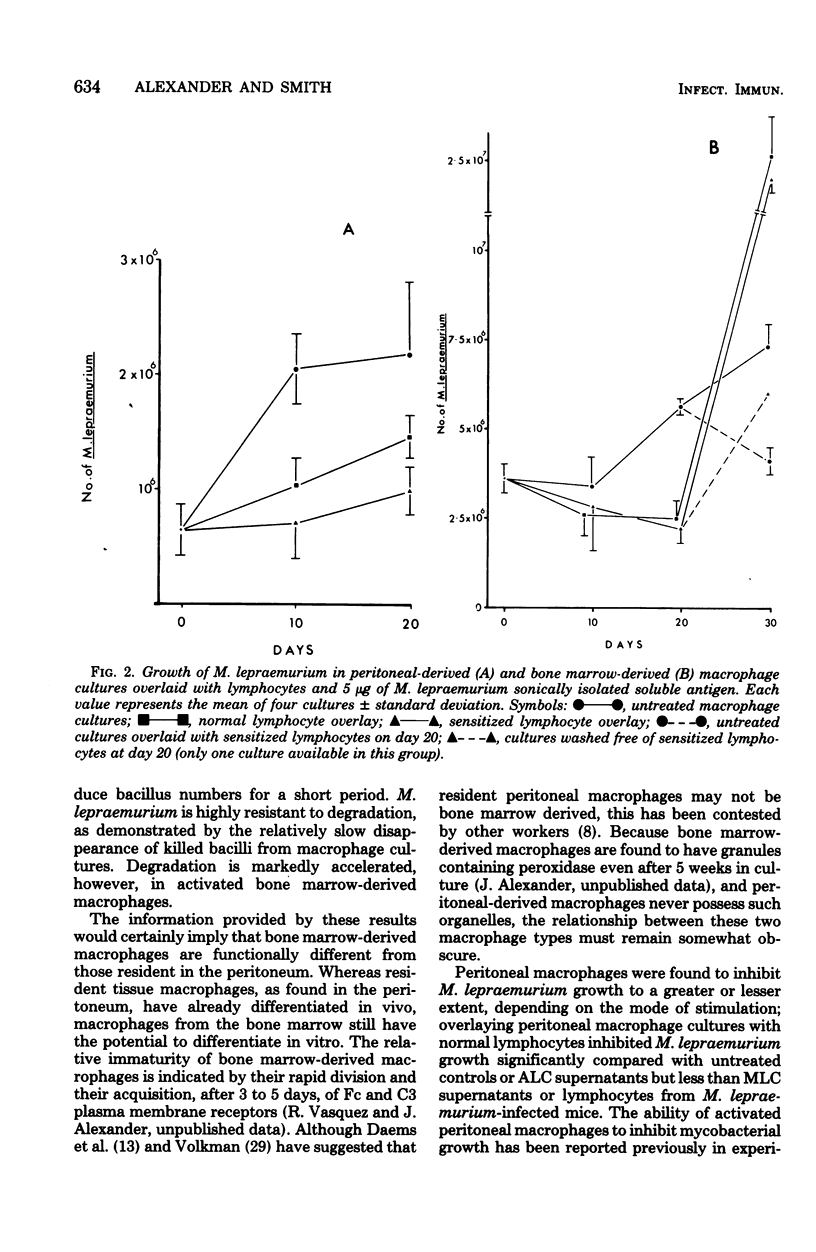
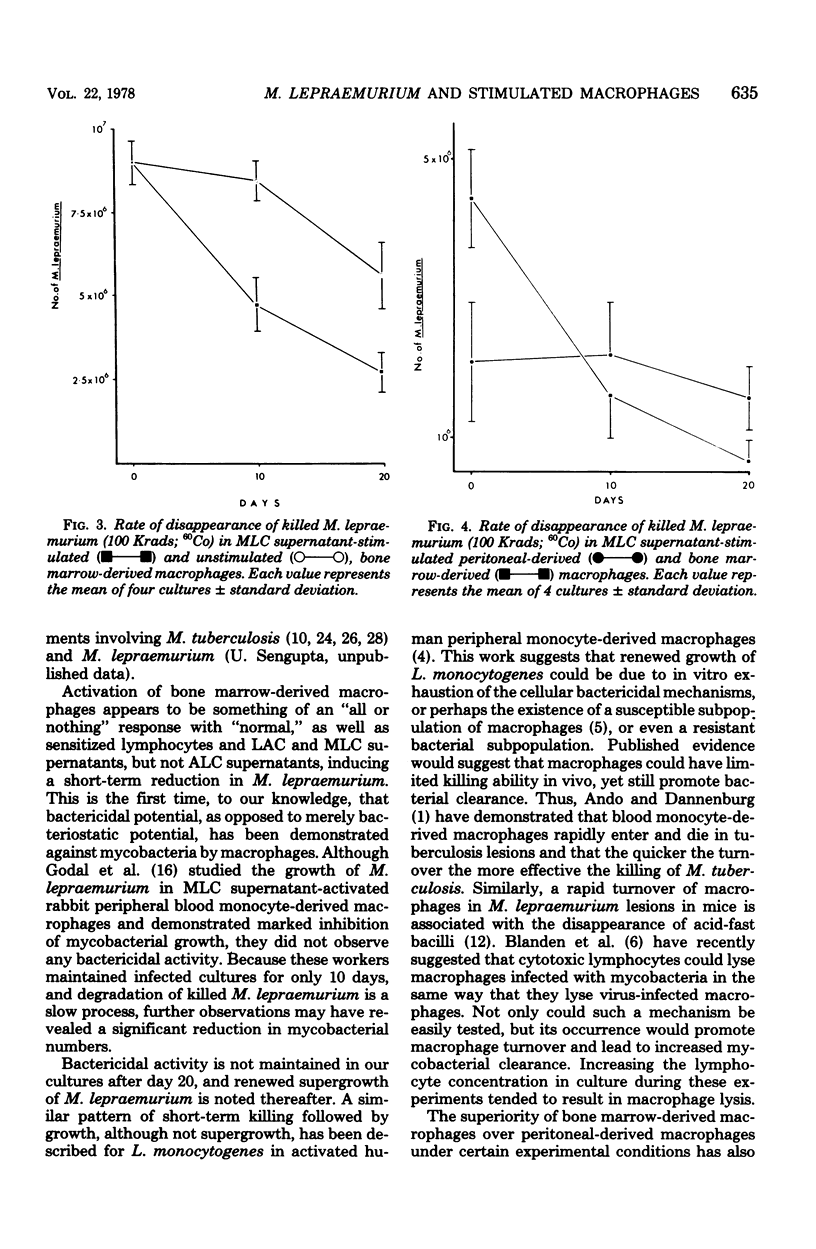
Mycobacterium lepraemurium cells were found to multiply in normal mouse peritoneal-derived and bone marrow-derived macrophages in vitro. Whereas activated peritoneal-derived macrophages demonstrated marked bacteriostasis for M. lepraemurium, significant bactericidal activity was exhibited by activated bone marrow-derived macrophages. However, only a small proportion of the bacterial were killed by activated bone marrow-derived macrophages with subsequent and enhanced bacteria growth. It is suggested that a rapid turnover of monocytes in active lesions is required to control mycobacterial infections in vivo. These results would suggest that careful consideration be given to the choice of the host cell in studies involving obligate intracellular parasites.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando M., Dannenberg A. M., Jr Macrophage accumulation, division, maturation, and digestive and microbicidal capacities in tuberculous lesions. IV. Macrophage turnover, lysosomal enzymes, and division in healing lesions. Lab Invest. 1972 Nov;27(5):466–472. [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med. 1975 Jul 1;142(1):1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behin R., Mauel J., Biroum-Noerjasin, Rowe D. S. Mechanisms of protective immunity in experimental cutaneous leishmaniasis of the guinea-pig. II. Selective destruction of different Leishmania species in activated guinea-pig and mouse macrophages. Clin Exp Immunol. 1975 May;20(2):351–358. [PMC free article] [PubMed] [Google Scholar]
- Biroum-Noerjasin Listericidal activity of non-stimulated and stimulated human macrophages in vitro. Clin Exp Immunol. 1977 Apr;28(1):138–145. [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V. Modification of macrophage function. J Reticuloendothel Soc. 1968 Jun;5(3):179–202. [PubMed] [Google Scholar]
- Bloom B. R. In vitro approaches to the mechanism of cell-mediated immune reactions. Adv Immunol. 1971;13:101–208. doi: 10.1016/s0065-2776(08)60184-4. [DOI] [PubMed] [Google Scholar]
- Bodel P. T., Nichols B. A., Bainton D. F. Appearance of peroxidase reactivity within the rough endoplasmic reticulum of blood monocytes after surface adherence. J Exp Med. 1977 Feb 1;145(2):264–274. doi: 10.1084/jem.145.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- CHANG Y. T. LONG-TERM CULTIVATION OF MOUSE PERITONEAL MACROPHAGES. J Natl Cancer Inst. 1964 Jan;32:19–35. [PubMed] [Google Scholar]
- Cahall D. L., Youmans G. P. Conditions for production, and some characteristics, of mycobacterial growth inhibitory factor produced by spleen cells from mice immunized with viable cells of the attenuated H37Ra strain of Mycobacterium tuberculosis. Infect Immun. 1975 Oct;12(4):833–840. doi: 10.1128/iai.12.4.833-840.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs O., Haugen O. A. Experimental murine leprosy. 3. Early local reaction to mycobacterium lepraemurium in C3H and C57/BL mice. Acta Pathol Microbiol Scand A. 1975 Jan;83(1):51–58. [PubMed] [Google Scholar]
- Draper P. The walls of Mycobacterium lepraemurium: chemistry and ultrastructure. J Gen Microbiol. 1971 Dec;69(3):313–324. doi: 10.1099/00221287-69-3-313. [DOI] [PubMed] [Google Scholar]
- Dumonde D. C., Wolstencroft R. A., Panayi G. S., Matthew M., Morley J., Howson W. T. "Lymphokines": non-antibody mediators of cellular immunity generated by lymphocyte activation. Nature. 1969 Oct 4;224(5214):38–42. doi: 10.1038/224038a0. [DOI] [PubMed] [Google Scholar]
- Godal T., Rees R. J., Lamvik J. O. Lymphocyte-mediated modification of blood-derived macrophage function in vitro; inhibition of growth of intracellular mycobacteria with lymphokines. Clin Exp Immunol. 1971 Apr;8(4):625–637. [PMC free article] [PubMed] [Google Scholar]
- HART P. D., REES R. J. Effect of macrocyclon in acute and chronic pulmonary tuberculous infection in mice as shown by viable and total bacterial counts. Br J Exp Pathol. 1960 Aug;41:414–421. [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Len L., Hirsch J. G. Assessment in vitro of immunity against Toxoplasma gondii. J Exp Med. 1975 Feb 1;141(2):466–482. doi: 10.1084/jem.141.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meerpohl H. G., Lohmann-Matthes M. L., Fischer H. Studies on the activation of mouse bone marrow-derived macrophages by the macrophage cytotoxicity factor (MCF). Eur J Immunol. 1976 Mar;6(3):213–217. doi: 10.1002/eji.1830060313. [DOI] [PubMed] [Google Scholar]
- Miller H. C., Twohy D. W. Cellular immunity to Leishmania donovani in macrophages in cultures. J Parasitol. 1969 Feb;55(1):200–207. [PubMed] [Google Scholar]
- North R. J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973 Apr;7(1):166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Demonstration in tissue culture of lymphocyte-mediated immunity to tuberculosis. Infect Immun. 1970 Jun;1(6):600–603. doi: 10.1128/iai.1.6.600-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTER E. Multiplication of tubercle bacilli within mononuclear phagocytes in tissue cultures derived from normal animals and animals vaccinated with BCG. J Exp Med. 1953 Feb 1;97(2):235–245. doi: 10.1084/jem.97.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTER E., RAMSEIAR H. CELLULAR REACTIONS IN INFECTION. Adv Immunol. 1964;27:117–173. doi: 10.1016/s0065-2776(08)60707-5. [DOI] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Enhancement of macrophage bactericidal capacity by antigenically stimulated immune lymphocytes. Cell Immunol. 1972 Jun;4(2):163–174. doi: 10.1016/0008-8749(72)90015-9. [DOI] [PubMed] [Google Scholar]
- Turcotte R., Des Ormeaux Y., Borduas A. G. Partial characterization of a factor extracted from sensitized lymphocytes that inhibits the growth of Mycobacterium tuberculosis within macrophages in vitro. Infect Immun. 1976 Aug;14(2):337–344. doi: 10.1128/iai.14.2.337-344.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


