Highlights
-
•
In PD, contralateral M1 showed greater beta power than ipsilateral M1.
-
•
Zolpidem reduced contralateral beta power while ipsilateral power was increased.
-
•
This resulted in a hemispheric power ratio that approached parity.
-
•
Changes were reflected in pre-movement desynchronization and post-movement rebound.
-
•
These changes underlie the symptomatic improvements afforded by zolpidem.
Abbreviations: AMC, age-matched control; EMG, electromyography; M1, primary motor cortex; MEG, magnetoencephalography; MRBD, movement-related beta desynchronization; MRI, magnetic resonance imaging; PD, Parkinson’s disease; PMBR, post-movement beta rebound; PSD, power spectral density; STN, subthalamic nucleus; UPDRS, Unified Parkinson’s Disease Rating Scale
Key words: magnetoencephalography, Parkinson’s disease, primary motor cortex, beta oscillations, GABAA receptors
Abstract
In Parkinson’s disease (PD), elevated beta (15–35 Hz) power in subcortical motor networks is widely believed to promote aspects of PD symptomatology, moreover, a reduction in beta power and coherence accompanies symptomatic improvement following effective treatment with l-DOPA. Previous studies have reported symptomatic improvements that correlate with changes in cortical network activity following GABAA receptor modulation. In this study we have used whole-head magnetoencephalography to characterize neuronal network activity, at rest and during visually cued finger abductions, in unilaterally symptomatic PD and age-matched control participants. Recordings were then repeated following administration of sub-sedative doses of the hypnotic drug zolpidem (0.05 mg/kg), which binds to the benzodiazepine site of the GABAA receptor. A beamforming based ‘virtual electrode’ approach was used to reconstruct oscillatory power in the primary motor cortex (M1), contralateral and ipsilateral to symptom presentation in PD patients or dominant hand in control participants. In PD patients, contralateral M1 showed significantly greater beta power than ipsilateral M1. Following zolpidem administration contralateral beta power was significantly reduced while ipsilateral beta power was significantly increased resulting in a hemispheric power ratio that approached parity. Furthermore, there was highly significant correlation between hemispheric beta power ratio and Unified Parkinson’s Disease Rating Scale (UPDRS). The changes in contralateral and ipsilateral beta power were reflected in pre-movement beta desynchronization and the late post-movement beta rebound. However, the absolute level of movement-related beta desynchronization was not altered. These results show that low-dose zolpidem not only reduces contralateral beta but also increases ipsilateral beta, while rebalancing the dynamic range of M1 network oscillations between the two hemispheres. These changes appear to underlie the symptomatic improvements afforded by low-dose zolpidem.
Introduction
Physiological neuronal network oscillations at beta frequency (15–35 Hz) occur spontaneously in the primary motor cortex (M1) of humans (Jensen et al., 2005; Hall et al., 2010a) and in animal models (Murthy and Fetz, 1996; Baker et al., 1997). The possible functional significance of beta oscillations is reflected by the power changes that occur during the various phases of movement. In self-paced movement, the pre-movement period is characterized by a bilateral reduction in the power of the spontaneous beta activity (Pfurtscheller and Berghold, 1989; Leocani et al., 1997; Jurkiewicz et al., 2006) up to two-seconds prior to movement initiation. Following this, whether self-paced or externally cued, there is a further bilateral reduction in beta power, referred to as movement-related beta desynchronization (MRBD) (Gaetz et al., 2011; Hall et al., 2011). Following movement termination, an increase in beta oscillatory power above the pre-movement baseline amplitude is observed (Pfurtscheller et al., 1996), a phenomenon referred to as post-movement beta rebound (PMBR).
In Parkinson’s disease (PD) exaggerated beta oscillations are observed in recordings from subcortical structures such as the subthalamic nucleus (STN) and cortex of both animal models (Sharott et al., 2005; Chen et al., 2007; Mallet et al., 2008) and PD patients (Cassidy et al., 2002; Pollok et al., 2012). Similarly, several studies report that this elevated beta power and coherence is attenuated following treatment with either l-DOPA (Silberstein et al., 2005; Kuhn et al., 2006) or deep brain stimulation (Kühn et al., 2008; Eusebio et al., 2011), both of which are associated with relief of PD symptoms.
A number of reports have highlighted that sub-sedative doses (2–5 mg) of the GABAA receptor modulator and hypnotic drug, zolpidem, improves cognitive and motor abilities of patients in persistent vegetative state (Clauss and Nel, 2006), brain injury (Cohen et al., 2004; Shames and Ring, 2008), dementia (Jarry et al., 2002) and idiopathic PD (Daniele et al., 1997; Chen et al., 2008; Huang et al., 2012). However, the mechanism underlying such benefits remains to be elucidated.
Our own previous study with a brain-injured participant revealed persistent pathological theta (4–10 Hz) and beta (15–35 Hz) oscillations in cortical areas surrounding the lesion (Hall et al., 2010b). Specifically, sensorimotor and language areas exhibited pathological beta and theta, consistent with altered gait and speech agnosia. Zolpidem greatly reduced the synchronous power of pathological oscillations in these loci, which correlated well with zolpidem uptake and restoration of function. These results suggest that specific motor and cognitive impairments are related to increased low-frequency oscillatory neuronal network activity, and that zolpidem is able to desynchronize such pathological low-frequency activity, restoring cognitive and motor function (for further details see Hall et al., 2010b).
Here, we have used low-dose zolpidem and magnetoencephalography (MEG) to perform a mechanistic evaluation of neuronal network activity of bilateral M1 cortices in early-stage, unilateral symptomatic, drug-naive PD patients. We hypothesized that zolpidem would reduce exaggerated cortical beta activity in PD patients and restore the dynamic range of MRBD and PMBR. We found that resting beta power contralateral to the symptomatic side is raised in PD and that power was reduced by zolpidem, while resting beta power ipsilateral to the symptomatic side was increased by zolpidem. When we analyzed changes in beta activity that accompany movement we found that following zolpidem, MRBD and PMBR were similar on contralateral and ipsilateral sides. This rebalancing of inter-hemispheric oscillatory dynamics appears to correlate with the symptomatic improvements afforded by low-dose zolpidem.
Experimental procedures
Participants
Nine early-stage right-handed PD participants (three females, six males with a mean age of 60.7 years, range 56–68 years) were recruited prior to the initiation of PD medication. All PD participants were recruited on the basis that they exhibited unilateral symptoms only and were assessed for symptomatic severity using the part III motor examination of the Unified Parkinson’s Disease Rating Scale (UPDRS).
In addition, nine right-handed healthy age-matched control (AMC) participants were recruited, (five females, four males with a mean age of 62.2 years, range 48–70 years), as a comparison for the measurement of spontaneous oscillatory activity and the effects of zolpidem on neural activity.
Throughout, contralateral and ipsilateral hemispheres were assigned with respect to symptom presentation in PD or dominant hand in AMC.
The study was performed in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the School of Life and Health Sciences at Aston University, U.K. Written, informed consent was obtained from all participants.
Structural magnetic resonance imaging (MRI) recordings
High resolution anatomical MR images were obtained using a 3-Tesla MRI system (Siemens, Erlangen, Germany). These structural brain scans were used for anatomical co-registration of MEG data, using 3-dimensional surface matching of the participants scalp and localization coils (Adjamian et al., 2004).
MEG recordings
Participants were seated in a 275-channel MEG system (VSM Medtech, Coquitlam, British Columbia, Canada) or 306-channel Elekta system (Neuromag Oy, Helsinki, Finland), for a period of 10 min. VSM data were acquired at a sampling rate of 1.2 kHz using a 3rd order gradiometer configuration with a 50-Hz notch filter and anti-aliasing 1–600-Hz band-pass filter while Elekta data were collected at 1 kHz with a 1–300-Hz band-pass filter.
In order to pool data collected from the two different MEG systems, data from individual participants were normalized to the absolute value of ipsilateral beta power and expressed as a percentage. This normalization process allows data from the two systems to be integrated without bias, but does preclude the direct comparison of absolute beta power values from PD patients and AMC participants.
Head position was monitored throughout MEG recordings, by matching the digitized position of three surface-mounted electromagnetic positioning coils (left and right pre-auricular and nasion), which were then monitored throughout the recording process to ensure no participant moved more than 5 mm from starting position during recording period.
Data were recorded during 1-min rest periods and during finger movement tasks (Fig. 1A, B) designed to localize left and right M1 cortices (Hall et al., 2011). Movement-related activity was recorded as discrete 3-min blocks. Participants were required to perform a visual reaction time task, in which they responded as quickly as possible to a change in fixation cross color with button press by abduction of either the left or right index finger. Stimuli were presented with a mean inter-stimulus duration of 6 s, with randomized jitter of ±1 s, with a total of 30 responses (Fig. 1A, B). Muscle activity was recorded using electromyography (EMG) (Delsys, Boston, MA, USA) in the first dorsal interosseus (FDI) muscle, which controls index-finger abduction. At the end of the first recording session, participants were administered oral zolpidem (0.05 mg/kg), consistent with previously reported effective sub-sedative doses (Hall et al., 2010b). An identical second MEG recording session was initiated 50 min after the zolpidem administration, with participants required to repeat the same rest and movement periods.
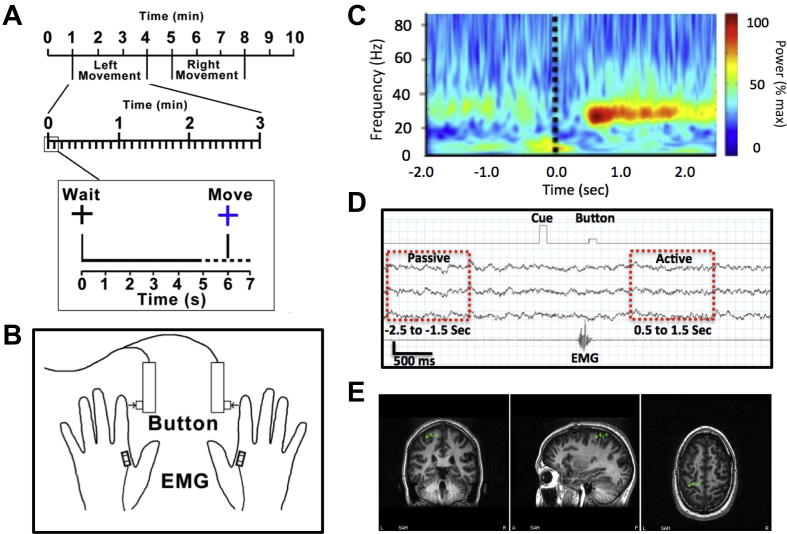
Fig. 1.
Recording of human primary motor cortex (M1) activity. (A) Participants completed a simple bi-manual FDI response task in response to visual cue. (B) Movement times were controlled and precisely recorded using a button press and EMG activity. (C) Data averaged with respect to the onset of EMG activity associated with the finger abduction. M1 can easily be identified by the generation of the PMBR following the offset of movement, seen in this example at 0.5 s post movement. (D) Active and passive window comparisons were made based upon pre-movement baseline and PMBR windows. (E) Single subject example of localized M1 cortex contralateral to movement (green) used as the location for virtual electrode recordings.
Data analysis
Previous studies have established that the post-movement increase in oscillatory activity, the PMBR, provides reliable localization of M1 hand area contralateral to the side of movement (Jurkiewicz et al., 2006; Hall et al., 2011). Therefore, in order to localize left and right M1, we used the synthetic aperture magnetometry (SAM) beamforming method (Vrba and Robinson, 2001; Hillebrand et al., 2005) to localize PMBR in M1 contralateral to the side of movement (Fig. 1). With time-zero defined as the offset of EMG activity associated with abduction of the index-finger (Fig. 1A–C), the difference image of the beta (15–35 Hz) frequency power between a pre-movement period (−2.5 to −1.5 s) and post-movement period (0.5–1.5 s) was computed (Fig. 1D). Using this approach, the 3-dimensional coordinates of the maximal t-score is a reflection of the neural generator of the PMBR, which is robustly localized to the M1 hand area contralateral to the side of the finger movement (Jurkiewicz et al., 2006). The maximal t-value in the sensorimotor cortex region provided the functional identification of M1 in each participant used as the location for virtual electrode recordings (Fig. 1E). The power profile of the oscillatory activity was determined using a 7-cycle Morlet wavelet time–frequency analysis of the virtual electrode output over the 1–100-Hz range in frequency bins of 0.5 Hz.
A pharmaco-MEG approach, as previously described (Hall et al., 2010a,b, 2011), was used to identify the changes in M1 as a consequence of zolpidem administration, visualized with power spectral density (PSD) plots. Changes in beta power were analyzed over the one-minute rest intervals, for the frequency (0.5 Hz resolution) where maximal PSD amplitude was observed in the pre-zolpidem condition in the 15–35-Hz range. In addition, the role of the balance in amplitude of beta power between hemispheres was determined by calculating the ratio of contralateral and ipsilateral beta before and after zolpidem. All data values reported in the main text are presented as mean ± S.D unless otherwise stated. Statistical differences in beta power as recorded between hemispheres or hemispheric ratios, both before and after zolpidem were determined by using paired t-tests.
The changes in beta activity related to movement were assessed at the individual beta peak. Results refer to pre-movement at rest measured at a time point 3 s before movement onset for 1 s, pre-EMG measured for 0.5 s immediately before movement. Post-EMG activity, analogous to MRBD was determined as the first point following the visual cue in which the beta band power fell below 2.5 standard deviations of the power in the baseline period and measured for 0.5 s. Early observations suggested that the duration of PMBR may be extended in PD patients. Therefore, measurement of PMBR was separated into an early-PMBR and late-PMBR commencing 1 s and 2.5 s after movement onset respectively.
Results
Robust localization of bilateral M1 was possible in eight of the nine PD patients and all nine AMC participants. Data collected from the remaining PD patient included non-experimental noise, that prevented reliable localization of M1 and therefore these data were excluded from further analysis. Overall, data were of sufficient quality in all seventeen PD and AMC participants to compare M1 resting state oscillations pre- and post-zolpidem.
Symptomatic severity and zolpidem related improvements
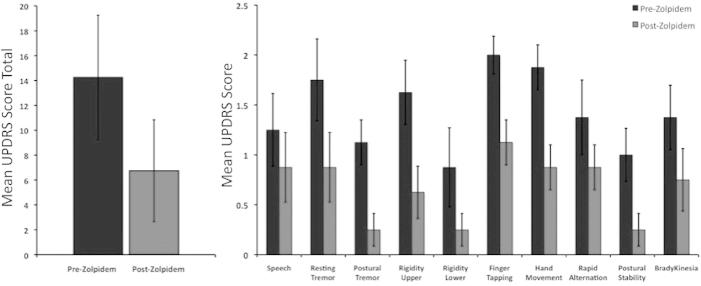
Assessment with the UPDRS confirmed that all nine PD patients presented with unilaterally dominant symptoms with varied levels of severity (UPDRS: mean 14.25 ± 5.0). In all cases, following zolpidem improvements were observed in the overall severity of the symptoms observed (UPDRS improvement: mean 7.5 ± 1.9; p = 0.006). Analysis of category revealed significant improvements in all UPDRS items except 14-speech (Fig. 2). Anecdotally, improvements were also observed in handwriting ability as noted from patient report and observation of consent form signatures obtained prior to MEG acquisition (pre-drug) and prior to MRI acquisition (post-drug).
Fig. 2.
Improvements in UPDRS following zolpidem administration. (left panel) Group mean UPDRS score (part III) before (dark) and after (light) zolpidem administration (0.05 mg/kg). (right panel) Group mean UPDRS scores for individual items before and after zolpidem administration.
Resting beta power
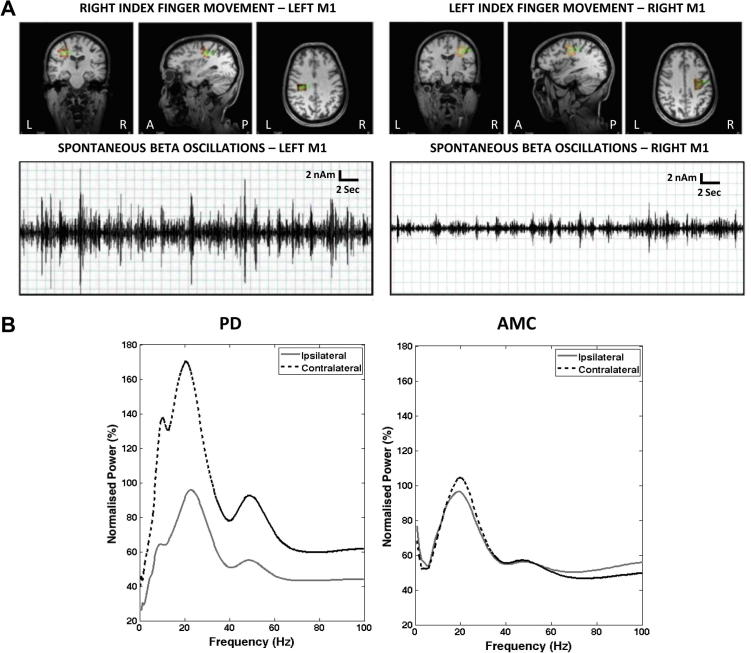
In PD patients abnormally elevated beta oscillations (15–35 Hz) were observed in contralateral M1 (Fig. 2A). This activity was characterized by prominent high amplitude irregular bursts. Irregular bursting activity was also observed in ipsilateral hemisphere but was of much lower amplitude (Fig. 3A).
Fig. 3.
Increased beta power contralateral to PD impairment. (A) Synthetic aperture magnetometry (SAM) peaks (orange) in left and right M1 in response to index finger movement (top panel). The middle panel shows representative band-pass filtered (15–35 Hz) virtual electrode (VE) traces of left and right M1 during passive rest periods. Prominent bursts of beta activity are seen in contralateral M1. (B) Power spectral density plots showing the percentage power (normalized to ipsilateral) in M1 contralateral and ipsilateral to symptomatic limbs in PD (n = 8, left panel) and the dominant hand in AMC (n = 9, right panel).
PSD analysis of PD data revealed beta activity in both contralateral and ipsilateral M1 (Fig. 3B). However, the beta power recorded in contralateral M1 was significantly higher than that of the ipsilateral region (180.29%, p = 0.003, Fig. 3B), consistent with previous reports of exaggerated beta frequency activity in PD. The frequency of the contralateral beta activity (18.2 ± 5.73 Hz) was not statistically different from the frequency of the ipsilateral (22.1 ± 5.44 Hz) beta activity (p = 0.2). A peak at mu (∼10 Hz) frequency was only observed in four of the nine controls and three of the eight PD patients and therefore statistical analysis was not carried out on this frequency. In AMC participants, there was no statistical difference in the power of the resting beta power recorded from the contralateral and ipsilateral M1 (p = 0.33).
Effects of zolpidem on resting beta power
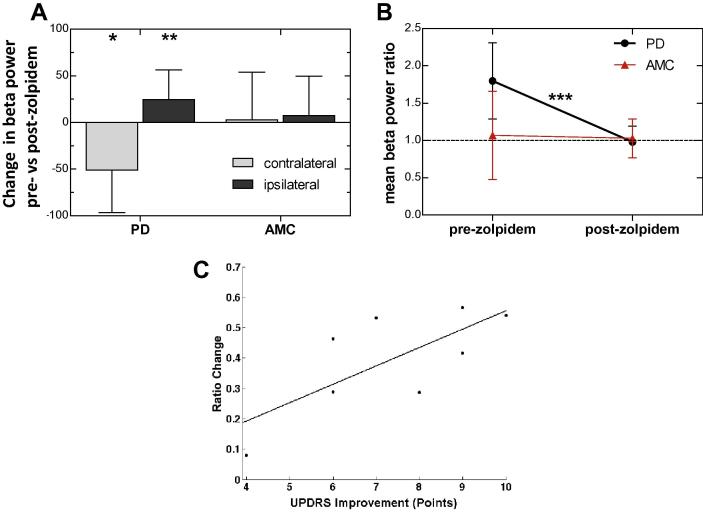
In PD participants, zolpidem reduced the power of contralateral beta by 51.1 ± 45.6%, p = 0.016, Fig. 4A) without significant change in frequency (18.2–21.8 Hz, p = 0.96). Conversely, zolpidem increased the power of ipsilateral beta by 24.4 ± 31.9%, p = 0.004, Fig. 4B) also without significant change in mean peak frequency (22.1–24.2 Hz, p = 0.27). The reduction in contralateral and increase in ipsilateral beta power (Fig. 4A, B) resulted in parity post zolpidem, (mean hemispheric (contralateral/ipsilateral) power ratio: pre-zolpidem 1.8 ± 0.51; post-zolpidem 0.98 ± 0.21, p = 0.0004, Fig. 4B) and no significant difference between hemispheres (p = 0.47).
Fig. 4.
Zolpidem modulation of inter-hemispheric M1 beta ratio in PD at rest. (A) Histograms showing percentage change in group mean beta power following administration of zolpidem in contralateral (light) and ipsilateral (dark) hemispheres in PD and AMC. (B) Mean ratio of beta power (contralateral/ipsilateral) before and after zolpidem in PD patients (n = 8) and AMC (n = 9). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. (C) Correlation between the change in inter-hemispheric beta ratio and change in UPDRS shows strong correspondence between ratio and severity (r2 = 0.52).
This address the question of functional significance the ratio of contralateral to ipsilateral beta power was determined for each patient before and after zolpidem administration. This was compared with the UPDRS for the corresponding patient (Fig. 4C). Calculation of Pearson product moment revealed a strong and significant linear dependence between inter-hemispheric M1 beta ratio change and UPDRS improvement (r2 = 0.52, p = 0.040). Further analysis of linear dependence between beta ratio and specific UPDRS items revealed strongly significant prediction of items 19: finger tapping (r2 = 0.59, p = 0.016) and 20: hand movement (r2 = 0.48, p = 0.039).
Following zolpidem administration to AMC participants there was no significant change in either contralateral beta power (2.89 ± 48.1%, p = 0.87) or ipsilateral beta power (7.4 ± 42.1%, p = 0.61, Fig. 3A). There was also no significant change in the mean beta power ratio (pre-zolpidem 1.07 ± 0.59; post-zolpidem 1.03 ± 0.26, p = 0.35, Fig. 3B).
Effects of zolpidem on movement-related changes in beta power
Finally, we tracked the absolute power changes that occurred in M1 regions, both contralateral and ipsilateral to symptom presentation, during the various phases of cued movement. Specifically, we focused upon pre-movement at rest, pre-EMG activity, post-EMG activity (MRBD), early PMBR and late PMBR. Analysis of absolute power at the peak-beta frequency in each individual, pre- versus post-zolpidem, revealed significant differences in the contralateral hemisphere pre-EMG (p = 0.026) and late PMBR (p = 0.044) (Fig. 5A upper panel) and in the ipsilateral hemisphere resting beta (p = 0.022), pre-EMG (p = 0.032) and late PMBR (p = 0.042) were all significantly different (Fig. 5A lower panel). In each case, low-dose zolpidem had no significant effect on the post-EMG (MRBD). To provide a temporal representation of the differences observed, a post hoc continuous t-test of beta power in each sample was used to illustrate the periods of significant difference. These were then superimposed upon the beta power profiles (Fig. 5).
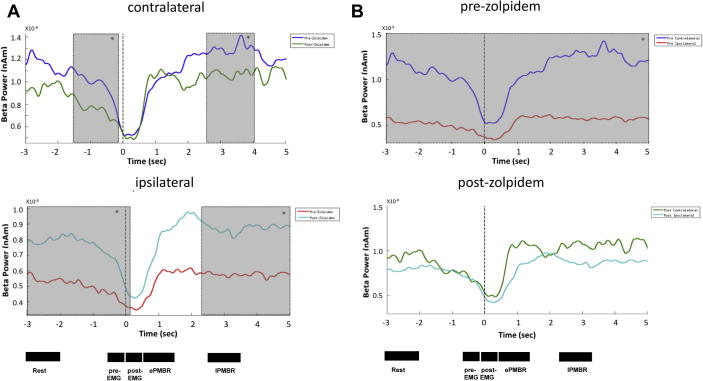
Fig. 5.
Effects of zolpidem on movement-related changes in beta activity. Comparative group images (n = 8) of beta power during the movement task, where dotted line at time 0 indicates the onset of EMG activity. (A) Upper panel compares M1 beta power contralateral to symptom presentation. Pre-zolpidem (blue) and post-zolpidem (green). The lower panel compares data from ipsilateral to symptom presentation. Pre-zolpidem (red) and post-zolpidem (light blue). (B) The upper panel shows pre-zolpidem data from ipsilateral and contralateral hemispheres, while the lower panel compares the data post-zolpidem. The specific time intervals indicated were used to assess differences in the pre-movement rest, pre-EMG, post-EMG (MRBD), early (e) PMBR and late (l) PMBR. Shaded areas indicate periods of significant differences as determined by a post hoc continuous t-test of beta power.
A comparison of M1 contralateral and ipsilateral beta power pre-zolpidem showed significant differences at all time points pre and post movement (rest, p = 0.022, pre-EMG, p = 0.02, post-EMG, p = 0.016, early PMBR, p = 0.039, late PMBR, p = 0.021, Fig. 5B upper panel). In contrast, the contralateral versus ipsilateral beta power post-zolpidem, showed no time points of significant difference (Fig. 5B lower panel). Hence, zolpidem appeared to induce hemispheric parity in both resting and movement-related dynamic beta.
Discussion
In this study we have shown prominent disparity in inter-hemispheric resting beta power in the motor cortex in early-stage PD participants. Furthermore, modulation of GABAA receptors using zolpidem re-normalized inter-hemispheric beta power such that the ratio approached parity. These changes correlated with symptomatic improvement. These changes in response to zolpidem were also reflected in pre-movement beta desynchronization and the late PMBR.
Exaggerated contralateral beta power
For a number of years exaggerated beta oscillations have been observed in subcortical structures such as STN and globus pallidus in both PD patients and animal models of PD (Brown and Marsden, 2001; Cassidy et al., 2002). This increase in beta power is accompanied by increased synchrony and coherence across networks and is thought to limit computational capacity (Hammond et al., 2007) through reduced information coding and consequent inability to uncouple and re-recruit appropriate motor networks. However, in the cortex, pathologically exaggerated oscillations have not been typically observed in PD patients. This may be due to the fact that the majority of studies focus on late-stage patients who are usually taking dopamine replacement therapies which are known to reduce beta power (Silberstein et al., 2005; Kuhn et al., 2006; Kühn et al., 2009; Jenkinson and Brown, 2011) or collapse of cortical beta generation in later stages of the disease. The results presented here confirm those in a previous investigation in early-stage PD patients which did report enhanced beta in M1 contralateral to the most severely affected side (Pollok et al., 2012). Anecdotally, a number of the patients (e.g. Fig. 3B) exhibited pronounced temporal fluctuation in beta band power (so-called bursting), which may be of importance with regard to functional abnormalities as observed in previous intraoperative recordings (Little et al., 2012).
Hemispheric parity of beta power
At face value, the effects of low-dose zolpidem on contralateral beta power are analogous to that of l-DOPA, with both drugs decreasing exaggerated beta and thus removing a functional block allowing appropriate desynchronization to take place prior to movement. However, a recent report has indicated that increased resting beta power is normal in healthy aging (Rossiter et al., 2014), an effect which is more pronounced contralateral than ipsilateral to the dominant hand. Given that most of the healthy-aged population do not show parkinsonian symptoms, it seems unlikely that exaggerated resting beta alone underlies PD symptoms. In this context, most of the reports to date have investigated beta power in PD on one side of the brain only, due to clinical and/or methodological considerations. In the current study, we have shown that, in contrast to the inhibitory effects of zolpidem on contralateral beta power, ipsilateral beta power is augmented, resulting in an equalisation of inter-hemispheric power at rest. These data suggest strongly that the inter-hemispheric power ratio is a critical determinant and potential biomarker of symptomatic PD. In support of this, in control participants, beta power on both sides of the brain is augmented by zolpidem, suggesting that beta power per se is irrelevant in the context of PD, so long as the power is equably distributed bilaterally.
This observation offers a rather simplistic explanation for drug-induced improvements in motor function; however, it is worth considering the implications of lateral equity in motor function. The performance of a finger movement is accompanied by a reduction in beta power that occurs bilaterally in the sensorimotor cortex (Pfurtscheller and Berghold, 1989; Leocani et al., 1997). While the general assumption is that the process of desynchronization represents an uncoupling of units in the contralateral hemisphere to engage and elicit a specific motor output, the consistent observation of this bilateral modulation would suggest that interaction between hemispheres is an intrinsic part of the process; imbalance in this relationship therefore represents a potential problem for any dependent communication.
Zolpidem effects on MRBD and PMBR
Our analyses of zolpidem-induced effects on beta power in relation to cued movement shows clear differences in the pre-EMG, consistent with the observation of elevated spontaneous beta power in M1 in PD. However, we observed no significant difference in MRBD following zolpidem administration. This is perhaps surprising, given that previous studies have observed increases in MRBD during GABAergic modulation (Hall et al., 2011; Muthukumaraswamy et al., 2013). However, these findings are supportive of the suggestion that movement generation is not mechanistically dependent upon MRBD per se, as previous studies have demonstrated independence of MRBD and motor function (Hall et al., 2011; McAllister et al., 2013). The fact that MRBD is not changed may indicate that MRBD is not a driver of motor output or indeed that this motor output is not impaired in early stage PD.
Functionally, it is possible that the zolpidem-induced normalization of the pre-EMG represents resolution of a cognitive load problem. In this scenario, differences in the pre-EMG phase might be due to alterations in the ability to plan before making the movement. Hence, the preparatory pre-EMG phase could have a potential role in the preparation and initiation of movement (i.e. bradykinesia), with the amplitude of synchronization before movement being related to the size of the network recruited to achieve the force required.
Here, we observe that early-PMBR appears unaffected by zolpidem, consistent with our previous observation that GABAergic modulation does not modify the amplitude of this feature (Hall et al., 2011). However, we observe significant differences in late-PMBR, which remains elevated for several seconds in the pre-zolpidem condition. Functionally, if elevated beta power is problematic for the initiation of movement, this may compound the problem of making continual, smooth movements because disengagement from previous motor programs may be impaired. Equally, the change in this feature may represent an effect, rather than a cause, of improvement in movement.
GABA-mediated network changes
The method used here does not offer the sensitivity to determine whether changes in beta power are the direct result of GABA modulation of M1 or secondary effects of GABA modulation in sub-cortical regions or both. However, evidence from rodent M1 models demonstrates that M1 beta is increased following zolpidem (100 nM) administration through increased phasic interneuron drive (Yamawaki et al., 2008, consistent with previously described models, see Whittington et al., 1995; Traub et al., 1996). Furthermore, recent work describes how zolpidem produces dose-specific effects on oscillations in M1 through an alpha-1 subunit-mediated reduction in beta power at low concentration of 10 nM and increased beta power at concentrations above 30 nM, the effects of low-dose zolpidem being mediated by selective modulation of interneuron-specific tonic current (Prokic et al., unpublished observation).
Conclusion
We have shown that low-dose zolpidem improves PD symptoms, reduces exaggerated pathological contralateral M1 cortical beta and increases ipsilateral beta power resulting in parity of hemispheric beta power ratio which directly correlates with symptom severity.
Acknowledgments
We would like to thank funding support from Parkinson’s UK (G-1008), the Biotechnology and Biological Sciences Research Council (BB/H003894/1) and the Dr Hadwen Trust and Lord Dowding Fund for financing the MEG and MRI. The Elekta MEG system was funded by The Wellcome Trust (grant 088314/Z/09/Z).
References
- Adjamian P., Barnes G.R., Hillebrand A., Holliday I.E., Singh K.D., Furlong P.L., Harrington E., Barclay C.W., Route P.J.G. Co-registration of magnetoencephalography with magnetic resonance imaging using bite-bar-based fiducials and surface-matching. Clin Neurophysiol. 2004;115:691–698. doi: 10.1016/j.clinph.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Baker S.N., Olivier E., Lemon R.N. Coherent oscillations in monkey motor cortex and hand muscle EMG show task-dependent modulation. J Physiol. 1997;501(1):225–241. doi: 10.1111/j.1469-7793.1997.225bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P., Marsden J.F. Cortical network resonance and motor activity in humans. Neuroscientist. 2001;7:518–527. doi: 10.1177/107385840100700608. [DOI] [PubMed] [Google Scholar]
- Cassidy M., Mazzone P., Oliviero A., Insola A., Tonali P., Di Lazzaro V., Brown P. Movement-related changes in synchronization in the human basal ganglia. Brain. 2002;125:1235–1246. doi: 10.1093/brain/awf135. [DOI] [PubMed] [Google Scholar]
- Chen C.C., Litvak V., Gilbertson T., Kühn A., Lu C.S., Lee S.T., Tsai C.H., Tisch S., Limousin P., Hariz M., Brown P. Excessive synchronization of basal ganglia neurons at 20 Hz slows movement in Parkinson’s disease. Exp Neurol. 2007;205:214–221. doi: 10.1016/j.expneurol.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Chen Y.Y., Sy H.N., Wu S.L. Zolpidem improves akinesia, dystonia and dyskinesia in advanced Parkinson’s disease. J Clin Neurosci. 2008;15:955–956. doi: 10.1016/j.jocn.2007.07.082. [DOI] [PubMed] [Google Scholar]
- Clauss R., Nel W. Drug induced arousal from the permanent vegetative state. NeuroRehabilitation. 2006;21:23–28. [PubMed] [Google Scholar]
- Cohen L., Chaaban B., Habert M.O. Transient improvement of aphasia with zolpidem. N Engl J Med. 2004;350:949–950. doi: 10.1056/NEJM200402263500922. [DOI] [PubMed] [Google Scholar]
- Daniele A., Albanese A., Gainotti G., Gregori B., Bartolomeo P. Zolpidem in Parkinson’s disease. Lancet. 1997;349:1222–1223. doi: 10.1016/S0140-6736(05)62416-6. [DOI] [PubMed] [Google Scholar]
- Eusebio A., Thevathasan W., Doyle Gaynor L., Pogosyan A., Bye E., Foltynie T., Zrinzo L., Ashkan K., Aziz T., Brown P. Deep brain stimulation can suppress pathological synchronisation in parkinsonian patients. J Neurol Neurosurg Psychiatry. 2011;82(5):569–573. doi: 10.1136/jnnp.2010.217489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W., Edgar J.C., Wang D.J., Roberts T.P. Relating MEG measured motor cortical oscillations to resting gamma-aminobutyric acid (GABA) concentration. Neuroimage. 2011;55:616–621. doi: 10.1016/j.neuroimage.2010.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S.D., Barnes G.R., Furlong P.L., Seri S., Hillebrand A. Neuronal network pharmacodynamics of GABAergic modulation in the human cortex determined using pharmaco-magnetoencephalography. Hum Brain Mapp. 2010;31:581–594. doi: 10.1002/hbm.20889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S.D., Yamawaki N., Fisher A.E., Clauss R.P., Woodhall G.L., Stanford I.M. GABA(A) alpha-1 subunit mediated desynchronization of elevated low frequency oscillations alleviates specific dysfunction in stroke – a case report. Clin Neurophysiol. 2010;121:549–555. doi: 10.1016/j.clinph.2009.11.084. [DOI] [PubMed] [Google Scholar]
- Hall S.D., Stanford I.M., Yamawaki N., Mcallister C.J., Rönnqvist K.C., Woodhall G.L., Furlong P.L. GABAergic modulation of motor function related neuronal network activity. Neuroimage. 2011;56(3):1506–1510. doi: 10.1016/j.neuroimage.2011.02.025. [DOI] [PubMed] [Google Scholar]
- Hammond C., Bergman H., Brown P. Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci. 2007;30:357–364. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Hillebrand A., Singh K.D., Holliday I.E., Furlong P.L., Barnes G.R. A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapp. 2005;25:199–211. doi: 10.1002/hbm.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.Y., Hsu Y.T., Wu Y.C., Chiou S.M. Zolpidem improves neuropsychiatric symptoms and motor dysfunction in a patient with Parkinson’s disease after deep brain stimulation. Acta Neurol Taiwan. 2012;21:84–86. [PubMed] [Google Scholar]
- Jarry C., Fontenas J.P., Jonville.-Bera A.P., Utret-Leca E. Beneficial effect of zolpidem for dementia. Ann Pharmacother. 2002;36:1808. doi: 10.1345/aph.1C087. [DOI] [PubMed] [Google Scholar]
- Jenkinson N., Brown P. New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci. 2011;34:611–618. doi: 10.1016/j.tins.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Jensen O., Goel P., Kopell N., Pohja M., Hari R., Ermentrout B. On the human sensorimotor-cortex beta rhythm: sources and modeling. Neuroimage. 2005;26:347–355. doi: 10.1016/j.neuroimage.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz M.T., Gaetz W.C., Bostan A.C., Cheyne D. Post-movement beta rebound is generated in motor cortex: evidence from neuromagnetic recordings. Neuroimage. 2006;32:1281–1289. doi: 10.1016/j.neuroimage.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Kuhn A.A., Kupsch A., Schneider G.H., Brown P. Reduction in subthalamic 8–35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease. Eur J Neurosci. 2006;23:1956–1960. doi: 10.1111/j.1460-9568.2006.04717.x. [DOI] [PubMed] [Google Scholar]
- Kühn A.A., Kempf F., Brücke C., Doyle L.G., Martinez-Torres L., Pogosyan A., Trottenberg T., Kupsch A., Schneider G.-H., Hariz M.I., Vandenberghe W., Nuttin B., Brown P. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory B activity in patients with Parkinson’s disease in parallel with improvement in motor performance. J Neurosci. 2008;28(24):6165–6173. doi: 10.1523/JNEUROSCI.0282-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn A.A., Tsui A., Aziz T., Ray N., Brücke C., Kupsch A., Schneider G.H., Brown P. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity. Exp Neurol. 2009;215(2):380–387. doi: 10.1016/j.expneurol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Leocani L., Toro C., Manganotti P., Zhuang P., Hallett M. Event-related coherence and event-related desynchronization/synchronization in the 10 Hz and 20 Hz EEG during self-paced movements. Electroencephalogr Clin Neurophysiol. 1997;104:199–206. doi: 10.1016/s0168-5597(96)96051-7. [DOI] [PubMed] [Google Scholar]
- Little S., Pogosyan A., Kuhn A.A., Brown P. Beta band stability over time correlates with parkinsonian rigidity and bradykinesia. Exp Neurol. 2012;236:383–388. doi: 10.1016/j.expneurol.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N., Pogosyan A., Sharott A., Csicsvari J., Bolam J.P., Brown P., Magill P.J. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J Neurosci. 2008;28:4795–4806. doi: 10.1523/JNEUROSCI.0123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcallister C.J., Ronnqvist K.C., Stanford I.M., Woodhall G.L., Furlong P.L., Hall S.D. Oscillatory beta activity mediates neuroplastic effects of motor cortex stimulation in humans. J Neurosci. 2013;33(18):7919–7927. doi: 10.1523/JNEUROSCI.5624-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy V.N., Fetz E.E. Oscillatory activity in sensorimotor cortex of awake monkeys: synchronization of local field potentials and relation to behavior. J Neurophysiol. 1996;76:3949–3967. doi: 10.1152/jn.1996.76.6.3949. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy S.D., Myers J.F.M., Wilson S.J., Nutt D.J., Lingford-Hughes A., Singh K.D., Hamandi K. The effects of elevated endogenous GABA levels on movement-related network oscillations. Neuroimage. 2013;66:36–41. doi: 10.1016/j.neuroimage.2012.10.054. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G., Berghold A. Patterns of cortical activation during planning of voluntary movement. Electroencephalogr Clin Neurophysiol. 1989;72:250–258. doi: 10.1016/0013-4694(89)90250-2. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G., Stancak A., Neuper C. Post-movement beta synchronization. A correlate of an idling motor area? Electroencephalography Clin Neurophysiol. 1996;98:281–293. doi: 10.1016/0013-4694(95)00258-8. [DOI] [PubMed] [Google Scholar]
- Pollok B., Krause V., Martsch W., Wach C., Schnitzler A., Sudmeyer M. Motor-cortical oscillations in early stages of Parkinson’s disease. J Physiol. 2012;590:3203–3212. doi: 10.1113/jphysiol.2012.231316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter H.E., Davis E.M., Clark E.V., Boudrias M.-H., Ward N.S. Beta oscillations reflect changes in motor cortex inhibition in healthy ageing. Neuroimage. 2014;91:360–365. doi: 10.1016/j.neuroimage.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shames J.L., Ring H. Transient reversal of anoxic brain injury-related minimally conscious state after zolpidem administration: a case report. Arch Phys Med Rehab. 2008;89:386–388. doi: 10.1016/j.apmr.2007.08.137. [DOI] [PubMed] [Google Scholar]
- Sharott A., Magill P.J., Harnack D., Kupsch A., Meissner W., Brown P. Dopamine depletion increases the power and coherence of beta-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat. Eur J Neurosci. 2005;21:1413–1422. doi: 10.1111/j.1460-9568.2005.03973.x. [DOI] [PubMed] [Google Scholar]
- Silberstein P., Pogosyan A., Kühn Aa., Hotton G., Tisch S., Kupsch A., Dowsey-Limousin P., Mi Hariz., Brown P. Cortico-cortical coupling in Parkinson’s disease and its modulation by therapy. Brain. 2005;128(6):1277–1291. doi: 10.1093/brain/awh480. [DOI] [PubMed] [Google Scholar]
- Traub R.D., Whittington M.A., Colling S.B., Buzsaki G., Jefferys J.G.R. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. J Physiol-Lond. 1996;493:471–484. doi: 10.1113/jphysiol.1996.sp021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrba J., Robinson S.E. Signal processing in magnetoencephalography. Methods. 2001;25:249–271. doi: 10.1006/meth.2001.1238. [DOI] [PubMed] [Google Scholar]
- Whittington M.A., Traub R.D., Jefferys J.G. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;73:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Yamawaki N., Stanford I.M., Hall S.D., Woodhall G.L. Pharmacologically induced and stimulus evoked rhythmic neuronal oscillatory activity in the primary motor cortex in vitro. Neuroscience. 2008;151:386–395. doi: 10.1016/j.neuroscience.2007.10.021. [DOI] [PubMed] [Google Scholar]