Abstract
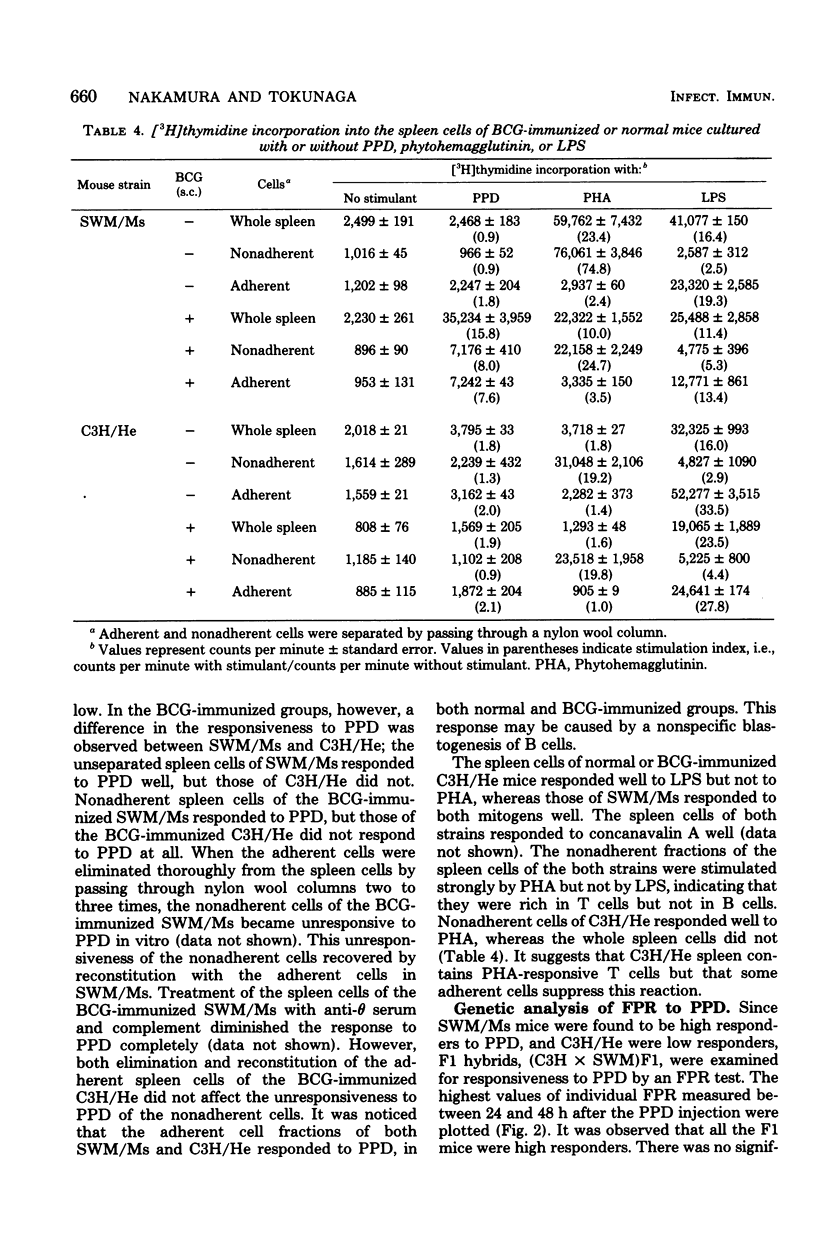
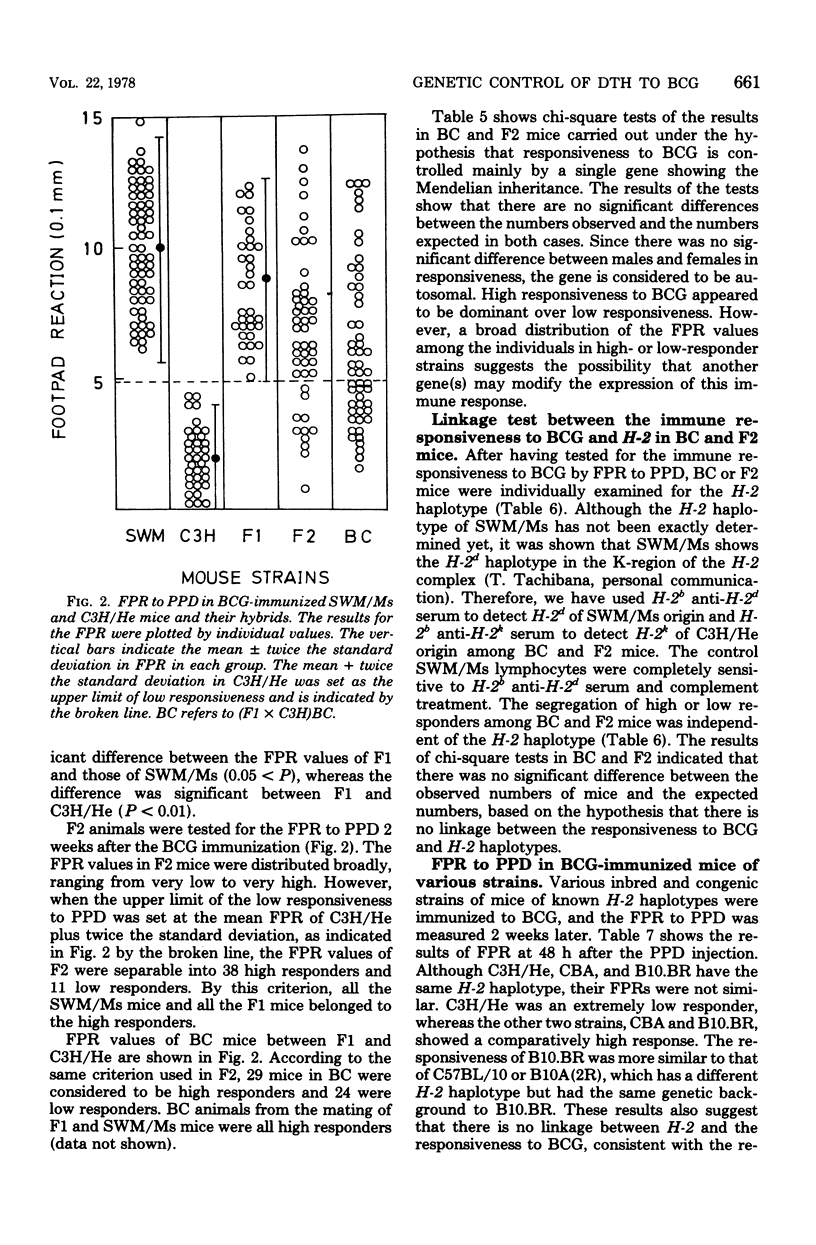
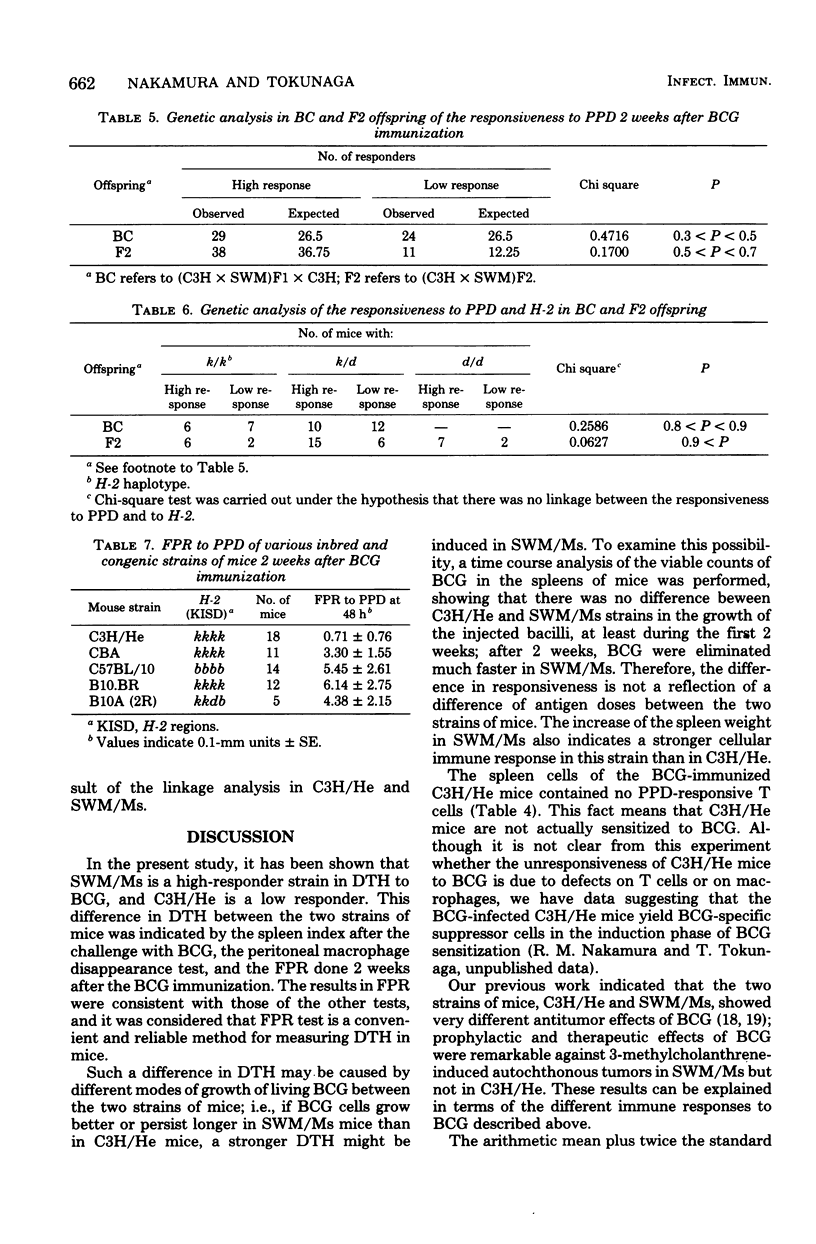
Delayed-type hypersensitivity to BCG was remarkably different in two inbred strains of mice, SWM/Ms and C3H/He, when measured by the spleen index, the disappearance of peritoneal macrophage, or the footpad reaction. High responsiveness in the SWM/Ms strain appeared to be dominant over low responsiveness in the C3H/He strain. Results of the footpad reaction test if F1, F2, and backcross hybrids of these two strains of mice suggested that the delayed-type hypersensitivity was mainly controlled by a gene which was transmitted under Mendel's laws and was possibly non-H-2 linked. The spleen cells and their nylon wool nonadherent fraction from BCG-infected C3H/He mice were not reactive to purified protein derivative in vitro, whereas both the spleen cells and the nylon wool nonadherent fraction from BCG-infected SWM/Ms mice reacted well to purified protein derivative. Possible mechanisms of the different responses in the delayed-type hypersensitivity to BCG were discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen E. M., Moore V. L., Stevens J. O. Strain variation in BCG-induced chronic pulmonary inflammation in mice. I. Basic model and possible genetic control by non-H-2 genes. J Immunol. 1977 Jul;119(1):343–347. [PubMed] [Google Scholar]
- Benacerraf B., McDevitt H. O. Histocompatibility-linked immune response genes. Science. 1972 Jan 21;175(4019):273–279. doi: 10.1126/science.175.4019.273. [DOI] [PubMed] [Google Scholar]
- Civil R. H., Mahmoud A. A. Genetic differences in BCG-induced resistance to Schistosoma mansoni are not controlled by genes within the major histocompatibility complex of the mouse. J Immunol. 1978 Mar;120(3):1070–1072. [PubMed] [Google Scholar]
- Davis S., Shearer G. M., Mozes E., Sela M. Genetic control of the murine cell-mediated immune response in vivo. II. H-2 linked responsiveness to the synthetic polypeptide poly(Tyr,Glu)-poly(DL-Ala)--poly(Lys). J Immunol. 1975 Dec;115(6):1530–1532. [PubMed] [Google Scholar]
- Fachet J., Andó I. Genetic control of contact sensitivity to oxazolone in inbred, H-2 congenic and intra-H-2 recombinant strains of mice. Eur J Immunol. 1977 Apr;7(4):223–226. doi: 10.1002/eji.1830070407. [DOI] [PubMed] [Google Scholar]
- Gasser D. L. Genetic control of the immune response in mice. I. Segregation data and localization to the fifth linkage group of a gene affecting antibody production. J Immunol. 1969 Jul;103(1):66–70. [PubMed] [Google Scholar]
- Gasser D. L., Silvers W. K. Genetic determinants of immunological responsiveness. Adv Immunol. 1974;18:1–66. doi: 10.1016/s0065-2776(08)60307-7. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Karakoz I., Krejci J., Hála K., Blaszczyk B., Hraba T., Pekárek J. Genetic determination of tuberculin hypersensitivity in chicken inbred lines. Eur J Immunol. 1974 Aug;4(8):545–548. doi: 10.1002/eji.1830040805. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B., Auclair D. J., Lagrange P. H. Immunopotentiation with BCG. I. Immune response to different strains and preparations. J Natl Cancer Inst. 1973 Nov;51(5):1655–1667. doi: 10.1093/jnci/51.5.1655. [DOI] [PubMed] [Google Scholar]
- McDevitt H. O., Benacerraf B. Genetic control of specific immune responses. Adv Immunol. 1969;11:31–74. doi: 10.1016/s0065-2776(08)60477-0. [DOI] [PubMed] [Google Scholar]
- Mozes E., Shearer G. M., Sela M. Cellular basis of the genetic control of immune responses to synthetic polypeptides. I. Differences in frequency of splenic precursor cells specific for a synthetic polypeptide derived from multichain polyproline ((T,G)-Pro--L) in high and low responder inbred mouse strains. J Exp Med. 1970 Oct 1;132(4):613–622. doi: 10.1084/jem.132.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELSON D. S., BOYDEN S. V. The loss of macrophages from peritoneal exudates following the injection of antigens into guinea-pigs with delayed-type hypersensitivity. Immunology. 1963 May;6:264–275. [PMC free article] [PubMed] [Google Scholar]
- Nomoto K., Makidono R., Takeya K. Immune response against hamster erythrocytes in the low-responder mouse strains. IV. Delayed hypersensitivity against solubilized hamster erythrocytes in mice. Jpn J Microbiol. 1972 Sep;16(5):415–423. doi: 10.1111/j.1348-0421.1972.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Polák L., Barnes J. M., Turk J. L. The genetic control of contact sensitization to inorganic metal compounds in guinea-pigs. Immunology. 1968 May;14(5):707–711. [PMC free article] [PubMed] [Google Scholar]
- Rosenstreich D. L., Glode L. M. Difference in B cell mitogen responsiveness between closely related strains of mice. J Immunol. 1975 Sep;115(3):777–780. [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S. Defective tumoricidal capacity of macrophages from C3H/HeJ mice. J Immunol. 1978 Jan;120(1):329–334. [PubMed] [Google Scholar]
- Tokunaga T., Yamamoto S., Nakamura R. M., Kataoka T. Immunotherapeutic and immunoprophylactic effects of BCG on 3-methylcholanthrene-induced autochthonous tumors in Swiss mice. J Natl Cancer Inst. 1974 Aug;53(2):459–463. doi: 10.1093/jnci/53.2.459. [DOI] [PubMed] [Google Scholar]
- Tokunaga T., Yamamoto S., Nakamura R. M., Kurosawa A., Murohashi T. Mouse-strain difference in immunoprophylactic and immunotherapeutic effects of BCG on carcinogen-induced autochthonous tumors. Jpn J Med Sci Biol. 1978 Apr;31(2):143–154. doi: 10.7883/yoken1952.31.143. [DOI] [PubMed] [Google Scholar]


