Abstract
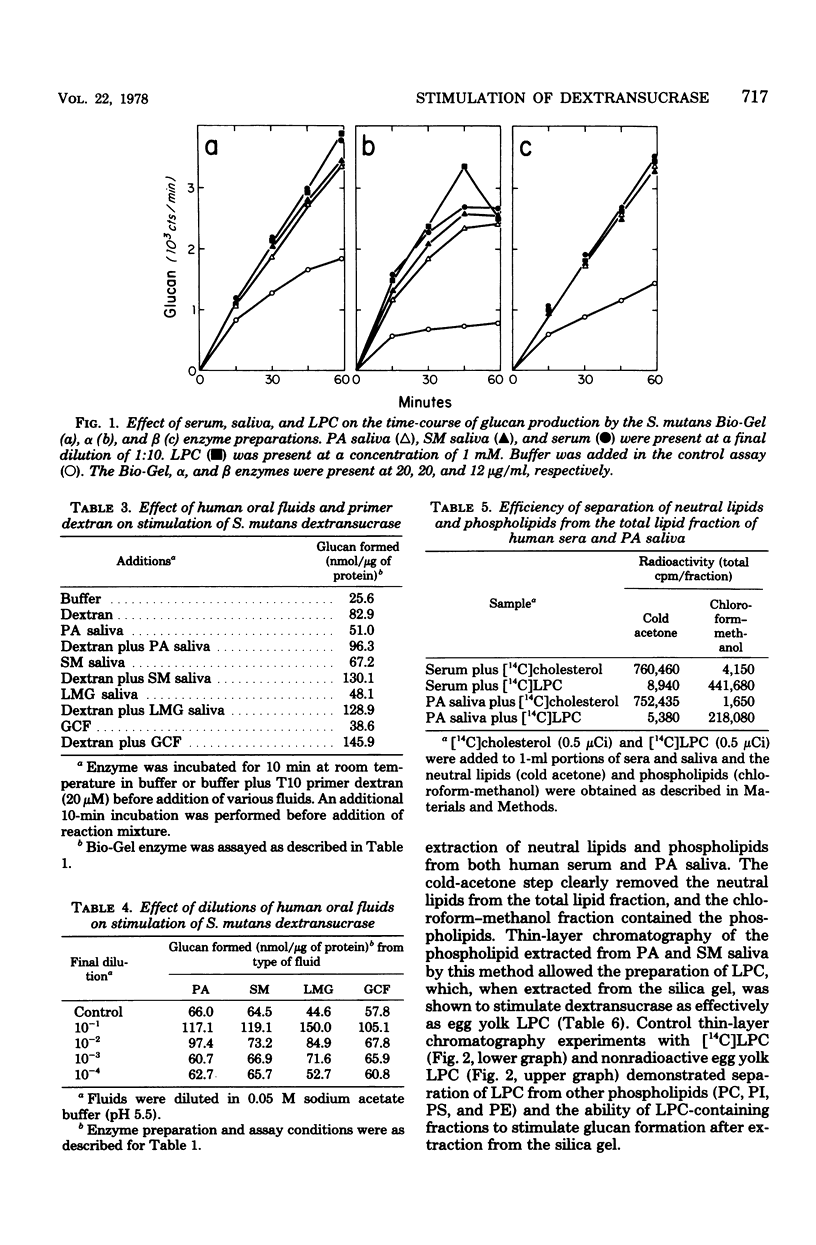
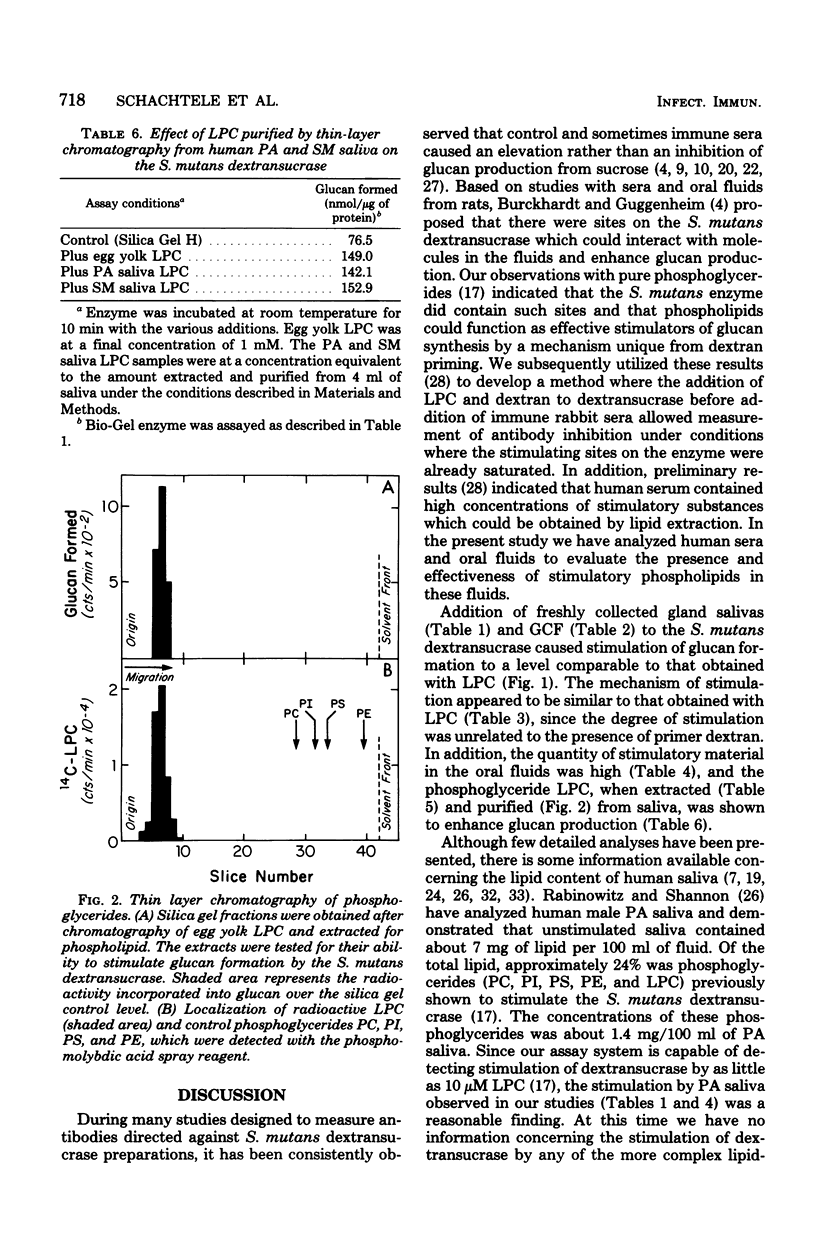
Serum, gingival crevicular fluid, and parotid, submandibular, and labial minor gland saliva from four individuals stimulated glucan formation from sucrose by the Streptococcus mutans strain 6715 dextransucrase (EC 2.4.1.5). At final dilutions of 1:10 all of the fluids stimulated crude enzyme preparations approximately 1.8-fold. The fluids stimulated the purified water-insoluble glucan-synthesizing form of the dextransucrase approximately 3.2-fold and the water-soluble glucan-producing form of the enzyme approximately 2.4-fold. The fluids all contained concentrations of stimulatory material that could be reduced to undetectable levels only after dilutions of greater than 1:1,000. The increased rates of glucan formation caused by the fluids and dextran were additive, indicating that stimulation by the fluids was primarily due to interactions with entities other than glucan primer molecules. In contrast, the elevated levels of glucan formation in the presence of the fluids was not further enhanced by the addition of lysophosphatidylcholine. Lysophosphatidylcholine purified from parotid and submandibular saliva by solvent extraction and thin-layer chromatography stimulated the dextransucrase as effectively as egg yolk lysophosphatidylcholine. Thus, phospholipids normally found in human oral fluids can enhance the activity of an enzyme believed to be directly associated with the cariogenic potential of S. mutans.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berlin R., Oldfelt C. O., Vikrot O. Lipid changes after incubation of normal plasma. Acta Med Scand. 1969 May;185(5):427–431. doi: 10.1111/j.0954-6820.1969.tb07359.x. [DOI] [PubMed] [Google Scholar]
- Burckhardt J. J., Guggenheim B. Interactions of antisera, sera, and oral fluid with glucosyltransferases. Infect Immun. 1976 Apr;13(4):1009–1022. doi: 10.1128/iai.13.4.1009-1022.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courts F. J., Boackle R. J., Fudenberg H. H., Silverman M. S. Detection of functional complement components in gingival crevicular fluid from humans with periodontal diseases. J Dent Res. 1977 Mar;56(3):327–331. doi: 10.1177/00220345770560032001. [DOI] [PubMed] [Google Scholar]
- Crawford J. M., Taubman M. A., Smith D. J. Minor salivary glands as a major source of secretory immunoglobin A in the human oral cavity. Science. 1975 Dec 19;190(4220):1206–1209. doi: 10.1126/science.1198107. [DOI] [PubMed] [Google Scholar]
- DIRKSEN T. R. LIPID CONSTITUENTS OF WHOLE AND PAROTID SALIVA. J Dent Res. 1963 Jul-Aug;42:920–924. doi: 10.1177/00220345630420041601. [DOI] [PubMed] [Google Scholar]
- EGELBERG J. CELLULAR ELEMENTS IN GINGIVAL POCKET FLUID. Acta Odontol Scand. 1963 Aug;21:283–287. doi: 10.3109/00016356309028193. [DOI] [PubMed] [Google Scholar]
- Evans R. T., Genco R. J. Inhibition of glucosyltransferase activity by antisera to known serotypes of Streptococcus mutans. Infect Immun. 1973 Feb;7(2):237–241. doi: 10.1128/iai.7.2.237-241.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui K., Fukui Y., Moriyama T. Some Immunochemical Properties of Dextransucrase and Invertase from Streptococcus mutans. Infect Immun. 1974 Nov;10(5):985–990. doi: 10.1128/iai.10.5.985-990.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Chludzinski A. M., Schachtele C. F. Streptococcus mutans dextransucrase: requirement for primer dextran. J Bacteriol. 1974 Oct;120(1):287–294. doi: 10.1128/jb.120.1.287-294.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Harlander S. K., Leung W. L., Schachtele C. F. Streptococcus mutans dextransucrase: functioning of primer dextran and endogenous dextranase in water-soluble and water-insoluble glucan synthesis. Infect Immun. 1977 May;16(2):637–648. doi: 10.1128/iai.16.2.637-648.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F., Chludzinski A. M. Rapid filter paper assay for the dextransucrase activity from Streptococcus mutans. J Dent Res. 1974 Nov-Dec;53(6):1355–1360. doi: 10.1177/00220345740530061101. [DOI] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F. Streptococcus mutans dextransucrase: mode of interaction with high-molecular-weight dextran and role in cellular aggregation. Infect Immun. 1976 Feb;13(2):365–372. doi: 10.1128/iai.13.2.365-372.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub L. M., Kleinberg I. Gingival crevicular fluid: a new diagnostic aid in managing the periodontal patient. Oral Sci Rev. 1976;(8):49–61. [PMC free article] [PubMed] [Google Scholar]
- Gordon D. F., Jr, Gibbons R. J. Glycolytic activity of Streptococcus mitis grown in vitro and in gnotobiotic animals. J Bacteriol. 1967 May;93(5):1735–1736. doi: 10.1128/jb.93.5.1735-1736.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlander S. K., Schachtele C. F. Streptococcus mutans dextransucrase: stimulation of glucan formation by phosphoglycerides. Infect Immun. 1978 Feb;19(2):450–456. doi: 10.1128/iai.19.2.450-456.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K., Ingersoll L. Differential inhibition of Streptococcus mutans in vitro adherence by anti-glucosyltransferase antibodies. Infect Immun. 1976 Jun;13(6):1775–1777. doi: 10.1128/iai.13.6.1775-1777.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEPAGE M. ISOLATION AND CHARACTERIZATION OF AN ESTERIFIED FORM OF STERYL GLUCOSIDE. J Lipid Res. 1964 Oct;5:587–592. [PubMed] [Google Scholar]
- Linzer R., Slade H. D. Characterization of an anti-glucosyltransferase serum specific for insoluble glucan synthesis by Streptococcus mutans. Infect Immun. 1976 Feb;13(2):494–500. doi: 10.1128/iai.13.2.494-500.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löe H. The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol. 1967 Nov-Dec;38(6 Suppl):610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- Mandel I. D., Eisenstein A. Lipids in human salivary secretions and salivary calculus. Arch Oral Biol. 1969 Feb;14(2):231–233. doi: 10.1016/0003-9969(69)90067-3. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G., Hampton J. R., Mitchell J. R. Cyclical changes in plasma-lysolecithin induced by oral contraceptives. Lancet. 1971 Dec 25;2(7739):1428–1429. doi: 10.1016/s0140-6736(71)90709-4. [DOI] [PubMed] [Google Scholar]
- Rabinowitz J. L., Shannon I. L. Lipid changes in human male parotid saliva by stimulation. Arch Oral Biol. 1975 Jul;20(7):403–406. doi: 10.1016/0003-9969(75)90223-x. [DOI] [PubMed] [Google Scholar]
- Russell M. W., Challacombe S. J., Lehner T. Serum glucosyltransferase-inhibiting antibodies and dental caries in rhesus monkeys immunized against Streptococcus mutans. Immunology. 1976 May;30(5):619–627. [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Harlander S. K., Ostrum L. C., Bracke J. W., Babb J. L. Streptococcus mutans dextransucrase: phosphoglycerides and the detection of inhibitory antibodies in sera. Adv Exp Med Biol. 1978;107:717–725. doi: 10.1007/978-1-4684-3369-2_81. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Loken A. E., Knudson D. J. Preferential utilization of the glucosyl moiety of sucrose by a cariogenic strain of Streptococcus mutans. Infect Immun. 1972 Apr;5(4):531–536. doi: 10.1128/iai.5.4.531-536.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkein H. A., Genco R. J. Gingival fluid and serum in periodontal diseases. I. Quantitative study of immunoglobulins, complement components, and other plasma proteins. J Periodontol. 1977 Dec;48(12):772–777. doi: 10.1902/jop.1977.48.12.772. [DOI] [PubMed] [Google Scholar]
- Shannon I. L., Chauncey H. H. A parotid fluid collection device with improved stability characteristics. J Oral Ther Pharmacol. 1967 Sep;4(2):93–97. [PubMed] [Google Scholar]
- Slomiany B. L., Slomiany A. ABH-blood-group antigens and glycolipids of human saliva. Eur J Biochem. 1978 Apr;85(1):249–254. doi: 10.1111/j.1432-1033.1978.tb12233.x. [DOI] [PubMed] [Google Scholar]
- Slomiany B. L., Slomiany A. Partial characterization of glyceroglucolipids from human saliva. Biochem Biophys Res Commun. 1977 Nov 7;79(1):61–66. doi: 10.1016/0006-291x(77)90060-2. [DOI] [PubMed] [Google Scholar]
- VIKROT O. QUANTITATIVE DETERMINATION OF PLASMA PHOSPHOLIPIDS IN PREGNANT AND NON-PREGNANT WOMEN, WITH SPECIAL REFERENCE TO LYSOLECITHIN. Acta Med Scand. 1964 Apr;175:443–453. doi: 10.1111/j.0954-6820.1964.tb00592.x. [DOI] [PubMed] [Google Scholar]


